Abstract
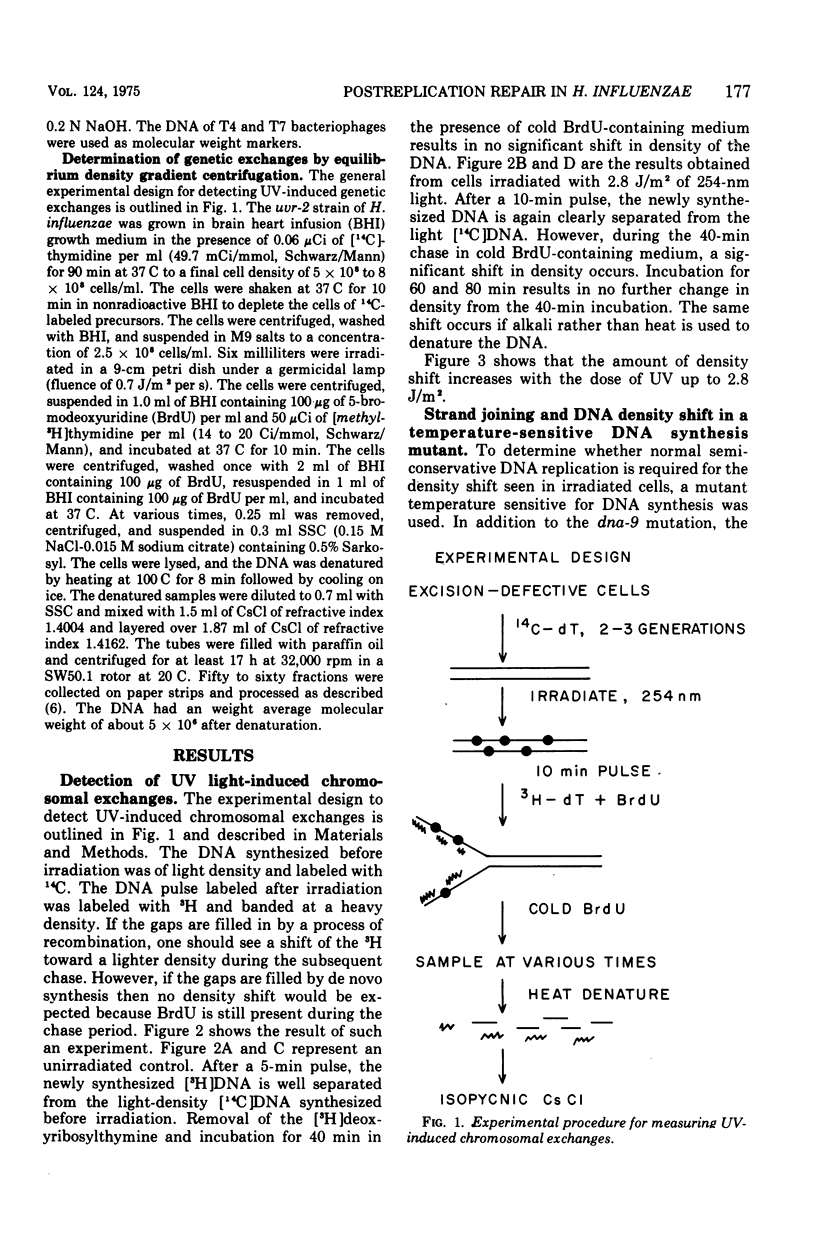
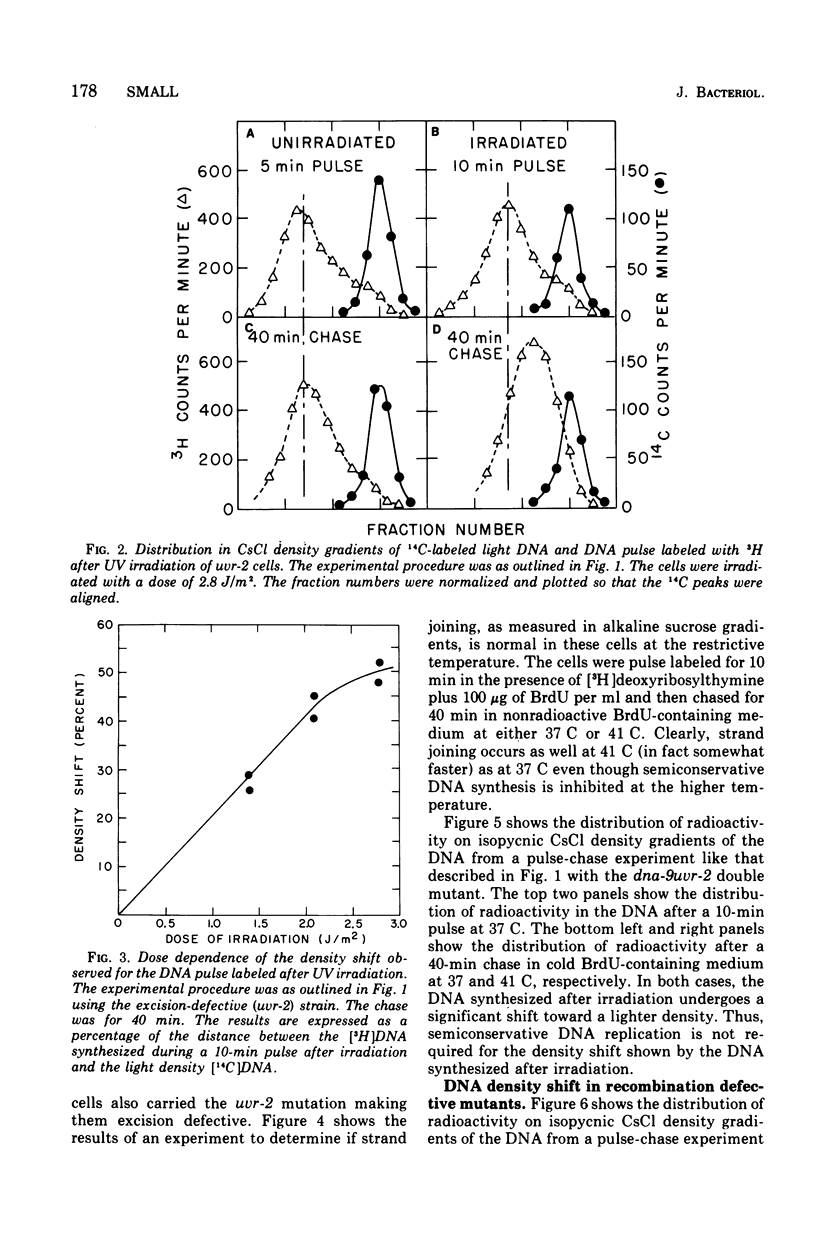
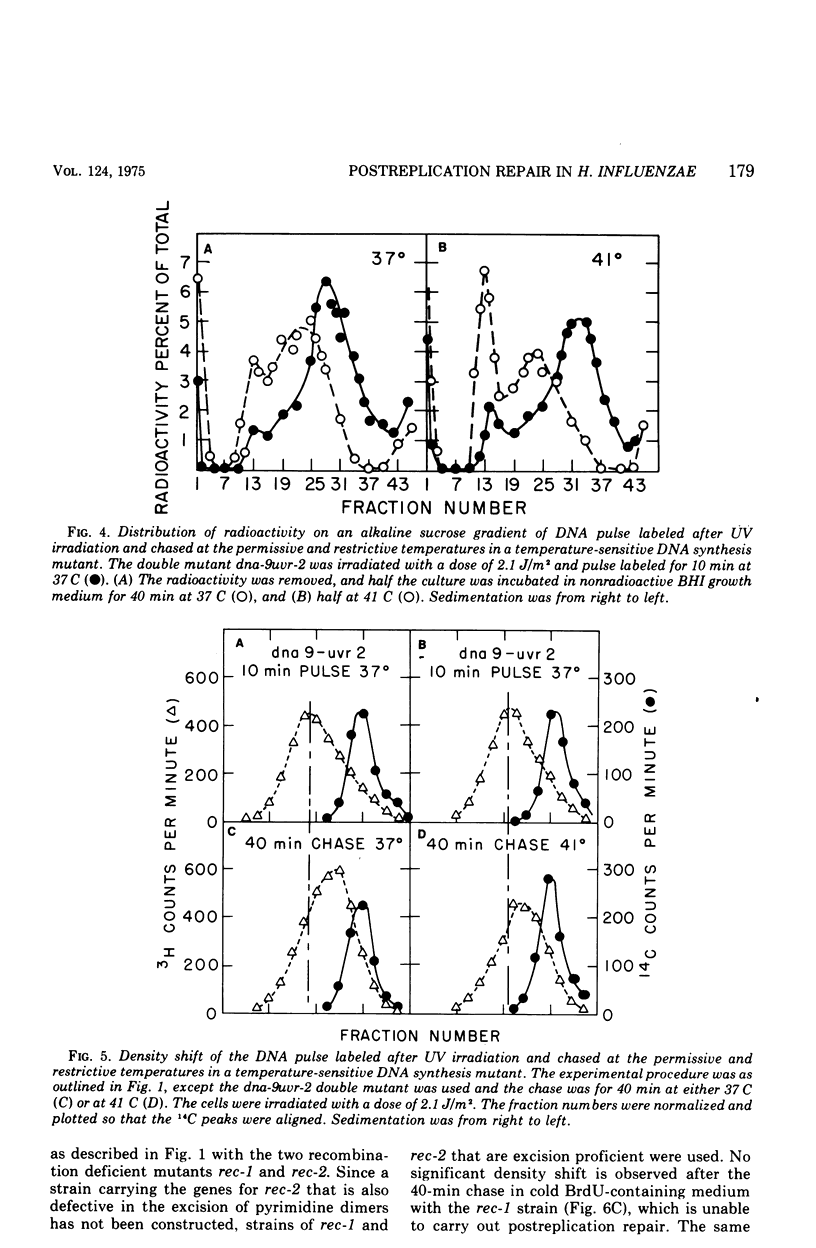
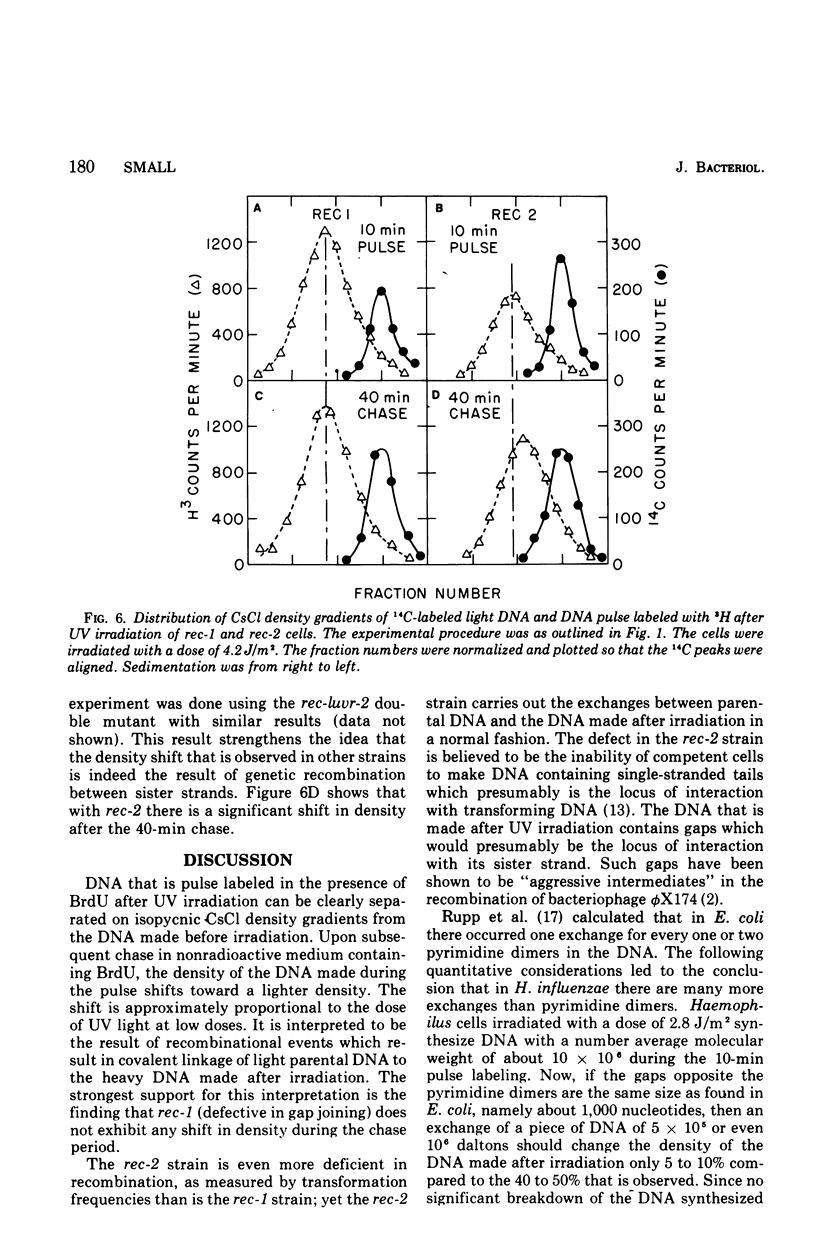
Deoxyribonucleic acid (DNA), pulse labeled after ultraviolet irradiation of excision-defective mutants of Haemophilus influenzae, is of lower single strand molecular weight than that of unirradiated cells but approaches the size of DNA from unirradiated cells upon further incubation in growth medium. This gap-filling process is controlled by the rec-1 gene. Gap-filling occurs normally in a temperature-sensitive DNA synthesis mutant at the restrictive temperature showing that normal semiconservative DNA synthesis is not necessary for gap-filling. To test for recombinational events after irradiation, the DNA synthesized after irradiation was radioactively labeled for a short time in medium containing 5-bromodeoxyuridine followed by incubation for various times in non-radioactive, 5-bromodeoxyuridine-containing medium. The DNA was denatured and analyzed isopycnically. The labeled DNA was initially "heavy," but later shifted toward lighter densities. This shift occurred in the temperature-sensitive DNA synthesis mutant at the restrictive temperature and in the recombination-defective mutant rec-2, but was not seen in the rec-1 mutant. The density shift can be interpreted as evidence that rather extensive exchanges occurred between parental DNA and the DNA made after irradiation. These results suggest that such exchanges are important for gap-filling in H. influenzae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie K. L. N-Nitrosocarbaryl-induced mutagenesis in Haemophilus influenzae strains deficient in repair and recombination. Mutat Res. 1975 Feb;27(2):201–217. doi: 10.1016/0027-5107(75)90079-2. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. DNA repair in Potorous tridactylus. Biophys J. 1974 Oct;14(10):791–803. doi: 10.1016/S0006-3495(74)85949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Stillman R. M., Setlow R. B., Regan J. D. DNA chain elongation and joining in normal human and xeroderma pigmentosum cells after ultraviolet irradiation. Biophys J. 1972 Sep;12(9):1183–1191. doi: 10.1016/S0006-3495(72)86154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Characteristics of DNA synthesis following ultraviolet light irradiation in mouse L cells. Postreplication repair. Exp Cell Res. 1972 Dec;75(2):485–489. doi: 10.1016/0014-4827(72)90456-9. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Rupp W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim Biophys Acta. 1971 Jan 1;228(1):117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Setlow J. K. Effects of combining ultraviolet repair and recombination mutations in Haemophilus influenzae. Nat New Biol. 1973 Feb 7;241(110):172–174. doi: 10.1038/newbio241172a0. [DOI] [PubMed] [Google Scholar]
- Leclerc J. E., Setlow J. K. Postreplication repair of ultraviolet damage in Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):930–934. doi: 10.1128/jb.110.3.930-934.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]



