Abstract
The gene trmA, responsible for the production of 5-methyluridine (ribothymidine) in transfer ribonucleic acid, has been located at 79 min on the chromosomal map of Escherichia coli K-12. In five-factor crosses the gene order was shown to be argH-trmA-rif-thiA-metA. The co-transduction frequency between argH and trmA was 65%. Furthermore, the trmA5 mutation was shown to be recessive, in agreement with the notion that the trmA gene is the structural gene for the transfer tibonucleic acid (5-methyluridine) methyltransferase.
Full text
PDF


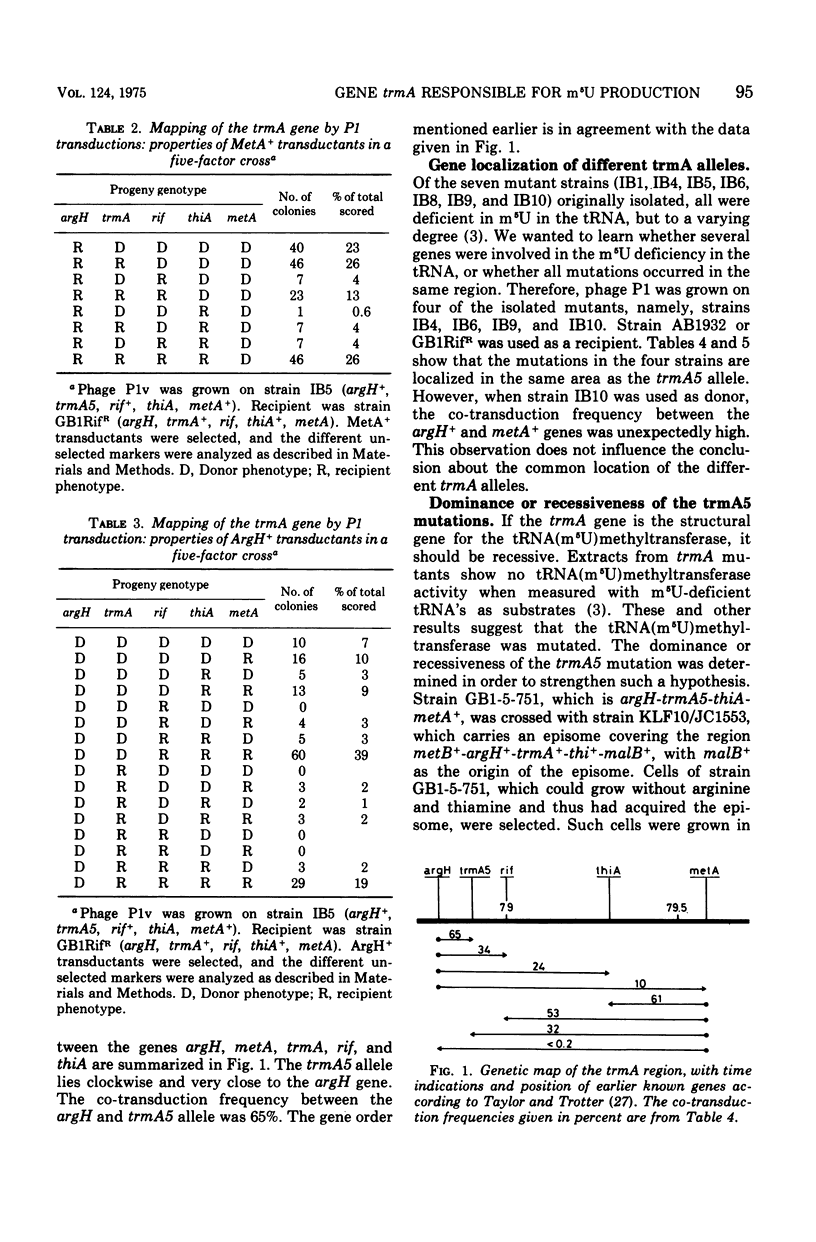
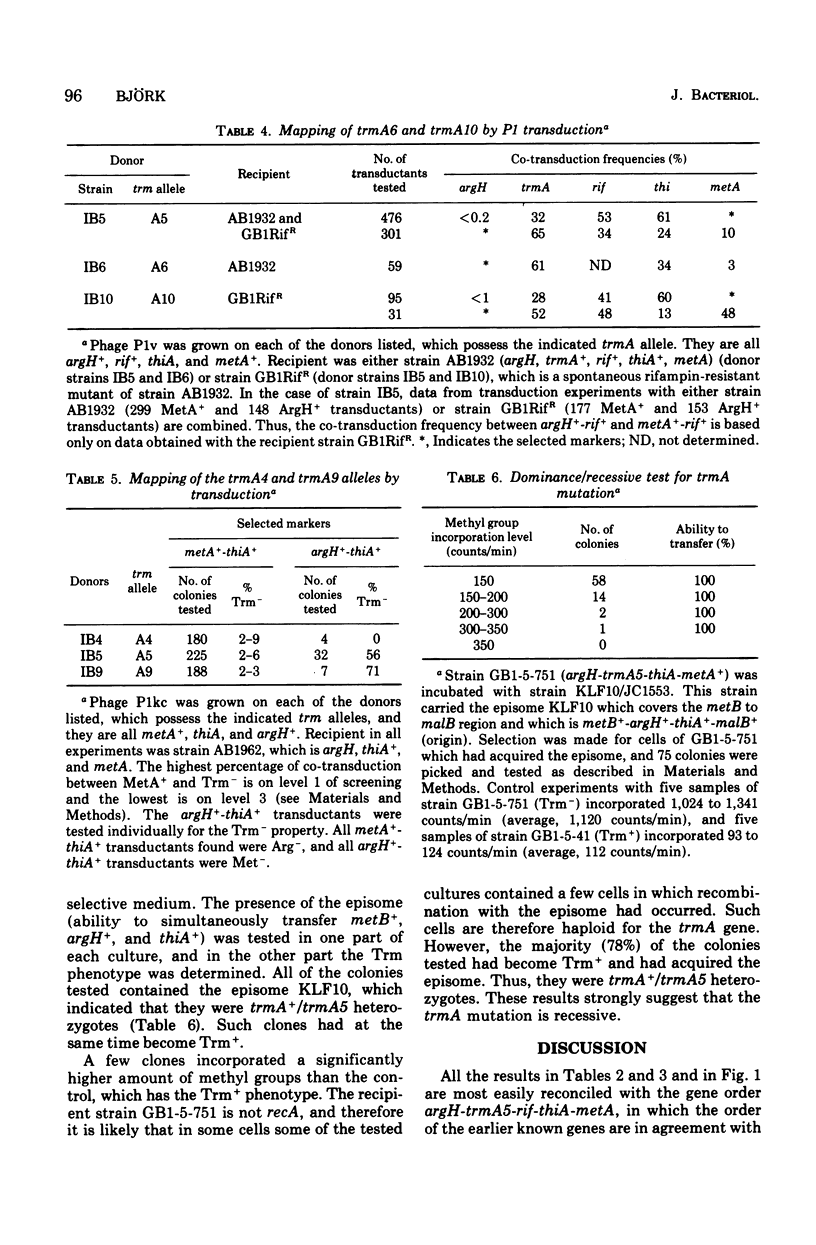


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Analysis of 5-methyluridine function in the transfer RNA of Escherichia coli. Cancer Res. 1971 May;31(5):706–709. [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G. Mapping of ochre suppressors in Escherichia coli. Genet Res. 1968 Feb;11(1):15–20. doi: 10.1017/s0016672300011150. [DOI] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Jasper P., Whitney E., Silver S. Genetic locus determining resistance to phage BF23 and colicins E 1 , E 2 and E 3 in Escherichia coli. Genet Res. 1972 Jun;19(3):305–312. doi: 10.1017/s0016672300014555. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Nakata T., Nose Y. Genetic mapping with a thiamine-requiring auxotroph of Escherichia coli K-12 defective in thiamine phosphate pyrophosphorylase. J Bacteriol. 1968 Apr;95(4):1483–1485. doi: 10.1128/jb.95.4.1483-1485.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R., Söll D., Kwong T. C. Isolation and partial characterization of three Escherichia coli mutants with altered transfer ribonucleic acid methylases. J Bacteriol. 1975 Apr;122(1):257–265. doi: 10.1128/jb.122.1.257-265.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Chromosomal location of a gene for fructose 6-phosphate kinase in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1108–1109. doi: 10.1128/jb.100.2.1108-1109.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Gartner T. K., Lannan J. E., Betlach M. Close linkage between ochre and missense suppressors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1125–1133. doi: 10.1128/jb.109.3.1125-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science. 1970 Jan 2;167(3914):56–58. doi: 10.1126/science.167.3914.56. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S. Ribosome development and the methylation of ribosomal ribonucleic acid. J Bacteriol. 1968 May;95(5):1844–1850. doi: 10.1128/jb.95.5.1844-1850.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- ZAMIR A., HOLLEY R. W., MARQUISEE M. EVIDENCE FOR THE OCCURRENCE OF A COMMON PENTANUCLEOTIDE SEQUENCE IN THE STRUCTURES OF TRANSFER RIBONUCLEIC ACIDS. J Biol Chem. 1965 Mar;240:1267–1273. [PubMed] [Google Scholar]



