Abstract
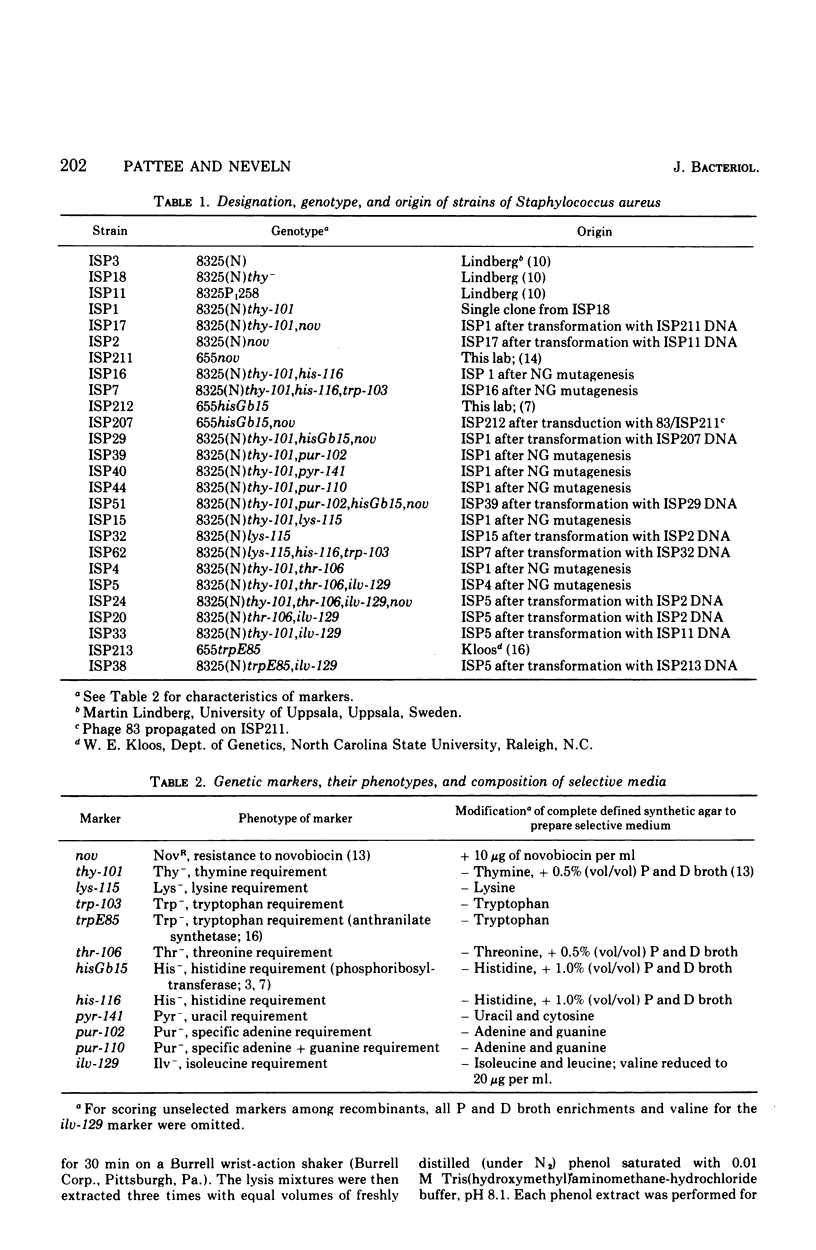
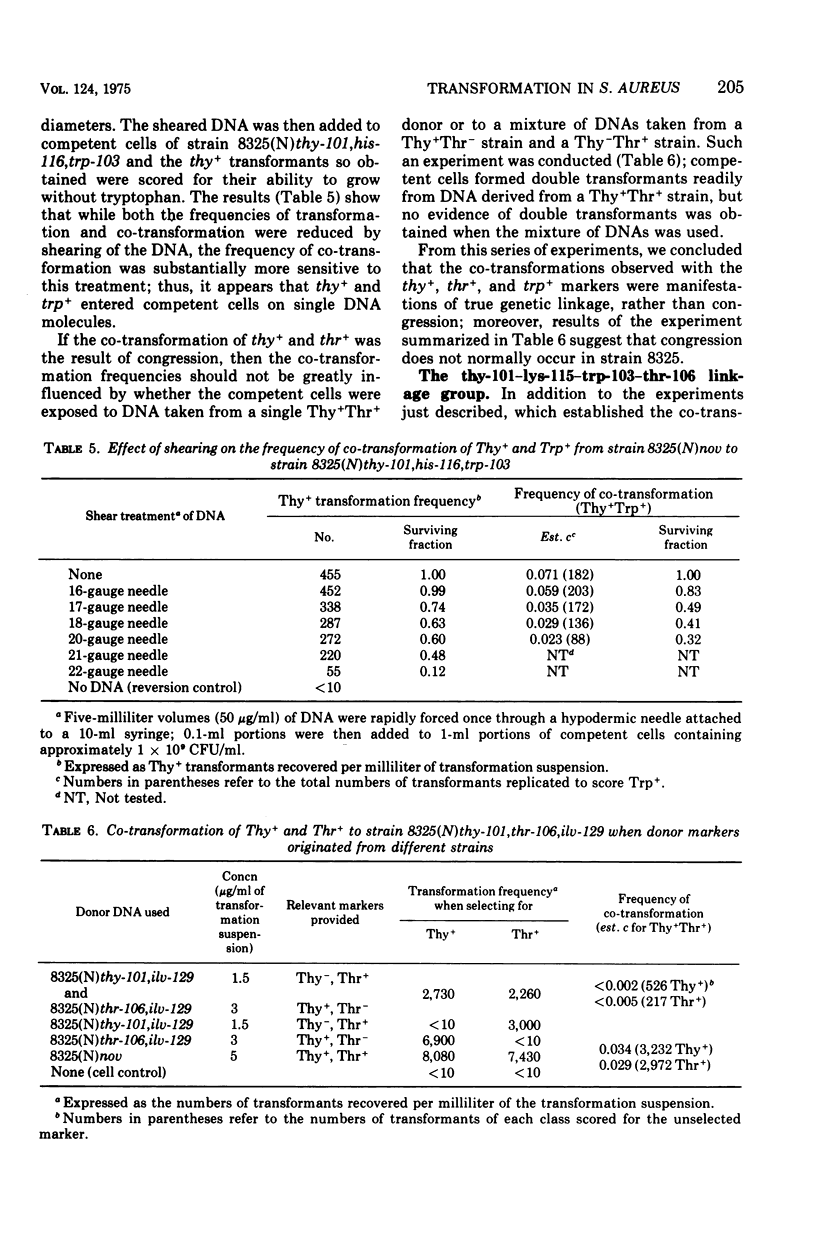
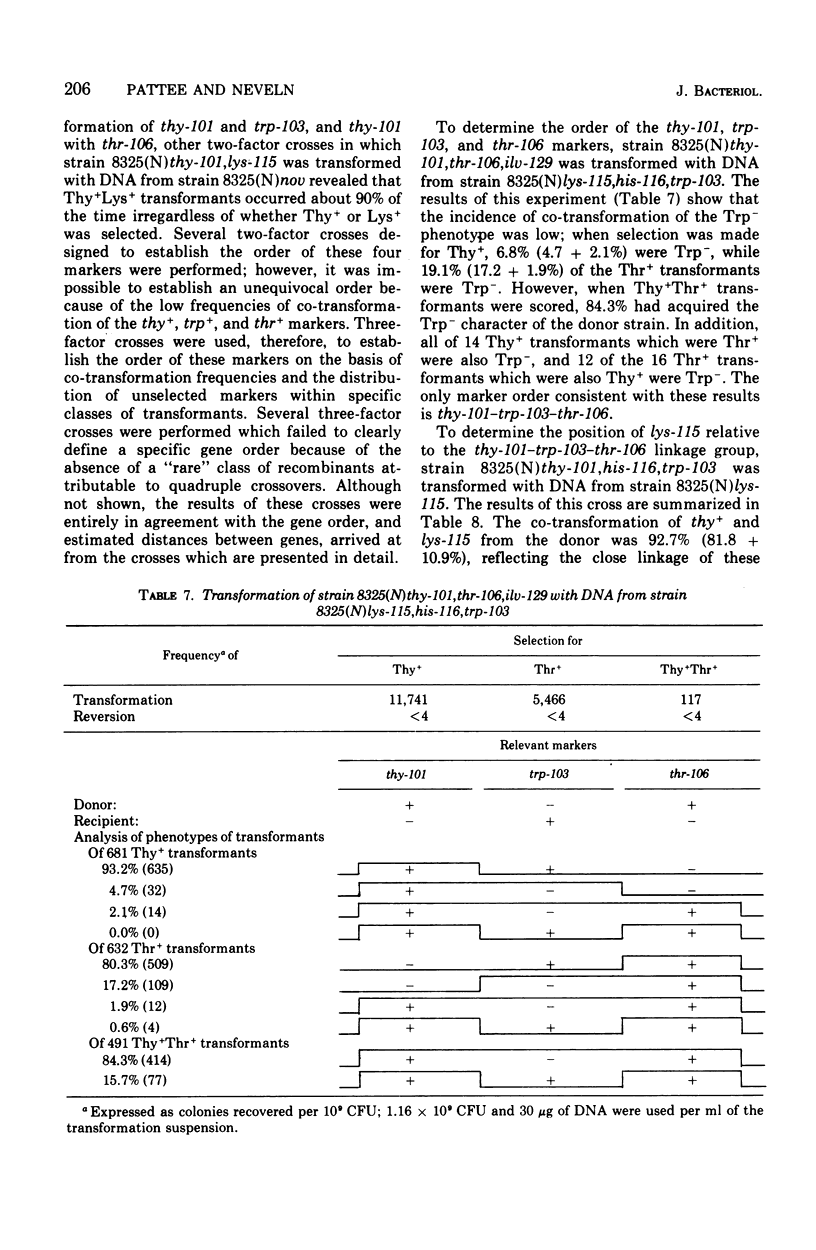
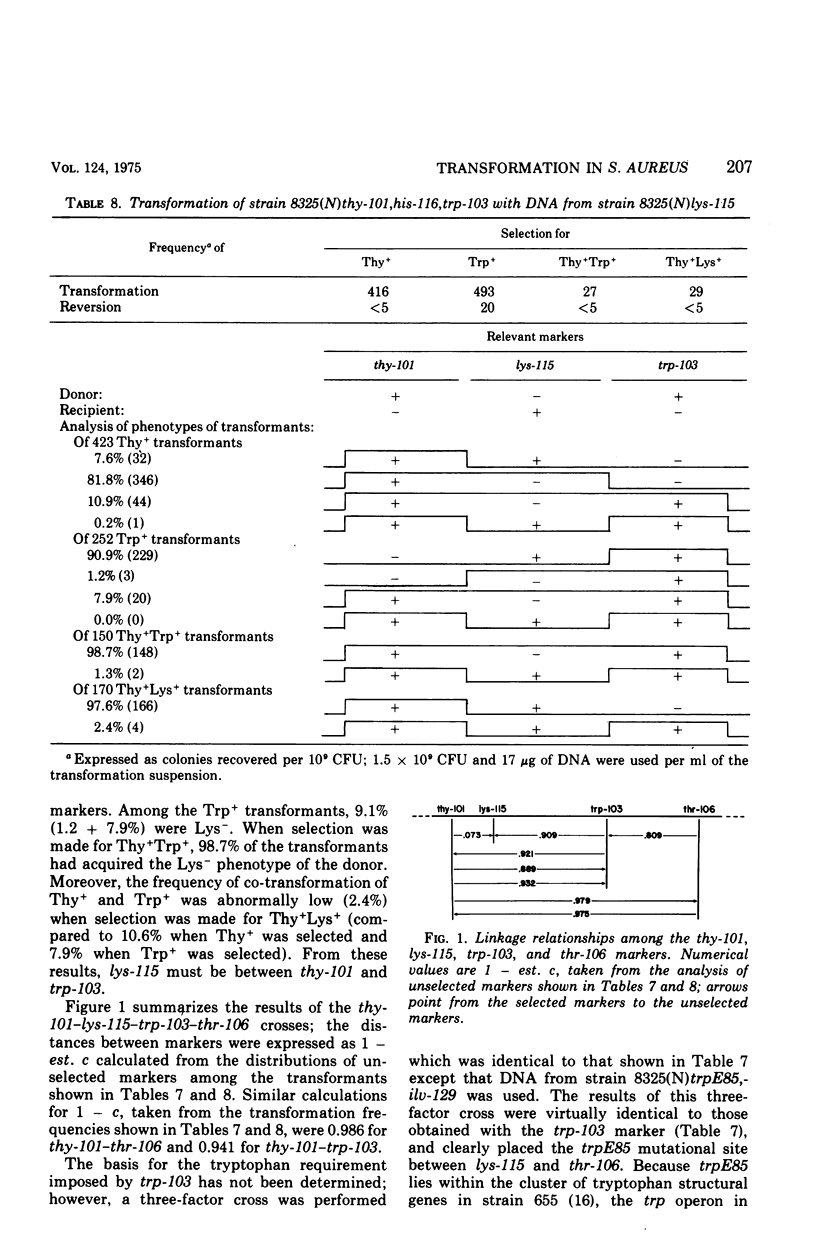
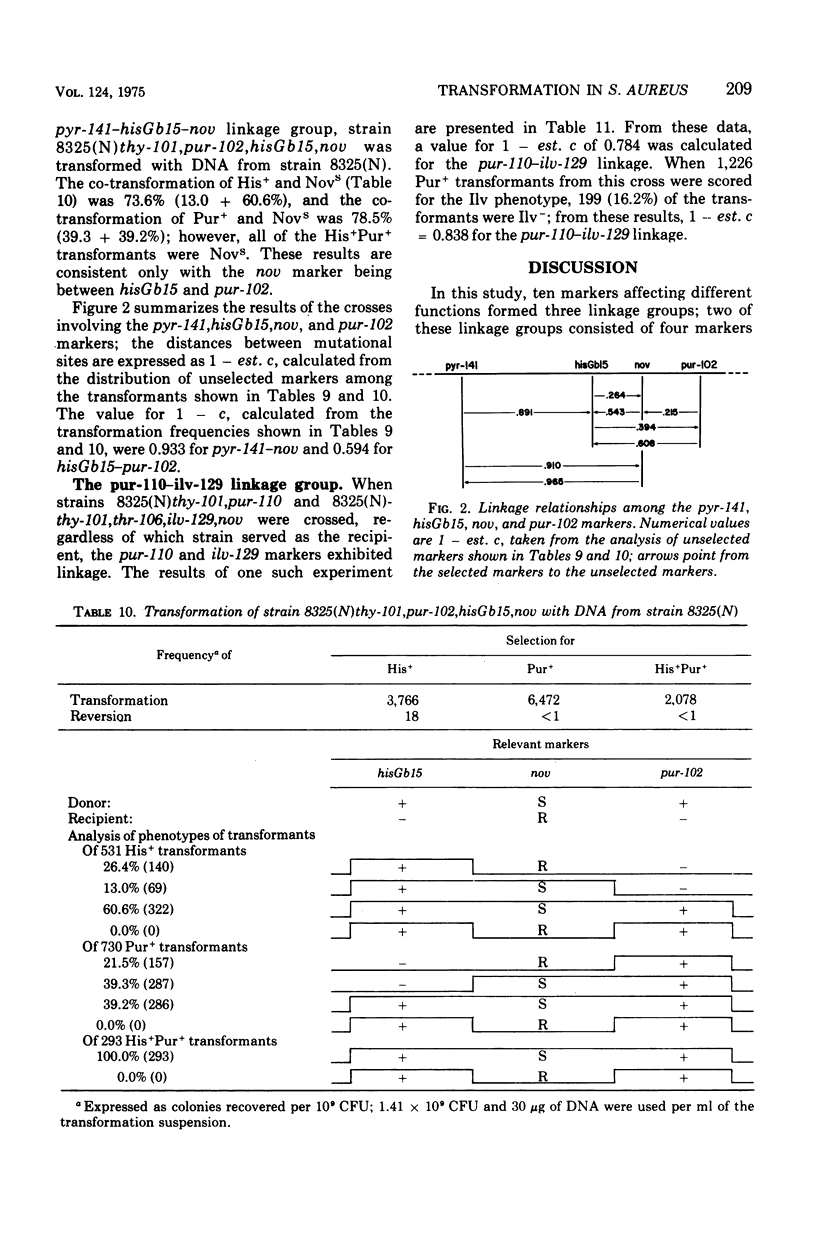
While studying a set of multiply marked mutants of Staphylococcus aureus strain 8325 by transformation, several instances of apparent genetic linkage were encountered. After showing that these linked transformations were readily inactivated by shearing of the deoxyribonucleic acid (DNA) but were resistant to dilution of the DNA, and showing that mixtures of DNA failed to form double transformants, it was concluded that the linkages were legitimate rather than the result of congression. Three linkage groups were defined: thy-101-lys-115-trp-103-thr-106, pyr-141-hisGb15-nov-pur-102, and pur-110-ilv-129. The positions of the previously studied trp and his operons corresponded to the trp-103 and hisGb15 loci. The ilv-129 position adjacent to pur-110 probably corresponds to the ilv-leu gene cluster. The distance over which linkage was detected was greater by transformation than by generalized transduction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes I. J., Bondi A., Fuscaldo K. E. Genetic analysis of lysine auxotrophs of Staphylococcus aureus. J Bacteriol. 1971 Feb;105(2):553–555. doi: 10.1128/jb.105.2.553-555.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L., Pattee P. A. Purine biosynthesis in Staphylococcus aureus. Arch Mikrobiol. 1970;71(1):40–48. doi: 10.1007/BF00412233. [DOI] [PubMed] [Google Scholar]
- Burke M. E., Pattee P. A. Histidine biosynthetic pathway in Staphylococcus aureus. Can J Microbiol. 1972 May;18(5):569–576. doi: 10.1139/m72-090. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H. Studies on transformations of Hemophilus influenzae. IV. Linked and unlinked transformations. J Gen Physiol. 1961 Nov;45:205–228. doi: 10.1085/jgp.45.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M. S., PRITCHARD R. H. UNSTABLE LINKAGE BETWEEN GENETIC MARKERS IN TRANSFORMATION. J Bacteriol. 1965 May;89:1314–1321. doi: 10.1128/jb.89.5.1314-1321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOSS W. E., PATTEE P. A. TRANSDUCTION ANALYSIS OF THE HISTIDINE REGION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:195–207. doi: 10.1099/00221287-39-2-195. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L. TRANSDUCTION BY STAPHYLOCOCCAL BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1959 May;45(5):722–727. doi: 10.1073/pnas.45.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTEE P. A., BALDWIN J. N. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol. 1961 Dec;82:875–881. doi: 10.1128/jb.82.6.875-881.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Kloos W. E., Bodensteiner J. B., Zara A. Homogeneity in a Staphylococcus aureus transducing fragment. J Virol. 1968 Jun;2(6):652–654. doi: 10.1128/jvi.2.6.652-654.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Schutzbank T., Kay H. D., Laughlin M. H. Genetic analysis of the leucine biosynthetic genes and their relationship to the ilv gene cluster. Ann N Y Acad Sci. 1974 Jul 31;236(0):175–186. doi: 10.1111/j.1749-6632.1974.tb41490.x. [DOI] [PubMed] [Google Scholar]
- Proctor A. R., Kloos W. E. The tryptophan gene cluster of Staphylococcus aureus. J Gen Microbiol. 1970 Dec;64(3):319–327. doi: 10.1099/00221287-64-3-319. [DOI] [PubMed] [Google Scholar]
- RITZ H. L., BALDWIN J. N. A transduction analysis of complex loci governing the synthesis of tryptophan by Staphylococcus aureus. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:667–671. doi: 10.3181/00379727-110-27611. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Pattee P. A. Biochemical and genetic analysis of isoleucine and valine biosynthesis in Staphylococcus aureus. J Bacteriol. 1967 Jun;93(6):1832–1838. doi: 10.1128/jb.93.6.1832-1838.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Imsande J. Regulation of penicillinase synthesis: a mutation in Staphylococcus aureus unlinked to the penicillinase plasmid that reduced penicillinase inducibility. J Bacteriol. 1972 Jan;109(1):116–121. doi: 10.1128/jb.109.1.116-121.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



