Abstract
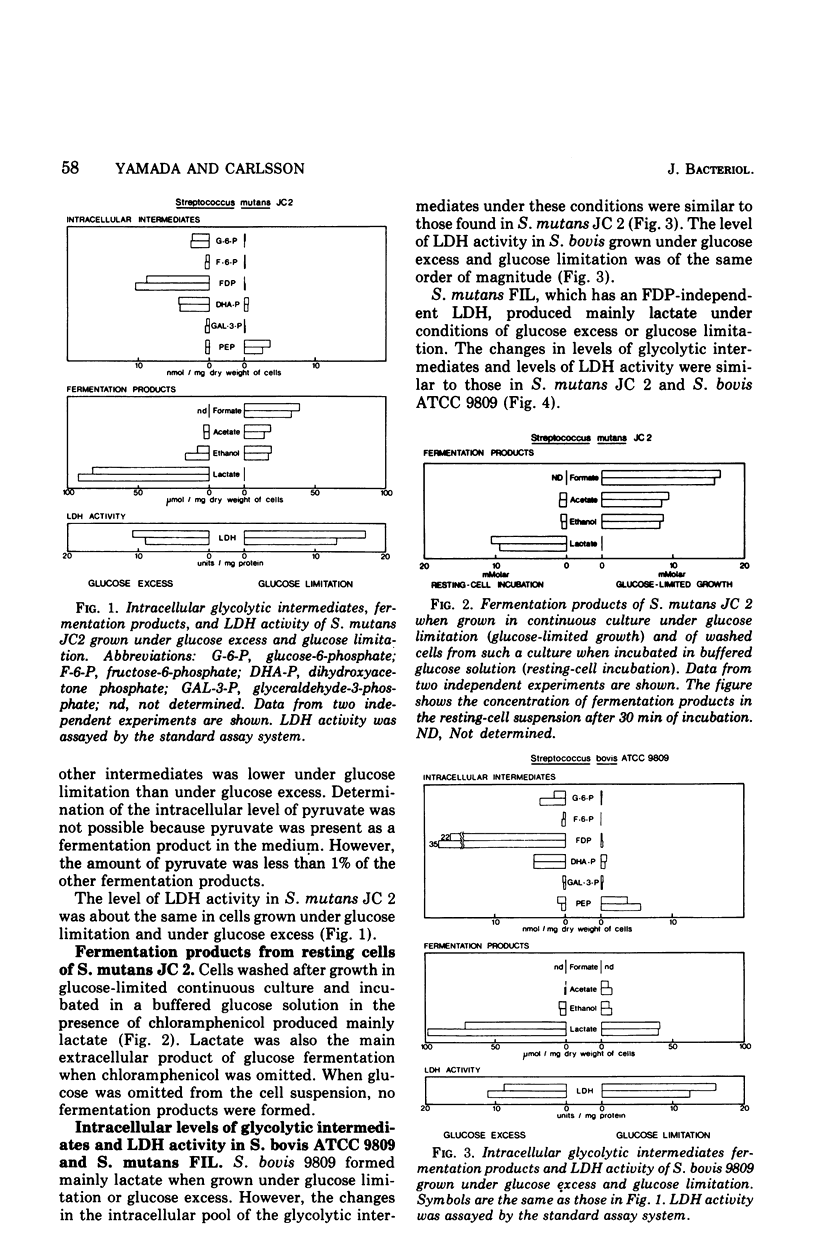
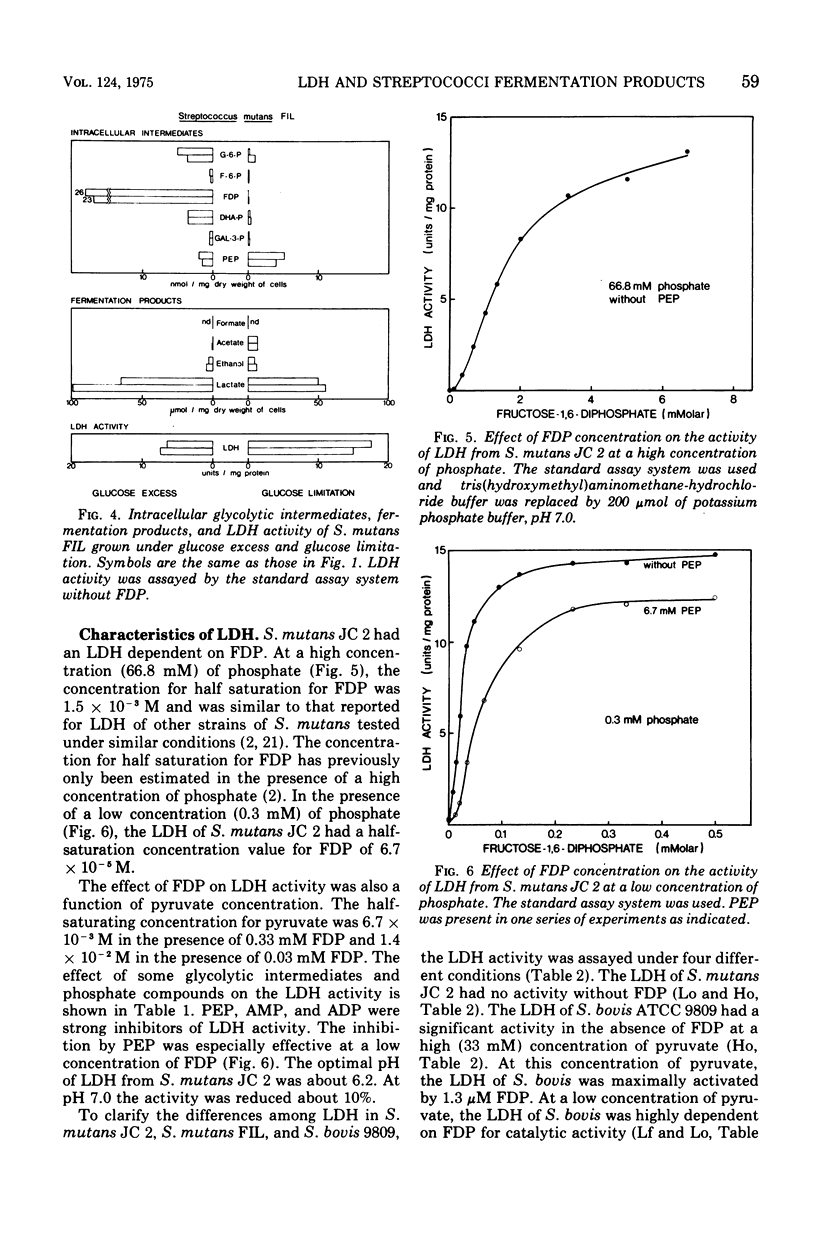
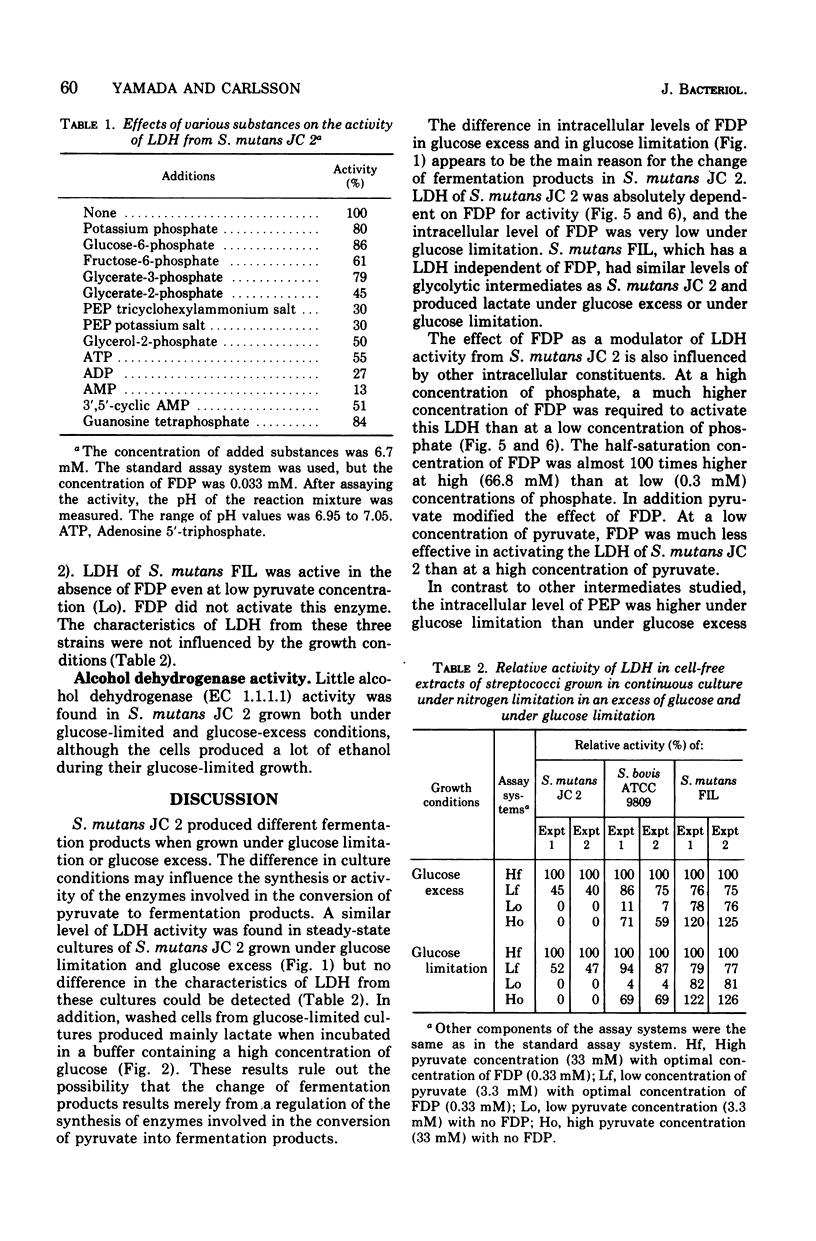
Streptococcus mutans JC 2 produced mainly lactate as a fermentation product when grown in nitrogen-limited continuous culture in the presence of an excess of glucose and produced formate, acetate, and ethanol, but no lactate, under glucose-limited conditions. The levels of lactate dehydrogenase (LDH) in these cultures were of the same order of magnitude, and the activity of LDH was completely dependent on fructose-1,6-diphosphate (FDP). The intracellular level of FDP was high and the level of phosphoenolpyruvate (PEP) was low under the glucose-excess conditions. In the glucose-limited cultures, all glycolytic intermediates studied, except PEP, were low. S. mutans FIL, which had an FDP-independent LDH and similar levels of glycolytic intermediates as S. mutans JC2, produced mainly lactate under glucose-excess or under glucose-limited conditions. LDH of Streptococcus bovis ATCC 9809 was dependent on FDP for activity at a low concentration of pyruvate but had a significant activity without FDP at a high concentration of pyruvate. This strain also produced mainly lactate both under glucose-excess and glucose-limited conditions. The levels and characteristics of these LDHs were not changed by the culture conditions. These results indicate that changes in the intracellular level of FDP regulate LDH activity, which in turn influences the type of fermentation products produced by streptococci. PEP, adenosine 5'-monophosphate, adenosine 5'-diphosphate, and inorganic phosphate significantly inhibited LDH activity from S. mutans JC 2 and may also participate in the regulation of LDH activity in other streptococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Ethanol production and alcohol dehydrogenase activity in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):127–131. doi: 10.1016/0003-9969(73)90027-7. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus salivarius. J Gen Microbiol. 1971 Jul;67(1):69–76. doi: 10.1099/00221287-67-1-69. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Simplified gas chromatographic procedure for identification of bacterial metabolic products. Appl Microbiol. 1973 Feb;25(2):287–289. doi: 10.1128/am.25.2.287-289.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Griffith C. J., Carlsson J. Mechanism of ammonia assimilation in streptococci. J Gen Microbiol. 1974 Jun;82(2):253–260. doi: 10.1099/00221287-82-2-253. [DOI] [PubMed] [Google Scholar]
- Jonas H. A., Anders R. F., Jago G. R. Factors affecting the activity of the lactate dehydrognease of Streptococcus cremoris. J Bacteriol. 1972 Aug;111(2):397–403. doi: 10.1128/jb.111.2.397-403.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., Lauer E. Neuere Vorstellungen zur Taxonomie der Bifidobacterien. Zentralbl Bakteriol Orig A. 1974;228(1):29–45. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Mou L., Mulvena D. P., Jonas H. A., Jago G. R. Purification and properties of nicotinamide adenine dinucleotide-dependent D- and L- lactate dehydrogenases in a group N streptococcus. J Bacteriol. 1972 Aug;111(2):392–396. doi: 10.1128/jb.111.2.392-396.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark H., Tung M. C. Properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Acholeplasma laidlawii type A. J Bacteriol. 1973 Jun;114(3):1025–1033. doi: 10.1128/jb.114.3.1025-1033.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Glucose-6-phosphate-dependent pyruvate kinase in Streptococcus mutans. J Bacteriol. 1975 Oct;124(1):562–563. doi: 10.1128/jb.124.1.562-563.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Phosphoenolpyruvate carboxylase and ammonium metabolism in oral streptococci. Arch Oral Biol. 1973 Jul;18(7):799–812. doi: 10.1016/0003-9969(73)90051-4. [DOI] [PubMed] [Google Scholar]
- de Vries W., Gerbrandy S. J., Stouthamer A. H. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967 Apr 25;136(3):415–425. doi: 10.1016/0304-4165(67)90001-3. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stouthamer A. H. Fermentation of glucose, lactose, galactose, mannitol, and xylose by bifidobacteria. J Bacteriol. 1968 Aug;96(2):472–478. doi: 10.1128/jb.96.2.472-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



