Abstract
A mutant of Escherichia coli that contains essentially no detectable glutathione has been isolated. The mutant contains a very low level of the enzyme glutathione synthetase and accumulates lambda-glutamyl cysteine at a concentration approximately equal to the level of glutathione found in its parent. No significant differences in growth were observed between the mutant and its parent. However, the activity of at least one enzyme was found to be affected by the absence of glutathione; the specific activity of the B1 subunit of ribonucleoside diphosphate reductase was greatly reduced. The possibility that the decreased B1 activity is due to a mutation in the structural gene coding for B1 or its regulatory gene could be eliminated. This suggests that one role of glutathione in the cell is to maintain at least this one protein in an active state. We propose the designation gshB for the gene coding for glutathione synthetase.
Full text
PDF


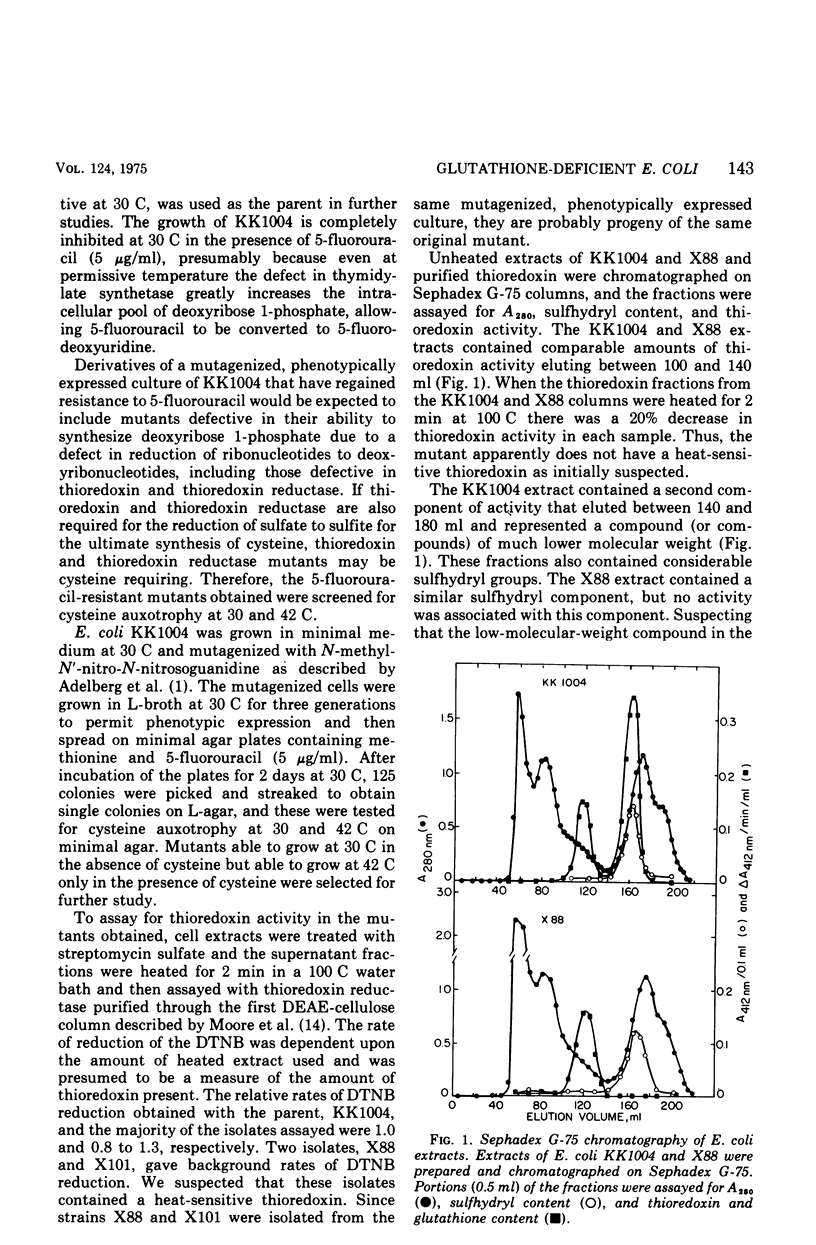
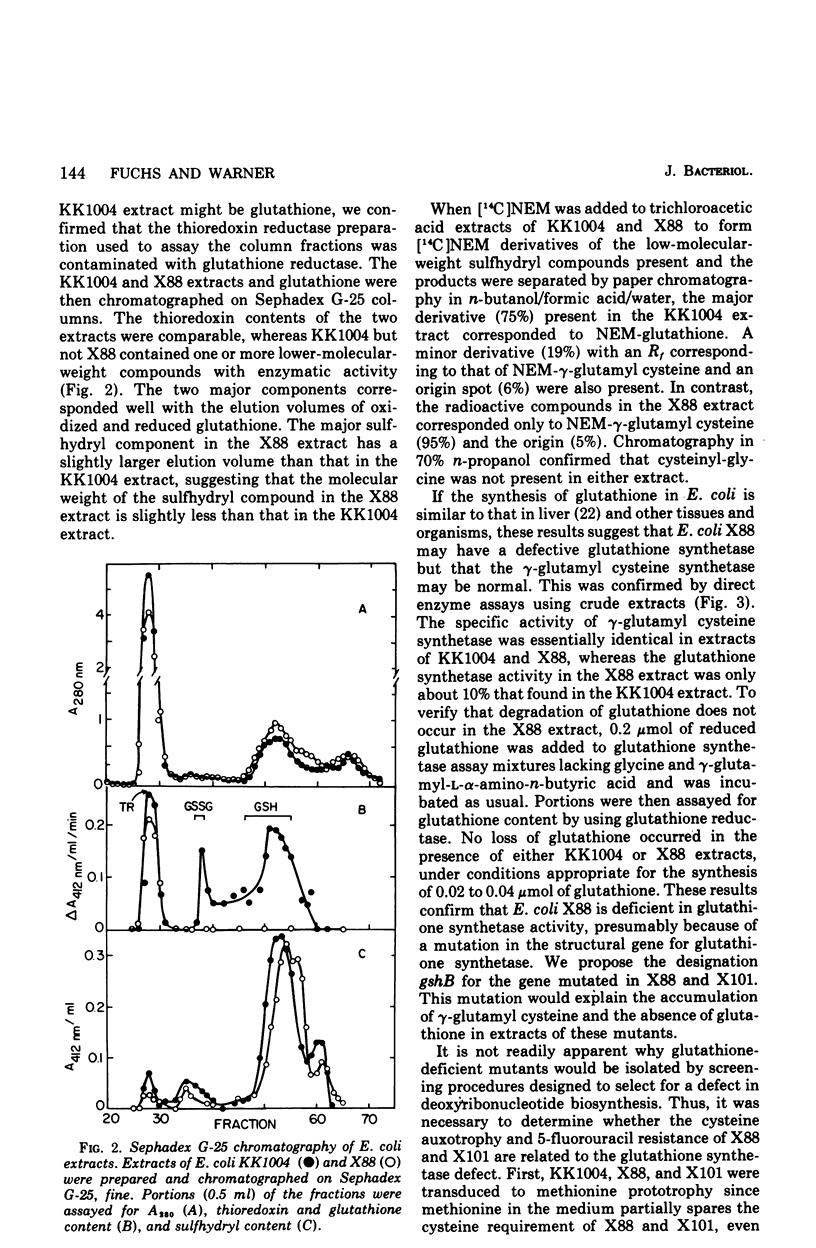
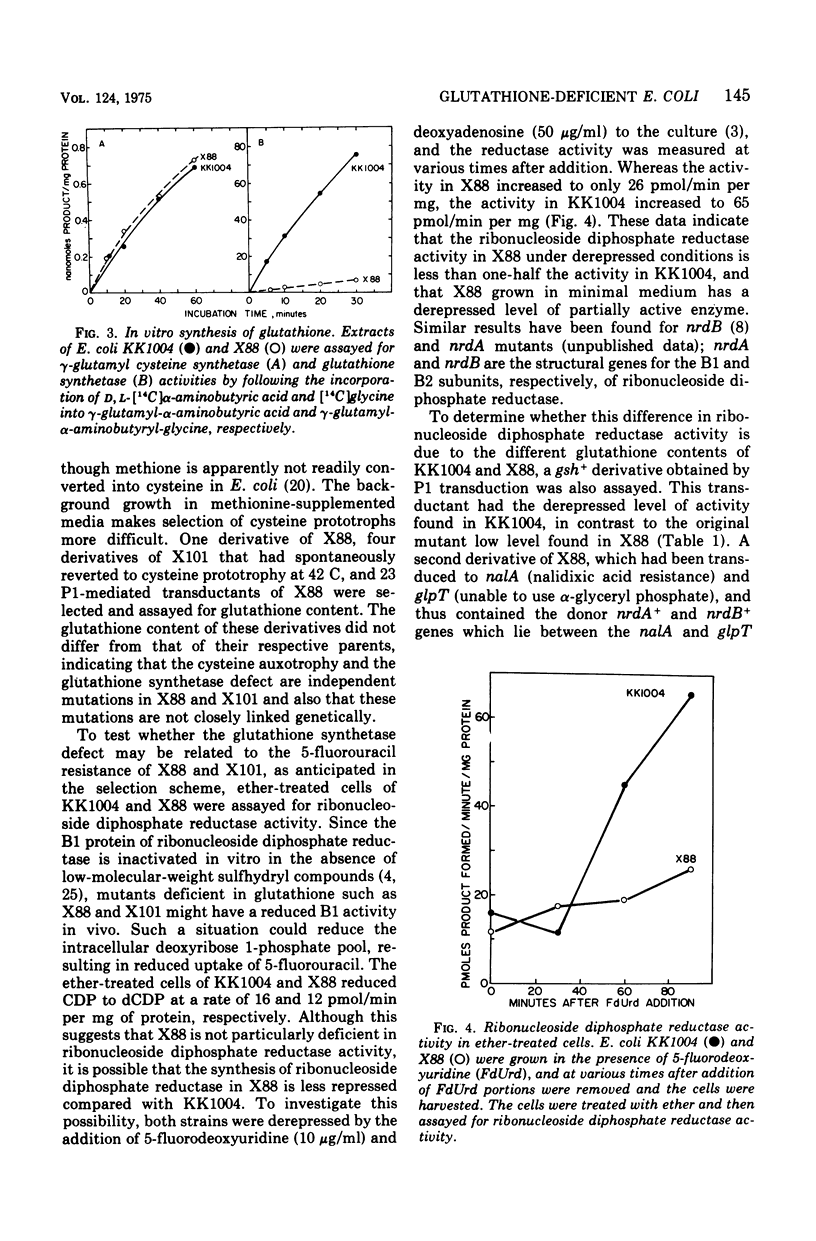



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C., Hardy J., Beck W. S. Release of repressor control of ribonucleotide reductase by thymine starvation. J Biol Chem. 1965 Sep;240(9):3631–3640. [PubMed] [Google Scholar]
- Brown N. C., Canellakis Z. N., Lundin B., Reichard P., Thelander L. Ribonucleoside diphosphate reductase. Purification of the two subunits, proteins B1 and B2. Eur J Biochem. 1969 Jul;9(4):561–573. doi: 10.1111/j.1432-1033.1969.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. A mutant of Escherichia coli defective in ribonucleosidediphosphate reductase. 2. Characterization of the enzymatic defect. Eur J Biochem. 1973 Feb 1;32(3):457–462. [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O., Warner H. R., Reichard P. Defective gene product in dnaF mutant of Escherichia coli. Nat New Biol. 1972 Jul 19;238(81):69–71. doi: 10.1038/newbio238069a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- REICHARD P. Enzymatic synthesis of deoxyribonucleotides. I. Formation of deoxycytidine diphosphate from cytidine diphosphate with enzymes from Escherichia coli. J Biol Chem. 1962 Nov;237:3513–3519. [PubMed] [Google Scholar]
- Rossman T., Norris C., Troll W. Inhibition of macromolecular synthesis in Escherichia coli by protease inhibitors. Specific reversal by glutathione of the effects of chloromethyl ketones. J Biol Chem. 1974 Jun 10;249(11):3412–3417. [PubMed] [Google Scholar]
- SNOKE J. E., BLOCH K. Formation and utilization of gamma-glutamylcysteine in glutathione synthesis. J Biol Chem. 1952 Nov;199(1):407–414. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L. Physicochemical characterization of ribonucleoside diphosphate reductase from Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4591–4601. [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R. Properties of ribonucleoside diphosphate reductase in nucleotide-permeable cells. J Bacteriol. 1973 Jul;115(1):18–22. doi: 10.1128/jb.115.1.18-22.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Rosenberg E., Kosower N. S., Kosower E. M. Effect of the thiol-oxidizing agent diamide on the growth of Escherichia coli. J Bacteriol. 1970 Mar;101(3):1092–1093. doi: 10.1128/jb.101.3.1092-1093.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



