Abstract
We have developed a multiwalled carbon nanotube/dihydropyran (MWCNT/DHP) composite sensor for the electrochemical detection of insulin in a microfluidic device. This sensor has been employed for physiological measurements of secreted insulin from pancreatic islets in a Cytosensor® previously modified to be a multianalyte microphysiometer (MAMP). When compared with other established electrochemical insulin sensors, the MWCNT/DHP composite film sensor presented improved resistance to fluidic shear forces, while achieving enhanced electrode kinetics. In addition, the preparation of the composite film is straightforward and facile with a self-polymerizing monomer, DHP, used to add mechanical stability to the film. The sensor film was able to detect insulin concentrations as low as 1 µM in the MAMP during calibration experiments. The MWCNT/DHP composite sensor has been successfully used for the direct detection of insulin secreted by islets in the microphysiometer.
Keywords: Insulin, Multiwalled Carbon Nanotube, Multianalyte Microphysiometer, Cytosensor®, Dihydropyran, Sensor
1. Introduction
The ability to detect insulin directly and rapidly is crucial in the study of the diabetes mellitus and in particular pancreatic islet metabolism. Currently, insulin secretion is most commonly studied using standard static or perifusion testing. In a typical standard static test, two samples of 5 or 10 islets are incubated for a period of time, typically one to two hours, one sample in media containing a low concentration of glucose (2.8–3.3 mM) and the other sample in media with a high concentration of glucose (16.7–20 mM). The amount of insulin in the media is determined, and the stimulation index is calculated as the ratio of the insulin content in the high glucose media to the insulin content of the low glucose media [1–3]. The stimulation index is used to quantify the insulin secreting capability of the islets; this measure is used in the analysis of islets for human transplants [4]. In perifusion experiments, islets are immobilized in a fluid path, such as between glass frits in a tube, and media is flown past. Typically, the islets equilibrate in low glucose media (~ 3 mM) for approximately 30 minutes to one hour, and then the glucose concentration is increased (~17 mM). Samples of the media outflow are collected throughout the experiment, every one to two minutes, and the insulin content is then determined to obtain a rate of insulin release. The number of islets used in perifusion experiments varies from 5 to as many as 50 [5–9]. In perifusion experiments, the concentration vs. time profile of secreted insulin can be compared to the expected glucose stimulated insulin response for healthy islets [10,11]. Both of these techniques require ex situ analysis of insulin content from collected samples, and as these methods necessitate testing of sample fractions, the true dynamic insulin release profile is not observed because time averaging occurs. Assay methods for the quantitative analysis of insulin in these samples include enzyme linked immunosorbent assay, radioimmunoassay, and chromatography. While these assays can have very low detection limits, these methods can be time consuming and require the labeling of insulin with either fluorescent tags or radioisotopes [12–14].
Electrochemistry and fluorescence have also been used to quantify islet secretion with time resolution that reaches real-time in some cases; however, these studies primarily have involved detecting zinc, calcium or other molecules such as 5-hydroxytryptamine that play a role in insulin secretion [15–19]. Maghasi et al. [15] detected secretion from β cells electrochemically through anodic stripping voltammetry of zinc at a bismuth coated electrode. This study proposed that the amount of zinc detected correlated to the amount of insulin secreted since insulin is stored primarily in crystalline form with encapsulated zinc ions. However, this detection method required preconcentration and ex situ analysis of the β cell culture supernatant [15]. Kennedy et al. have been able to study insulin secretion by single islets in a microfluidic device through electrophoresis-based immunoassay of insulin [17] and also through fluorescence spectroscopy of zinc and intracellular calcium [19]. While these techniques have very low detection limits, they are unable to measure unlabeled native insulin directly.
Direct electrochemical detection of insulin is attractive as it can provide sensitivity and also reduced analysis times to enable continuous real-time measurements compared to the above methodologies. One of the first chemically modified electrodes developed to measure the oxidation of insulin was a ruthenium dioxide/cyanoruthenate film on a glassy carbon fiber electrode [20]; however this electrode operated at pH 2, making it impractical for physiological measurements. A ruthenium dioxide/cyanoruthenate film on a carbon fiber microelectrode was later utilized at pH 7.4 to monitor chemical secretion by single β cells in response to stimulation with glucose, tolbutamide, and potassium [21]. Redox mediators and electrocatalysts such as ruthenium oxide [22], ruthenium metallodendrimer [23], and iridium oxide [24] layers have been shown to promote the oxidation of insulin at physiological pH and have been used in amperometric detection and quantization of insulin. However, iridium oxide sensors have the disadvantages of pH sensitivity resulting from the proton mediated nature of electron transfer and also the slow nature of proton transport within the film that delays the electrode response [25]; ruthenium oxide and ruthenium metallodendrimer films have mixed redox states leading to complex electron transfer kinetics, with the electrochemical behavior of ruthenium oxide varying between crystal faces [26]. While these sensors have been used to detect insulin electrochemically, they have limited stability of their mixed redox state surface films leading to loss of insulin sensitivity.
Because of the difficulties associated with the above chemically modified electrodes, multiwalled carbon nanotubes (MWCNTs) are an attractive possibility as an electrocatalyst for the oxidation of insulin. MWCNTs are long, multi-walled, thin cylinders of carbon that can be described as several two-dimensional graphite sheets rolled into a tube [27]. Recent studies have shown the ability of MWCNTs to promote electron transfer reactions of both important biomolecules [28,29] and redox centers embedded deep within proteins [30,31]. This electrocatalytic property, as well as the chemical stability and high mechanical strength of MWCNTs, has rendered them an appealing material for use in biosensors [32]. Also, MWCNT-modified electrodes have been shown to reduce surface fouling effects, like those seen in NADH oxidation at highly ordered pyrolytic graphite electrodes [33].
The direct oxidation of insulin with high sensitivity in physiological pH using a MWCNT modified glassy carbon electrode (GCE) cast from a suspension in N,N-dimethylformamide (DMF) was reported by Wang and Musameh [34]. No cross-linking agent or polymer was used in the preparation of this film. Because the film was produced upon the evaporation of DMF, there was little control of film morphology leading to agglomeration of the MWCNTs. Recent improvements by Wang and co-workers include a combination carbon nanotube/ruthenium oxide electrode [35]. Wu et al. reported that the stability of MWCNT films for the detection of small biomolecules is improved by addition of a fatty acid such as dihexadecyl phosphate to an aqueous suspension of MWCNTs [36]. Gorski et al. recently explored the use of a chitosan MWCNT composite film for insulin oxidation [37]. The addition of the polysaccharide chitosan was seen to facilitate the preparation of films for electrochemical sensors, but these sensors were not coupled to microfluidic analysis nor used for direct insulin measurements on islets. The preparation of the chitosan-based film includes the use of an acid solution at 80–90 °C and results in a film up to 10 µm thick [37]. In our microphysiometer setup, a thick film may prove an impediment in the microfluidic thin layer cell where the volume of the microfluidic chamber is 3 µL. The use of a small molecule, such as dihydropyran (DHP), as a stabilizing agent for the MWCNT film should result in a simpler preparation as DHP is simply added to the aqueous suspension. DHP is a vinyl ether that has been shown to self-polymerize [38], and therefore it has the ability to form a thinner stabilizing network for adhering the MWCNTs to the glassy carbon surface.
An electrochemical insulin sensor would be ideally suited for use in the physiological detection of insulin in a microfluidic device, such as the multianalyte microphysiometer (MAMP). The MAMP was developed from the commercially available Cytosensor® microphysiometer by Eklund et al. [39–42]. The MAMP was developed through the integration of four electrodes into the system to allow for the simultaneous measurement of three other metabolic parameters in addition to acidification [39,40]. The MAMP has since been used to measure the metabolic profiles of various types of live cells, including cancer cells and the effect of toxins on cellular metabolism [42]. In the MAMP, as in all microfluidic devices, the film electrodes must be able to withstand the shear forces generated in the detection chamber.
In this paper, we present an insulin sensor for electrochemical detection in a microfluidic device based on direct oxidation at a MWCNT/DHP composite film at physiological pH. Compared to sensors in literature, our sensor has improved surface coverage and adherence to the electrode surface. We have successfully adapted this sensor for the microfluidic chamber of the MAMP and used it to record the response of isolated murine islets to glucose stimulation.
2. Experimental Section
2.1 Chemicals
All materials were used as obtained unless otherwise noted. Insulin (from bovine pancreas, 27 USP units/mg), and dihexadecyl phosphate were purchased from Sigma. The insulin content appeared to vary as much as 20% between commercial lots. 3,4-Dihydro-2H-pyran (DHP), Tween-80, and DMF were purchased from Acros. Cell-Tak™ cell and tissue adhesive was purchased from BD Biosciences. Glassy carbon rods (1 mm diameter) were obtained from Structure Probe, Inc. (West Chester, PA). Platinum rod (2 mm) was purchased from VWR. All microphysiometer consumables were purchased from Molecular Devices (Sunnyvale, CA).
Insulin solutions were prepared from a stock of 10 mg/mL solution of insulin in 19.6 mM hydrochloric acid containing 0.02% v/v Tween-80. Dilutions were then made in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80) immediately before use in experiments. Male FVB mice were made available by the Islet Isolation Core of the Vanderbilt Diabetes Research and Training Center. FVB mice have been used previously as control models for basal levels in a number of studies of the effect of gene modification on insulin secretion [43–47]. Islets were isolated from these mice by Dr. Zhongyi Chen of the as described by Brissova et al. [48]. The islets were cultured overnight in RPMI with 10% fetal bovine serum and 11 mM glucose. Upon receipt the islets were stored in an incubator at 37 °C and 5% CO2 until use.
MWCNTs were obtained as a gift from Dr. William Hofmeister and were manufactured as a byproduct of diamond deposition by microwave assisted chemical vapor deposition using an iron catalyst. An Astex Large Area Diamond Deposition System with a 5 kW microwave source was used. Nanotubes were purified through treatment with nitric acid, following the method of Dujardin et al. [49], to remove the remaining catalyst and aggregates. Following purification, the nanotubes were stored in a desiccator.
2.2 Apparatus
All electrochemical measurements, with the exception of amperometric tests in the MAMP, were conducted using a CHI 660a potentiostat from CH Instruments (Austin, TX). The electrochemical cell was used in a three electrode configuration with 3 mm glassy carbon electrode (GCE) working electrode CHI104 (CH Instruments Austin, TX), Ag/AgCl (2 M KCl) reference electrode CHI 111 (CH Instruments Austin, TX), and platinum mesh counter electrode in all experiments but those in the MAMP. In the MAMP, the 2 mm platinum disk on the modified sensor head (Figure 1) was used as the counter and the 1 mm glassy carbon electrode was used as a working electrode, while the reference electrode (Ag/AgCl, 2 M KCl) was downstream from the microfluidic chamber, as is typical in the Cytosensor®. For amperometric tests in the MAMP, a four channel microphysiometer and Cytosoft® program (Molecular Devices) were used to control pump cycles. A multipotentiostat capable of simultaneous measurement of amperometric responses for three working electrodes in up to eight electrochemical cells, developed in house by the Vanderbilt Institute of Integrative Biosystem Research, was used to measure currents.
Fig. 1.
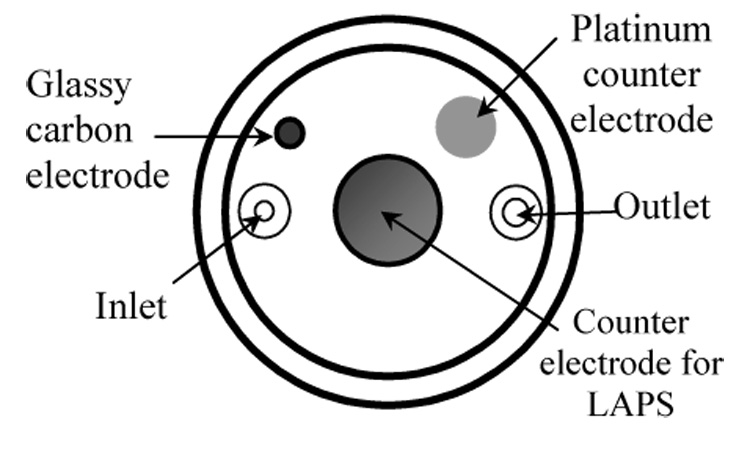
Modified sensor head showing 1 mm glassy carbon electrode, 2 mm platinum counter electrode, fluid inlet and outlet, and counter electrode for light addressable potentiometric sensor (LAPS).
2.3 Sensor Head Construction
For insulin determination in the MAMP, a sensor head was constructed with a 1 mm glassy carbon working electrode and a 2 mm platinum counter electrode, based on the method of Eklund et al. [40]. All electrodes were sealed with Hysol® Epoxi-Patch and polished until flush with the surface. The schematic of the modified sensor head can be seen in Figure 1.
2.4 Modified Electrodes for the Detection of Insulin
All films were formed by drop casting the suspension on to the electrode surface, with 20 µL used for a 3 mm GCE and 3 µL for a 1 mm sensor head GCE.
MWCNTs cast from DMF
A 10 mg/mL suspension of carbon nanotubes in DMF was sonicated for one hour [34].
MWCNT/dihexadecyl phosphate
A 10 mg/mL suspension of MWCNT in water was prepared with a 1:1 mass ratio of MWCNT:dihexadecyl phosphate. The suspension was sonicated for one hour [36].
MWCNT/DHP
A 10 mg/mL suspension of MWCNT in water was prepared with a 1:1 mass ratio of MWCNT:DHP. This suspension was sonicated for fifteen minutes.
2.5 Testing of Films for Insulin Sensitivity
Cyclic voltammograms (CVs) were obtained for each electrode film on the 3 mm GCE and 1 mm GCE of the modified sensor head in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80) with and without insulin, from 0 to 1 V at a scan rate of 50 mV/s. The MWCNT/DHP film on the 3 mm GCE was also calibrated outside of the MAMP in a batch test. After ~ 1 hour of electrode conditioning at +0.88 V vs. Ag/AgCl (2 M KCl) in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80), aliquots of buffer and insulin were injected to give concentrations of 0, 1.97 × 10−7, 3.94 × 10−7, 6.87 × 10−7, and 9.79 × 10−7 M insulin. The resulting data was filtered using a LabVIEW program with a Fourier transform low pass filter to remove the electromagnetic stirring noise.
To test the suitability of these films for use in the MAMP, the 1 mm sensor head GCE was coated with one of the three film types and then placed in the MAMP for 4.5 hours under stop flow conditions with a cycle of 180 s with a flow rate of 20 µL/min for 140 s and a 40 s stop flow period. The condition of the films was observed using an optical microscope before and after testing. Linear sweep voltammetry (LSV) in solutions of buffer and insulin was performed with a scan rate of 50 mV/s in the microphysiometer for films on the sensor head GCE at a constant flow rate of 20 µL/min. The MWCNT/DHP composite film was then calibrated amperometrically on the modified sensor head in the MAMP with ~ 1 hour of electrode conditioning following the same method used in the batch test of the film on the 3 mm GCE, with concentrations of 0, 1.001 × 10−6, 5.002 × 10−6, 1.001 × 10−5, 2.501 × 10−5 M insulin.
2.6 Testing of Islets in MAMP
The polycarbonate membranes of the Cytosensor® capsule cups were coated with Cell-Tak™, a combination of proteins from mussels. The cups were dried at 37 °C, rinsed with water, and dried again. Approximately 75–125 islets were hand picked from the culture media using a manual pipette, rinsed three times with 1 mM phosphate buffer with 100 mM NaCl (pH 7.0), and then placed onto the Cell-Tak™. The islets were then coated with 5 µL of agarose following a well established protocol for the use of non-adherent cells in the Cytosensor® [50–52]. A spacer and insert were placed inside the cup. Hank’s balanced salt solution (HBSS) [53] with 2.8 mM glucose was pipetted inside.
The capsule containing the islets was then placed in the MAMP, and the Cytosoft® data collection and pump control program started. The islets were allowed to equilibrate for 20 minutes in the MAMP at 37 °C at a flow rate of 20 µL/min, before data collection of insulin commenced. The pump cycle for islet experiments was 180 seconds, with 140 seconds at flow rate of 20 µL/min and a 40 second stop flow period. To validate that the sensor can detect secreted insulin, islets were initially perifused with HBSS with 2.8 mM glucose, referred to as low glucose. After 60 minutes, the perifusion media was switched to HBSS with 16.7 mM glucose, referred to as high glucose. Identical procedures were followed in the absence of islets to determine the effect of increased glucose levels on the sensor.
3. Results and discussion
We present the development of a MWCNT/DHP composite film for use in a microfluidic chamber inside the MAMP, yielding a sensor with almost instantaneous response time on the order of 1 s or less. This film was compared with established electrochemical insulin sensors based on MWCNTs, namely the MWCNT film cast from DMF of Wang and Musameh [34] and the MWCNT/dihexadecyl phosphate composite films of Wu et al. [36]. As this sensor has been designed for use in the MAMP, uniformity of coating and adherence of the film to the electrode surface are vital.
Figure 2 presents a preliminary comparison of these three film types. CVs were obtained in buffer (pH 7.4, 0.02% v/v Tween-80) as background and in insulin solutions (72.5 µM) prepared in the same buffer. As displayed in Figure 2A, a representative CV using the MWCNT film cast from DMF shows an irreversible oxidation peak at +0.759 V vs. Ag/AgCl, 2 M KCl, which is well resolved from the background buffer. The CV using the MWCNT/dihexadecyl phosphate film, solid line in Figure 2B, has an oxidation peak at +0.753 V vs. Ag/AgCl, 2 M KCl, and is not as well defined as the insulin oxidation peak for the MWCNT film cast from DMF or the MWCNT/DHP film. Because of the decreased magnitude of the insulin oxidation peak, the MWCNT/dihexadecyl phosphate film is the least desirable choice for use in insulin detection. The oxidation peak of insulin for the cyclic voltammogram of the MWCNT/DHP composite film, solid line in Figure 2C, has a peak potential of +0.765 V vs. Ag/AgCl, 2 M KCl, and is well defined compared to the background voltammogram in buffer (dotted line). The average peak current as a function of insulin concentration for cyclic voltammograms of each film is given in Table 1. The electron transfer kinetics, as indicated by the oxidation peak current divided by the insulin concentration, ip/C, for the MWCNT film cast from DMF and the MWCNT/DHP composite film were improved compared with the values for the MWCNT/dihexadecyl phosphate film. All three films show oxidation at similar potentials (Table 1), with the sensitivity of the MWCNT/dihexadecyl phosphate film being the lowest. The large standard deviations in the peak current values in Table 1 result from each film being hand cast. For this reason, each film must be calibrated individually prior to any experiments that involve insulin detection. By comparing the oxidation peak current as a function of concentration, it is clear that the MWCNT/DHP film and the MWCNT film cast from DMF have the best current response and kinetics and thus are the best leads for the detection of insulin from physiological samples; however, further testing is necessary to determine which film was most suitable for the microfluidic environment of the MAMP.
Fig. 2.
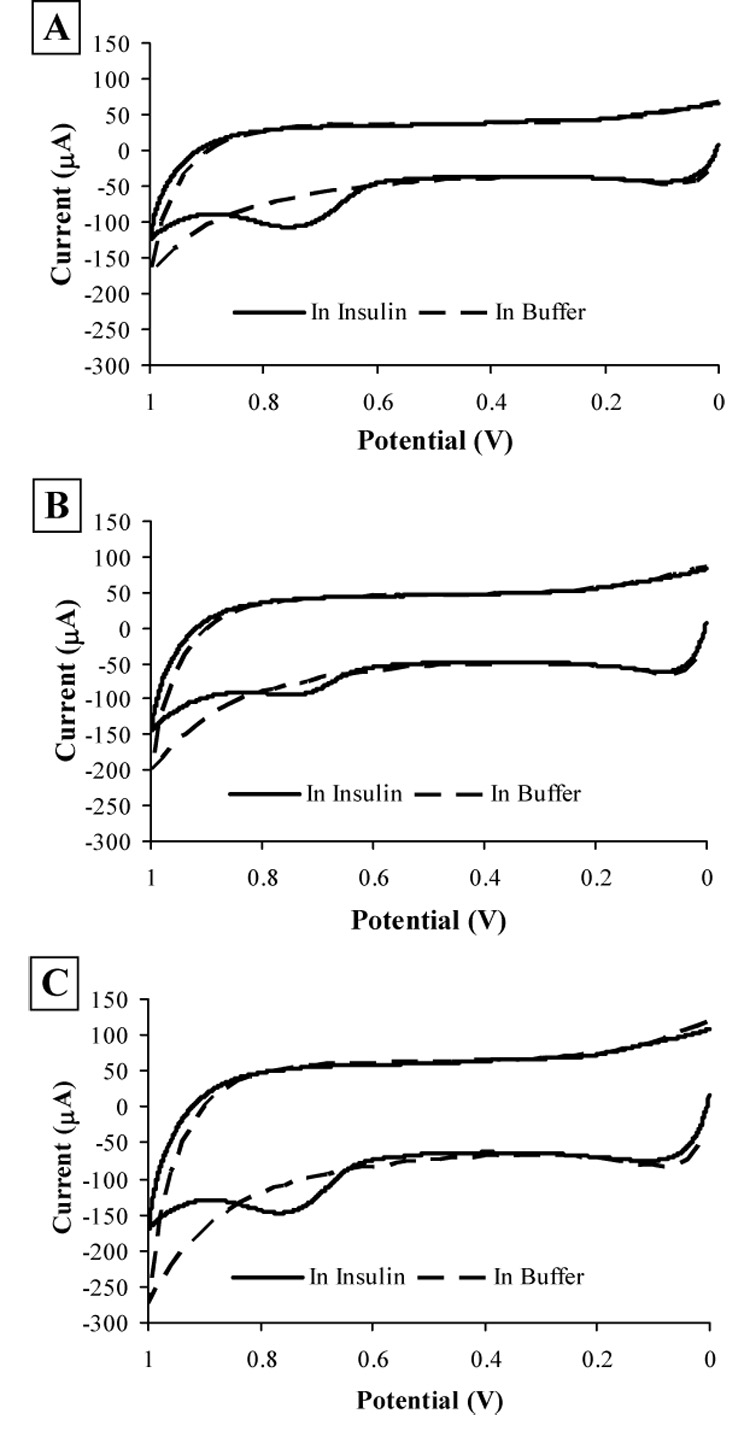
Cyclic voltammograms in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80) and 72.5 µM insulin solutions in the same buffer at a 3.0 mm GCE coated with each film type vs. Ag/AgCl (2 M KCl). Scan rate: 50 mV/s. (A) MWCNT cast from DMF; (B) MWCNT/dihexadecyl phosphate electrode; (C) MWCNT/DHP electrode.
Table 1.
The insulin oxidation peak currents from cyclic voltammograms of 1 mm GCEs modified with MWCNT cast from DMF, MWCNT/dihexadecyl phosphate films, and MWCNT/DHP films and the ratios of the peak current to the insulin concentrationa
| Film Type | Ep (V vs. Ag/AgCl) | ip (µA) | C (µM) | ip/C (µA/µM) |
|---|---|---|---|---|
| cast from DMF | +0.759 | 59 ± 5.0 | 72.5 | 0.81±0.07 |
| dihexadecyl phosphate | +0.753 | 35 ± 3.3 | 72.5 | 0.48±0.05 |
| DHP | +0.765 | 61 ± 4.9 | 72.5 | 0.84±0.06 |
CVs were run with scan rate of 50 mV/s and a Ag/AgCl (2 M KCl) reference electrode in insulin solutions in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80). At least three CVs were obtained for each film type.
To determine the resistance to shear force of these three film types under the microfluidic flow conditions of the MAMP, sensor head GCEs (Figure 1) were coated and tested under identical conditions. Representative films for each film type are shown in Figures 3A–C before testing and in Figures 3D–F after testing. Prior to testing in the MAMP, all three films appear to have complete surface coverage with some surface roughness seen in the MWCNT/dihexadecyl phosphate and MWCNT/DHP films. After 4.5 hours in the MAMP under stop flow conditions with 180 s cycles with a flow rate of 20 µL/min for 140 s and a 40 s stop flow period, the thicknesses of the MWCNT film cast from DMF and the MWCNT/dihexadecyl phosphate film, Figure 3D and 3E, are clearly reduced. The adherence and integrity of the films were compared by using the pre- and post-testing images and visually inspecting the optical density of the film remaining as the percentage of its original area. The ratio of the remaining film to the area of original film is defined as the percentage of film loss. The MWCNT film cast from DMF lost 53.6% of its original coverage. The loss of the MWCNT/dihexadecyl phosphate film was more dramatic, with a loss of 90.2% of the original film area. In comparing Figures 3C and 3F, visual inspection showed that none of the MWCNT/DHP film was lost, although some surface changes were observed. The MWCNT/DHP film maintained its integrity and had the best adherence to the electrode surface during the pulsed stop flow conditions in the MAMP out of the three film types tested. The MWCNT film cast from DMF and the MWCNT/dihexadecyl phosphate film are only physisorbed onto the electrode surface, while MWCNT/dihexadecyl phosphate film shows increased homogeneity of the MWCNT film resulting from the addition of a fatty acid to the suspension. In the case of the MWCNT/DHP film, there is physical crosslinking of the nanotubes from the self-polymerization of DHP [38]. Therefore, although the MWCNT/DHP film and the MWCNT film cast from DMF showed similar ip/C values (Table 1), the MWCNT/DHP film is best suited for use in the MAMP as it can better withstand the fluidic shear forces of the instrument, and consequently was used in all future experiments.
Fig. 3.
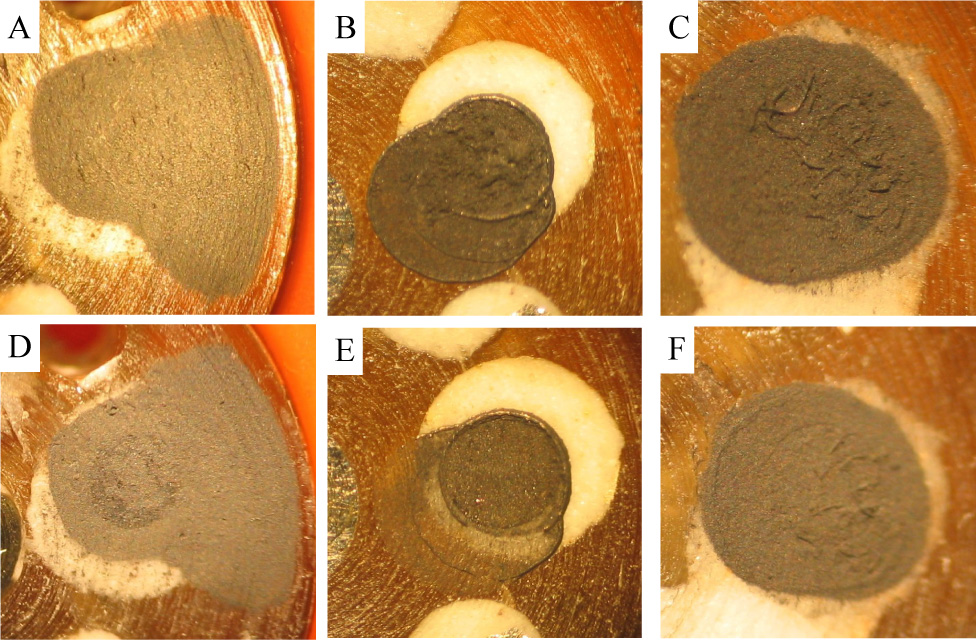
(A–C) Optical microscope images of (A) MWCNT cast from DMF, (B) MWCNT/dihexadecyl phosphate, and (C) MWCNT/DHP films on 1 mm GCE of sensor head prior to testing in MAMP. These particular sensor films (A–C) were subjected to 4.5 hours of 180 s cycles with a flow rate of 20 µL/min for 140 s and a stop flow period of 40 s in the MAMP, and their condition recorded through optical microscope images (D–F): (D) MWCNT cast from DMF (film (A) after 4.5 hours of testing in the MAMP), (E) MWCNT/dihexadecyl phosphate (film (B) after 4.5 hours of testing in the MAMP), and (F) MWCNT/DHP (film (C) after 4.5 hours of testing in the MAMP).
LSVs of the MWCNT/DHP composite film were also obtained in the MAMP to verify that the MWCNT/DHP composite film on the 1 mm glassy carbon electrode of the sensor head still has the ability to electrochemically detect insulin in a microfluidic system similar to the preliminary tests of Figure 2. Sample LSVs of the MWCNT/DHP composite film on the glassy carbon electrode of the modified sensor head in buffer and 300 µM insulin are shown in Figure 4. In the presence of insulin, a peak appears around + 0.81 V vs. Ag/AgCl, 2 M KCl demonstrating the oxidation of insulin at the MWCNT/DHP composite film on the GCE of the modified sensor head in a microfluidic device. This experiment indicates that the MWCNT/DHP film preserves electrochemical activity in the MAMP (Figure 4) similar to that of preliminary experiments (Figure 2).
Fig. 4.
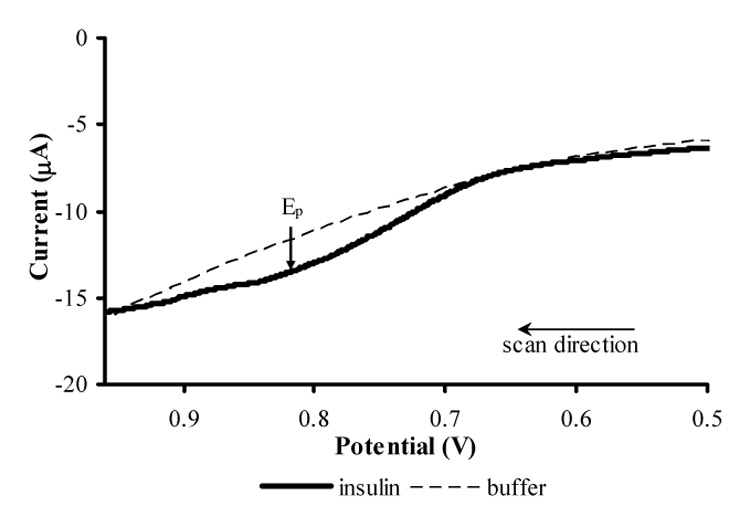
Linear sweep voltammograms of 300 µM insulin in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80) and of blank buffer at a 1 mm GCE on the modified sensor head coated with MWCNT/DHP composite film vs. Ag/AgCl (2 M KCl) in the MAMP. Scan rate: 50 mV/s.
Amperometric calibrations were performed on MWCNT/DHP films at + 0.88 V vs. Ag/AgCl, 2 M KCl on both 3 mm GCEs in solution under moderate stirring and the 1 mm GCE of the modified sensor head in the MAMP. The blank and insulin solutions were in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80). The current increase as a function of insulin concentration is given in Figure 5A for three distinct MWCNT/DHP sensor films on a 3 mm GCE, with each point on the graph representing twenty seconds or 200 collected data points. The similarity of the slopes for these three calibrations, given in the equations in Figure 5A, suggests that the sensitivity of the films is comparable, indicating the reproducibility of these films. An amperometric calibration of the MWCNT/DHP film in the MAMP was performed to ensure that the limit of detection is within the expected physiological concentration range. The current increase during flow as a function of insulin concentration increase is given in Figure 5B. Each calibration point on the graph represents at least five stop flow cycles, comprising 700 collected data points. Each film must be calibrated individually to account for variation in electrode coating due to manual handcasting of the MWCNT/DHP suspension. The current increase for the blank of buffer (0 µM insulin) was not visible above the peristaltic noise of the sensor. The presence of the large y-axis intercept in Figure 5B is due to the same peristaltic pump noise. We were able to detect changes in insulin concentration as low as 1 µM during calibration experiments, which falls within the expected physiological range. As there are no literature values for the secretion of insulin from islets of male FVB mice, for comparison purposes Bergsten’s study of individual islets isolated from ob/ob- mice was used, where insulin release of 3 ± 1 pmol g−1 s−1 was observed when the glucose concentration was 3 mM with an increase to 99 ± 10 pmol g−1 s−1 upon stimulation with 16 mM glucose. By extrapolating this data using the reported islet weight range of 8 – 15 µg and the MAMP volume of 3 µL, approximately 75 – 125 islets, and stop flow of 40 seconds, a concentration range of 0.8 – 2.5 µM could be expected at the end of the stop flow period [10]. While this value is for ob/ob-mice, it should be similar to the FVB mice used in our studies.
Fig. 5.
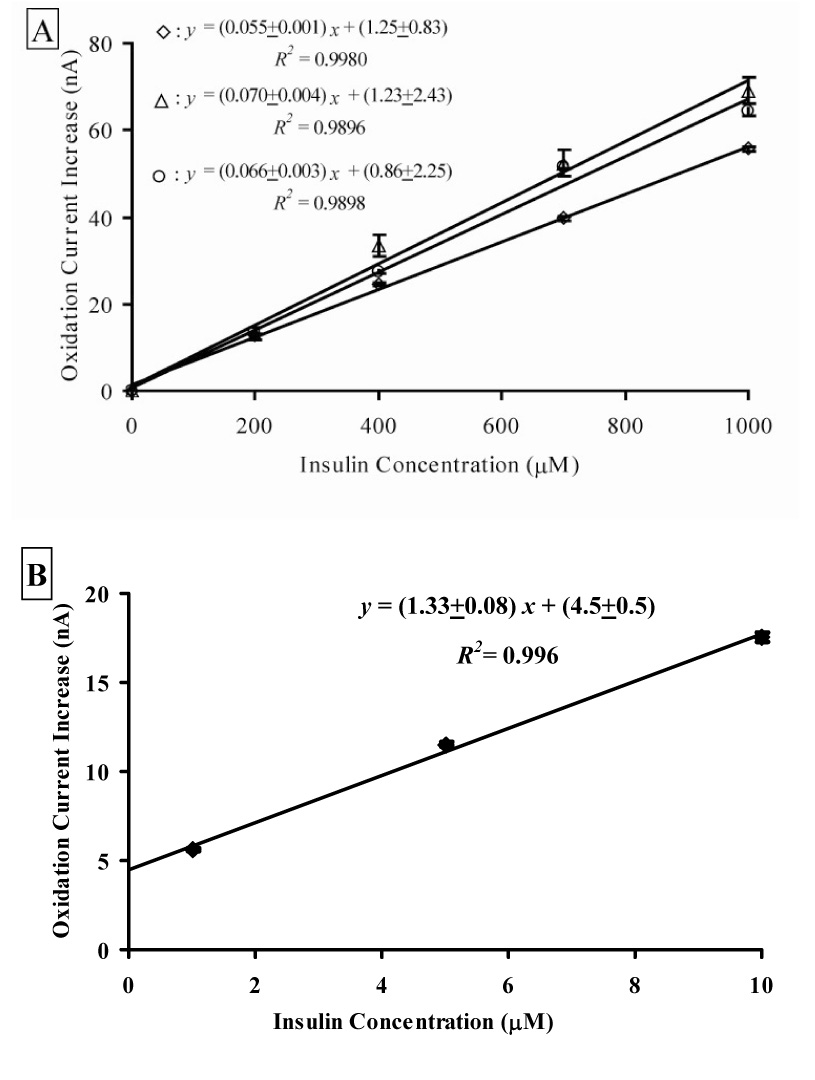
Insulin calibration curves of MWCNT/DHP films at + 0.88 V vs. Ag/AgCl (2 M KCl). Insulin was added as a solution in 50 mM phosphate buffer (pH 7.4, 0.02% v/v Tween-80). Buffer was also added as a control. (A) Calibration curves of three different films on a 3 mm GCEs, performed under moderate stirring with each point showing the current increase for that concentration. Each data point on the graph represents twenty seconds or 200 collected data points. (B) Calibration curve of a MWCNT/DHP film on a 1 mm GCE of a modified sensor head in the MAMP microfluidic chamber under diffusion control. Each data point on the graph represents at least five flow – stop flow cycles, comprising 700 collected data points.
To demonstrate the suitability of the MWCNT/DHP film in the MAMP for studying insulin with physiological samples, the response of the sensors in the presence of islets was measured. Figure 6 shows the amperometric current/time profile of a MWCNT/DHP composite film electrode during a typical stop-flow period in the presence of insulin secreting islets and the dynamics of the signal upon restart of flow. Also visible in Figure 6 is the spike in current corresponding to the perturbation in microfluidic flow caused by the peristaltic pump clamping. The shape of the current-time profile is the result of convective mass transfer during flow as well as decreased mass transport resulting only from diffusion during stop flow, when the secreted insulin must diffuse to sensors across the 100 µm gap of the microfluidic chamber. The peak height (ip), which was used in data analysis, is measured from convective flow steady-state current to the minimum of the stop flow diffusional current as shown in Figure 6. One reason for using ip in data analysis is that the stop flow data has a higher signal to noise ratio and lower detection limits than the convective flow data even after filtering out the high frequency noise of the peristaltic pump from flow data. The current at the sensor during stop flow is linear when graphed versus t−1/2, indicating that the time dependence profile of the insulin release results from diffusional mass transport from the cells to the sensor. This will be the focus of future studies and could likely be modeled as a two electrode system as analyzed by Bard and Faulkner [54]. Currently, peak height at the end of the stop flow phase is the most accurate way to analyze the insulin sensor data with islets as it represents the maximum physiological activity measured during the stop flow period.
Fig. 6.
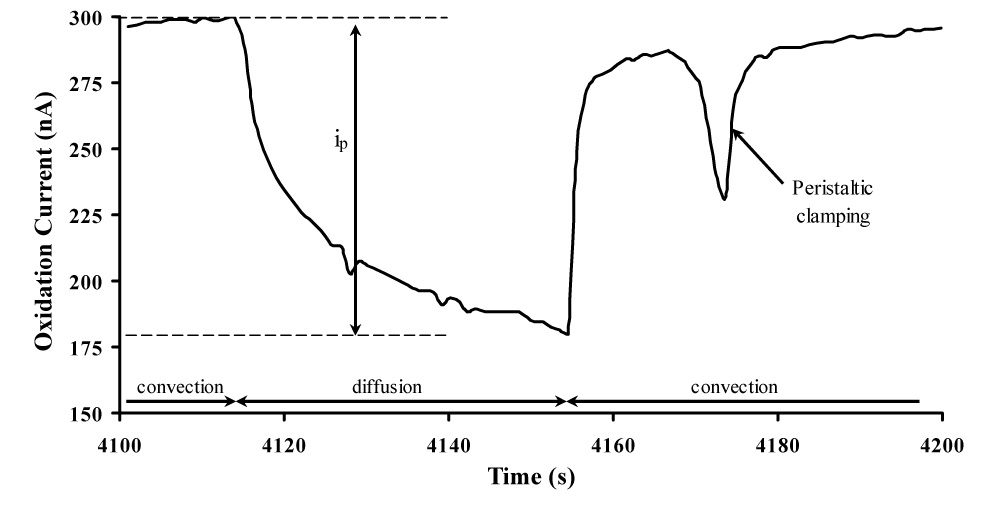
Typical flow – stop flow amperometric current profile of a MWCNT/DHP sensor film in MAMP at + 0.88 V vs. Ag/AgCl (2M KCl) showing the pump on/off periods, peak current (ip) and peristaltic pump clamping spike. This measurement was performed in low buffered HBSS media with 16.7 mM glucose in the presence of islets: 180 s cycle with a flow rate of 20 µL/min for 140 s and a stop flow period of 40 s and sample volume of 3 µL.
Confirmation of the detection of insulin release from pancreatic islets is demonstrated in Figure 7. The inset image in Figure 7 is the corresponding raw amperometric current response of the MWCNT/DHP sensor film in the presence of islets with 2.8 and 16.7 mM glucose. In this experiment, switching the glucose concentration in the HBSS media from low (2.8 mM) to high (16.7 mM) was used to stimulate insulin secretion by the islets. As shown in Figure 7, the stop flow peak current in the absence of islets (denoted by squares) exhibits no significant change (7.8 ± 6.3 %) when the glucose concentration of the HBSS media is increased from 2.8 mM (hollow squares) to 16.7 mM (solid squares). However, when islets are present in the microfluidic system (denoted by circles), a dramatic oxidation current increase of 228 ± 1 % is observed. At +0.88 V vs. Ag/AgCl (2 M KCl), the potential where insulin is detected with the MWCNT/DHP composite film, somatostatin is a possible interferent as it is secreted by the δ-cells of the islet [55] and has a peak oxidation potential of + 0.80 V vs. Ag/AgCl [56]. The dramatic current increase seen in Figure 7 (228 %) is unlikely to be due to somatostatin as β cells are stimulated to secrete insulin when islets are exposed to high levels of glucose [11] and the function of somatostatin is to inhibit β cells [57,58].
Fig. 7.
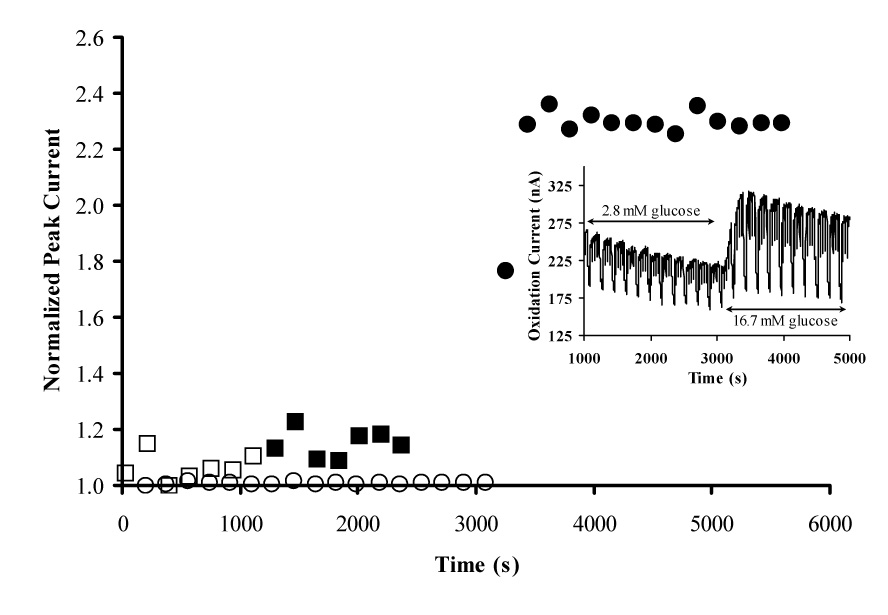
Normalized stop flow peak current response for MWCNT/DHP film on the 1 mm GCE of the sensor head in microfluidic chamber of MAMP upon stimulation with low and high glucose with and without islets. Measurements were performed in HBSS with 180 s cycle with a flow rate of 20 µL/min for 140 s and a stop flow period of 40 s and sample volume of 3 µL. □: No islets low glucose; ■: No islets high glucose; ○: Islets low glucose; ●: Islets high glucose. INSET: Amperometric response of MWCNT/DHP composite film at + 0.88 V vs. Ag/AgCl (2M KCl) with islets present. All data for this figure was collected with the same sensor, same coating, and same microfluidic chamber to reduce variability.
The currents in the presence and absence of islets were normalized to their corresponding stop flow peak current at 2.8 mM glucose to highlight the percent change seen when the media was switched to 16.7 mM glucose. The increase in peak height, seen in Figure 7, when the glucose content of the media is increased from 2.8 to 16.7 mM is ~50 nA and is due to the presence of islets. From calibration experiments of the MWCNT/DHP composite film on the 1 mm GCE of the sensor head, this oxidation current increase (~50 nA) corresponds to an insulin concentration increase of 100 µM in the confined volume of the microfluidic chamber. Thus, a major benefit of our microfluidic assay is the confined volume into which insulin is released compared with conventional methods. This setup enhances the detection of insulin as it allows insulin to accumulate during the stop-flow period. If the sensor was utilized outside of the MAMP stop-flow system, this benefit would be lost and the concentration detected from the same number of islets would be lower. While this concentration is easily measured; if necessary, the stop flow period could be lengthened to allow insulin to accumulate and increase the sensitivity of the technique, enabling the use of a smaller number of islets. These islet studies demonstrate the detection of insulin secreted by islets with our newly developed MWCNT/DHP composite sensor in the MAMP as a real-time method for characterizing islet function and physiology in microfluidic devices.
CONCLUSIONS
We have successfully developed a MWCNT/DHP composite film electrode for the electrochemical detection of insulin secreted by islets in a microfluidic system. This sensor enables direct label-free detection of insulin with improved electrocatalytic kinetics, facile preparation, and resistance to fluidic shear forces as compared to other insulin sensors. We were able to detect insulin concentrations as low as 1 µM in the MAMP during calibration experiments. This sensor was integrated into the multianalyte microphysiometer, and insulin secretion by islets was detected. The increase in concentration of insulin detected for 100 islets is much higher than the limit of detection and as high as 100 µM for the sensor because of the confined volume of the microfluidic chamber and the buildup of insulin molecules during a stop flow period.
ACKNOWLEDGEMENTS
We thank Dr. William Hofmeister, formerly of the Vanderbilt Department of Chemical Engineering and currently at the University of Tennessee Space Institute for the multiwall carbon nanotube samples. We also thank Dr. Alvin Powers and Dr. Zhongyi Chen of the Vanderbilt Diabetes Center for their guidance and for their aid in isolating islets. This work was supported in part by the Vanderbilt Institute of Integrative Biosystem Research, a pilot project grant from the Vanderbilt Diabetes Training and Research Center, which was supported by NIDDK (P60 DK20593-25), and by a grant from the National Institute of Allergy and Infectious Diseases (U01 AI061223).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shirouzu Y, Gu Y, Koga M, Sakurai T, Qi M, Hiura A, Sumi S, Inoue K. J Surg Res. 2006;133:167. doi: 10.1016/j.jss.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Bussiere CT, Lakey JRT, Shapiro AMJ, Korbutt GS. Diabetologia. 2006;49:2341. doi: 10.1007/s00125-006-0374-5. [DOI] [PubMed] [Google Scholar]
- 3.Poirier H, Rouault C, Clement L, Niot I, Monnot M-C, Guerre-Millo M, Besnard P. Diabetologia. 2005;48:1059. doi: 10.1007/s00125-005-1765-8. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. N. Engl. J. Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Persaud SJ, Muller D, Belin VD, Kitsou-Mylona I, Asare-Anane H, Papadimitriou A, Burns CJ, Huang GC, Amiel SA, Jones PM. Diabetes. 2007;56:197. doi: 10.2337/db06-0490. [DOI] [PubMed] [Google Scholar]
- 6.Cooksey RC, Pusuluri S, Hazel M, McClain DA. Am J Physiol Enocrinol Metab. 2006;290:E334. doi: 10.1152/ajpendo.00265.2005. [DOI] [PubMed] [Google Scholar]
- 7.Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis J, Reems J. Diabetes. 2004;53:401. doi: 10.2337/diabetes.53.2.401. [DOI] [PubMed] [Google Scholar]
- 8.Sugden MC, Greenwood GK, Smith ND, Holness MJ. Endocrinology. 2003;144:146. doi: 10.1210/en.2002-220811. [DOI] [PubMed] [Google Scholar]
- 9.Holness MJ, Greenwood GK, Smith ND, Sugden MC. Diabetes. 2006;55:3501. doi: 10.2337/db06-0666. [DOI] [PubMed] [Google Scholar]
- 10.Bergsten P. Am J Physiol Enocrinol Metab. 1998;274:796. [Google Scholar]
- 11.Straub SG, Sharp GWG. Diabetes Metab. Res. Rev. 2002:451. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 12.Porksen N, Nyholm B, Veldhuis JD, Butler PC, Schmitz O. Am. J. Physiol. Enocrinol. Metab. 1997;273:E908. doi: 10.1152/ajpendo.1997.273.5.E908. [DOI] [PubMed] [Google Scholar]
- 13.Cunha DA, Carneiro EM, Alves MdC, Jorge AG, Morais de Sousa S, Boschero AC, Saad MJA, Velloso LA, Rocha EM. Am. J. Physiol. Enocrinol. Metab. 2005;289:E768. doi: 10.1152/ajpendo.00469.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Huang H, Zhang H, Liu M. Anal. Lett. 2006;39:2463. [Google Scholar]
- 15.Maghasi AT, Halsall HB, Heineman WR, Rodriguez-Rilob HL. Anal. Biochem. 2004;326:183. doi: 10.1016/j.ab.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa RM, Silva AM, Tomé AR, Stamford JA, Santos RM, Rosário LM. Biochem. Biophys. Res. Commun. 1996;228:100. doi: 10.1006/bbrc.1996.1622. [DOI] [PubMed] [Google Scholar]
- 17.Roper MG, Shackman JG, Dahlgren GM, Kennedy RT. Anal. Chem. 2003;75:4711. doi: 10.1021/ac0346813. [DOI] [PubMed] [Google Scholar]
- 18.Shackman JG, Dahlgren GM, Peters JL, Kennedy RT. Lab on a Chip. 2005;5:56. doi: 10.1039/b404974h. [DOI] [PubMed] [Google Scholar]
- 19.Qian W-J, Peters JL, Dahlgren GM, Gee KR, Kennedy RT. Biotechniques. 2004;37:922. doi: 10.2144/04376BI01. [DOI] [PubMed] [Google Scholar]
- 20.Cox JA, Gray T. Anal. Chem. 1989;61:2462. doi: 10.1021/ac00196a027. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Shen H, Atkinson MA, Kennedy RT. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9608. doi: 10.1073/pnas.92.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorski W, Aspinwall CA, Lakey JRT, Kennedy RT. J. Electroanal. Chem. 1997;425:191. [Google Scholar]
- 23.Cheng L, Pacey GE, Cox JA. Anal. Chem. 2001;73:5607. doi: 10.1021/ac0105585. [DOI] [PubMed] [Google Scholar]
- 24.Pikulski M, Gorski W. Anal. Chem. 2000;72:2696. doi: 10.1021/ac000343f. [DOI] [PubMed] [Google Scholar]
- 25.Elsen HA, Slowinska K, Hull E, Majda M. Anal. Chem. 2006 doi: 10.1021/ac060449w. ASAP online article. [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z, You H. Electrochim. Acta. 2002;47:3037. [Google Scholar]
- 27.Baughman RH, Zakhidov AA, de Heer WA. Science. 2002;297:787. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q, Gan Z, Zhauang Q. Electroanalysis. 2002;14:1609. [Google Scholar]
- 29.Musameh M, Wang J, Merkoci A, Lin Y. Electrochem. Comm. 2002;4:743. [Google Scholar]
- 30.Gooding JJ, Wibowo R, Liu JQ, Yang W, Losic D, Orbons S, Mearns FJ, Shapter JG, Hibbert DB. J. Am. Chem. Soc. 2003;125:9006. doi: 10.1021/ja035722f. [DOI] [PubMed] [Google Scholar]
- 31.Yu X, Chattopadhyay D, Galeska I, Papadimitrakopoulos F, Rusling JF. Electrochem. Comm. 2003;5:408. [Google Scholar]
- 32.Wang J. Electroanalysis. 2005;17:7. [Google Scholar]
- 33.Wang J, Agnes L, Martinez T. Bioelectrochem. Bioenerg. 1992;29:215. [Google Scholar]
- 34.Wang J, Musameh M. Anal. Chim. Acta. 2004;511:33. doi: 10.1016/j.aca.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Tangkuaram T, Loyprasert S, Vazquez-Alvarez T, Veerasai W, Kanatharana P, Thavarungkul P. Anal. Chim. Acta. 2007;581:1. doi: 10.1016/j.aca.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Ji X, Fei J, Hu S. Nanotechnology. 2004;15:287. [Google Scholar]
- 37.Zhang M, Mullens C, Gorski W. Anal. Chem. 2005;77:6396. doi: 10.1021/ac0508752. [DOI] [PubMed] [Google Scholar]
- 38.Naumov S, Janovsky I, Knolle W, Mehnert R. Macromol. Chem. Phys. 2004;205:1530. [Google Scholar]
- 39.Eklund SE, Cliffel DE, Kozlov E, Prokop A, Wikswo J, Baudenbacher F. Anal. Chim. Acta. 2003;296:93. [Google Scholar]
- 40.Eklund SE, Taylor D, Kozlov E, Prokop A, Cliffel DE. Anal. Chem. 2004;76:519. doi: 10.1021/ac034641z. [DOI] [PubMed] [Google Scholar]
- 41.Eklund SE, Kozlov E, Taylor DE, Baudenbacher F, Cliffel DE. In: Meth. Mol. Biol. Rosenthal SJ, Wright DW, editors. Totowa, NJ: Humana Press Inc; 2005. p. 209. [Google Scholar]
- 42.Eklund SE, Snider RM, Wikswo J, Baudenbacher F, Prokop A, Cliffel DE. J. Electroanal. Chem. 2006;587:333. [Google Scholar]
- 43.Dong F, Fang CX, Yang X, Zhang X, Lopez FL, Ren J. Diabetologia. 2006;49:1421. doi: 10.1007/s00125-006-0230-7. [DOI] [PubMed] [Google Scholar]
- 44.Chen N, Liu L, Zhang Y, Ginsberg HN, Yu Y-H. Diabetes. 2005;54:3379. doi: 10.2337/diabetes.54.12.3379. [DOI] [PubMed] [Google Scholar]
- 45.Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Diabetologia. 2005;48:2412. doi: 10.1007/s00125-005-1940-y. [DOI] [PubMed] [Google Scholar]
- 46.Shavlakadze T, Davies M, White JD, Grounds MD. Journal of Histochemistry and Cytochemistry. 2004;52:873. doi: 10.1369/jhc.3A6177.2004. [DOI] [PubMed] [Google Scholar]
- 47.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. Endocrinology. 2004;145:3258. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 48.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Diabetes. 2004;53:1318. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 49.Dujardin E, Ebbesen TW, Krishnan A, Treacy MMJ. Adv. Mater. 1998;10:611. [Google Scholar]
- 50.Hafner F. Biosens. and Bioelectron. 2000;15:149. doi: 10.1016/s0956-5663(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 51.Bironaite D, Gera L, Stewart JM. Chemico-Biological Interactions. 2004;150:283. doi: 10.1016/j.cbi.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda K, Kobayashi S, Suzuki M, Miyata K, Yamada T, Honda K. Life Sciences. 1999;65:1569. doi: 10.1016/s0024-3205(99)00402-6. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson J, Allsop RH, Taylor RMR, Johnston IDA. Diabetologia. 1976;12:115. doi: 10.1007/BF00428975. [DOI] [PubMed] [Google Scholar]
- 54.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. New York: John Wiley and Sons, Inc; 2001. [Google Scholar]
- 55.Robertson RP. N. Engl. J. Med. 2004;350:694. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 56.Bennett GW, Brazell MP, Marsden CA. Life Sci. 1981;29:1001. doi: 10.1016/0024-3205(81)90458-6. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava S, Goren HJ. Diabetes. 2003;52:2049. doi: 10.2337/diabetes.52.8.2049. [DOI] [PubMed] [Google Scholar]
- 58.Sharp GW. Am. J. Physiol. Cell. 1996;271:C1781. doi: 10.1152/ajpcell.1996.271.6.C1781. [DOI] [PubMed] [Google Scholar]


