Abstract
The contractile characteristics of fast voluntary and electrically evoked unilateral isometric knee extensions were followed in 16 healthy men during 56 days of horizontal bed rest and assessed at bed rest days 4, 7, 10, 17, 24, 38 and 56. Subjects were randomized to either an inactive control group (Ctrl, n = 8) or a resistive vibration exercise countermeasure group (RVE, n = 8). No changes were observed in neural activation, indicated by the amplitude of the surface electromyogram, or the initial rate of voluntary torque development in either group during bed rest. In contrast, for Ctrl, the force oscillation amplitude at 10 Hz stimulation increased by 48% (P < 0.01), the time to reach peak torque at 300 Hz stimulation decreased by 7% (P < 0.01), and the half relaxation time at 150 Hz stimulation tended to be slightly reduced by 3% (P = 0.056) after 56 days of bed rest. No changes were observed for RVE. Torque production at 10 Hz stimulation relative to maximal (150 Hz) stimulation was increased after bed rest for both Ctrl (15%; P < 0.05) and RVE (41%; P < 0.05). In conclusion, bed rest without exercise countermeasure resulted in intrinsic speed properties of a faster knee extensor group, which may have partly contributed to the preserved ability to perform fast voluntary contractions. The changes in intrinsic contractile properties were prevented by resistive vibration exercise, and voluntary motor performance remained unaltered for RVE subjects as well.
Keywords: Maximal rate of torque rise, Time course, Unloading, Force oscillation amplitude, EMG
Introduction
Previous research has shown that exposure to actual or simulated spaceflight leads to pronounced muscle atrophy in humans. The associated muscle weakness significantly impairs the performance of various motor tasks (for reviews see Adams et al. 2003; Desplanches 1997; Edgerton and Roy 2000; Fitts et al. 2000). Muscle function (e.g., maximal isometric force) is often further impaired by adaptations in the central motor control system, as indicated by reductions in the amplitude of the surface electromyogram (EMG) during maximal voluntary contractions (Berg et al. 1997; Deschenes et al. 2002; di Prampero and Narici 2003; Gondin et al. 2004; Schulze et al. 2002).
Compared to steady-state contractions, much higher levels of neural activation are needed for contractions where torque develops as rapidly as possible. This is seen for both voluntary (de Ruiter et al. 2004) as well as for electrically evoked contractions (de Haan 1998; de Ruiter et al. 1999). In daily life, elderly individuals who lack sufficient motor speed or possess poor lower extremity strength have an increased risk of fall-related bone fractures (Shigematsu et al. 2006). The same may hold for astronauts suffering from muscle atrophy, neural deconditioning and increased bone fragility following space missions (Smith and Heer 2002).
The adaptive physiological responses in the human body as a consequence of real or simulated space flight may be offset by means of efficient countermeasures. With respect to the neuromuscular system, it appears that resistance exercise (strength training) is effective to maintain, or at least to minimize changes in muscle mass and strength during bed rest (Akima et al. 2001; Ferrando et al. 1997; Kawakami et al. 2001). Previously, we have shown by means of the twitch interpolation technique, as well as by means of EMG recordings that neural deconditioning was absent during 56 days of bed rest for maximal steady-state contractions, regardless of whether subjects participated in an exercise countermeasure program (Mulder et al. 2006, 2007). Nonetheless, based on the above, it can be hypothesised that the neural activation of explosive isometric contractions is more deteriorated by bed rest than the activation of steady-state contractions (Mulder et al. 2007). The first aim of the study was to test the hypothesis that, in the absence of an exercise countermeasure, the maximal rate of voluntary isometric torque development would show a greater decrease during 56 days of bed rest than the maximal steady-state torque, due to reduced neural drive during the fast voluntary contractions. Such an effect was expected to be prevented by combining daily resistive exercise training with vibration training, which was hypothesized to provide a better protection against musculoskeletal deconditioning during bed rest than heavy-resistance training only (Rittweger et al. 2006). Although the individual merits of resistance training versus vibration training cannot be quantified with such a study design, the efficacy of this combined countermeasure to preserve neural activation and maximal steady-state torque is reported elsewhere (Mulder et al. 2007).
Apart from neural activation, the rate at which muscle force develops under voluntary command is also determined by peripheral factors, such as the intrinsic contractile muscle fibre speed (Andersen and Aagaard 2006) and the stiffness of the series elastic component (Bojsen-Moller et al. 2005). Both factors are known to be altered by exposure to actual or simulated space flight, but their effect is opposite. Whereas faster intrinsic contractile speed characteristics (Talmadge 2000) can partly or fully compensate for the effect of atrophy on power output of single muscle fibres (Widrick et al. 1998), the power output of whole muscles (with intact tendons) would be diminished by decrements in tendon stiffness (Kubo et al. 2000; Reeves et al. 2005). The second aim of the study was to assess whether changes in peripheral factors influenced the voluntary rate of torque development during bed rest. The functional change in peripheral factors, i.e., the combined effect of intrinsic muscle and tendon characteristics, were investigated by applying percutaneous muscle stimulation. This is a frequently used methodology to assess muscle properties irrespective of central neural influences (Binder-Macleod et al. 1995; de Haan et al. 2000; Gerrits et al. 2001; Harridge et al. 1996). We hypothesized that the muscle–tendon complex of the knee extensors would acquire the intrinsic contractile properties of a faster muscle, which would be prevented by the current countermeasure design, conceivably due to the large number of contraction–relaxation cycles during resistive vibration exercise (Blottner et al. 2006).
Methods
Subjects
A total of 16 subjects participated in the present study. All subjects were in good health and were involved in normal physical activity before participation in the large-scale Berlin Bed Rest study (Rittweger et al. 2006). At the start of the study the subjects were randomly assigned to an experimental group or an inactive control group. The experimental group (RVE, n = 8; mean age, height and body mass ± SD: 33.0 ± 1.9 years, 1.84 ± 0.03 cm and 79.5 ± 3.8 kg) participated in a progressive resistive vibration exercise (RVE) training program during the bed rest. The subjects of the inactive control group (Ctrl, n = 8; mean age, height and body mass ± SD: 34.3 ± 2.5 years, 1.82 ± 0.02 cm and 76.8 ± 1.8 kg, respectively) were restricted to bed rest without exercise countermeasure. All subjects were familiarized with the concepts of the experiments, procedures, and the equipment during a familiarization session that was scheduled 3 days prior to the start of bed rest. The local Ethics committee of the Charité, Campus Benjamin Franklin Berlin approved the study and all participants gave their written informed consent.
General design
All subjects underwent 56 days of strict horizontal bed rest at the Charité Benjamin Franklin Hospital, Berlin, Germany. During the bed rest, the subjects were not allowed to stand up, to lift their trunk in bed more than to 30° of trunk flexion, to move their legs briskly, or to elicit large forces with their legs muscles other than during testing sessions or during training sessions. Adherence to this protocol was controlled for by continuous video surveillance and by force transducers in the frames of the bed. The diet was balanced using the Harris–Benedict equation and ingestion of alcohol or nicotine, excessive doses of caffeine, as well as the regular intake of any drug or medication was prohibited (details in Rittweger et al. 2006).
Exercise countermeasure
RVE subjects performed resistive exercises on a vibration system that was specifically developed for application under microgravity and bed rest conditions (Galileo Space; Novotec, Pforzheim, Germany). The applied equipment and protocol for countermeasure exercise are described in detail elsewhere (Rittweger et al. 2006). In short, the training device consists of a vibration platform, which is vertically suspended on a trolley. Elastic springs were attached to the trolley for the subjects to attach themselves through belts with their shoulders, hips, and hands. During bed rest, RVE subjects trained in the supine position, two times daily for 6 days/week. In each training session, four resistive exercises were performed in the following order: squats, heel raises, toe raises and explosive squats. All exercises were performed while the platform was vibrated at a frequency of 19 Hz. Vibration frequency was progressively increased during the 56-day bed rest period to ∼26 Hz at the end of bed rest (Rittweger et al. 2006).
Experimental set-up
Isometric force recordings were made from voluntary and electrically evoked contractions of the knee extensor group of the right leg. Subjects were tested in the supine position using the same equipment as previously described (Mulder et al. 2006). Force signals were digitized using a sampling rate of 1 kHz and stored on disc for immediate and off-line analysis. Torque (N m) was off-line calculated as the product of force and external moment arm.
Experimental procedures
During bed rest, all subjects participated in seven experimental sessions, which were scheduled at days: 4, 7, 10, 17, 24, 38 and 56, the latter being the last day of the bed rest period. The baseline experiment was conducted on the fourth day of bed rest (BR4) for logistical reasons. Although this prevents us to address rapid initial changes in strength and contractile characteristics of the quadriceps (Berg and Tesch 1996), which might result in an underestimation of the effect of bed rest on the measured parameters, it does not compromise the comparison between groups during bed rest, because the RVE subjects started their exercise training program on the fourth day of bed rest, which was scheduled after the completion of the functional testing conducted on that day. In addition, in this way all experiments were conducted under methodologically similar conditions. That is, at the baseline experiment subjects were already minimally 72 h bedridden, each subject was tested at the same time of day, and subjects of the RVE group were always tested before their morning training session.
To minimize potential damage to muscle and tendon structures due to maximal and explosive contractions after a long period of strict muscle inactivity, subjects started each experimental session by performing a standardised warm-up set that consisted of eight to ten unloaded dynamic contractions (right leg not yet strapped to the force transducer), followed by eight sub-maximal isometric contractions at 70° of knee flexion. Further force recordings were obtained at the individually determined pre-bed rest optimal knee flexion (either 60° or 70°, whereby a knee flexion angle of 0° corresponds to full knee extension; see Mulder et al. 2006 for details). Subjects were asked to perform two to three maximal voluntary contractions (MVC) of 2–4 s in duration. Attempts were interposed with 2 min of rest.
After this procedure, the quadriceps muscle was stimulated through two self-adhesive surface electrodes (model 283100, Schwa-Medico, Nieuw Leusden, The Netherlands) of 80 mm × 130 mm. The stimulation intensity was progressively increased until 40% of the MVC torque was obtained during a 700 ms tetanic contraction at 150 Hz (Mulder et al. 2006). The quadriceps muscle was then electrically stimulated with trains of the same single pulse intensity at low (7 pulses at 10 Hz) and high (24 pulses at 300 Hz) stimulation frequency. The trains were applied in this order and interposed with 1 min of rest.
Following the electrically evoked contractions, the stimulation electrodes were removed and the skin over the lateral vastus muscle was re-prepared for the positioning of a high-density surface EMG system (HD-sEMG, Active One, BioSemi Inc., Amsterdam, The Netherlands). The system consisted of 130 densely spaced skin-surface electrodes, arranged in a rectangular 10 × 13 matrix with 5 mm inter-electrode distance (Blok et al. 2002). Before mounting the grid to the skin, the skin was scrubbed with alcoholic pads and slightly rubbed with electrode paste. Prior to each test, the skin-electrode impedance was checked and, if necessary, the skin was re-prepared. The grid was positioned over the distal (third), anterio-lateral part of the right vastus lateralis muscle, such that the columns of 13 electrodes were aligned parallel to the muscle fibre orientation of the muscle and with the motor endplate zone around the centre of the columns of the grid (Mulder et al. 2007). The pre-amplified 130 monopolar signals (referenced to the patella) were bandpass filtered (16–400 Hz) and simultaneously AD-converted (16 bits with a resolution of 1 μV/bit at a rate of 2 kHz/channel). Data were stored on hard disk for subsequent off-line processing.
With the sEMG system properly positioned, each subject performed another MVC of 2–4 s. If the torque deviated more than 5% from the highest torque attained during the previous MVC-task (i.e., the highest value of the attempts before the application of electrical stimulation), another attempt was made.
Lastly, each subject performed three fast (explosive) isometric contractions to assess the neural control of these contractions, as well as the initial rate of torque development during these attempts. Subjects were instructed to contract “as fast and forcefully as possible” on a given signal from the test leader (3-2-1 “Go”). Subjects were required to reach a minimum of 80% of the current MVT and to maintain torque at the highest attained level for approximately 1 s, i.e., ‘kicks’ were always disqualified. Attempts with an initial countermovement (identified by a drop in the torque signal exceeding 1 N just before the onset of torque development) were also disqualified (Aagaard et al. 2002; de Ruiter et al. 2004). Attempts were interposed with 2 min of rest.
Data analysis
Fast voluntary isometric knee extensions
The neural activation of muscle fibres at the start of a contraction greatly determines the performance of specific voluntary motor functions, such as the rate at which muscle force develops during fast and forceful voluntary isomeric contractions (de Ruiter et al. 2004). Even so, the maximal rate of isometric torque development did not differentiate subjects according to their ability to generate high neural activation levels at the very start of the contraction. Instead, the time torque integral, calculated as the area under the time torque curve over the first 40 ms after the onset of torque development, showed to be more sensitive to the initial level of neural activation (de Ruiter et al. 2004). Based on these findings, we calculated the voluntary time torque integral (vTTI40) as an estimate of maximal isometric tension development under voluntary command (Fig. 1). The onset of torque development was thereby defined as the point at which the torque curve exceeded baseline torque by more than three standard deviations.
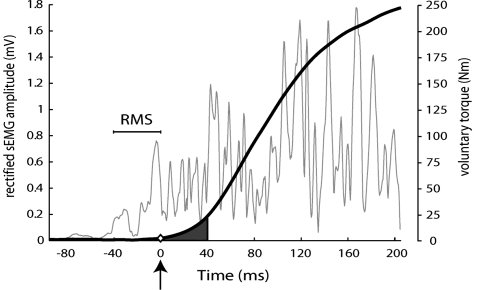
Fig. 1.
Voluntary torque (thick line) and rectified surface EMG of the vastus lateralis muscle (thin line) time traces, obtained from a representative subject during an isometric voluntary knee extension performed as fast and forcefully as possible. The arrow and diamond at time 0 ms indicate the start of voluntary torque development. The shaded area under the voluntary torque trace reflects the vTTI40, calculated the first 40 ms after onset of torque development. The horizontal bar indicates the 40 ms immediately preceding the onset of torque development (i.e., from −40 to 0 ms) for which the root mean square (RMS) of the surface EMG was calculated. Subsequently, both vTTI40 and RMS−40–0 were normalized to the steady state maximal isometric knee extension condition at the day of testing
Voluntary neural activation during the fast isometric knee extensions was assessed by averaging the amplitude (based on root mean square, RMS) of the monopolarly recorded sEMG signals (Fig. 1) over 40 ms before the onset of torque development (RMS−40–0). Unlike a single bipolar recording, the HD-sEMG system allowed for the assessment of monopolar recordings, and allowed for the spatial selection of the grid column with the highest amplitude. The latter was based on the mean of all electrodes within one column.
To investigate how neural activation related to the relative maximal rate of torque development for each individual, vTTI40 values were corresponded to RMS−40–0 data, whereby each accepted contraction of each session was included. For the evaluation of both RMS−40–0 and vTTI40 as a function of bed rest duration, only the data of one single fast contraction per session were incorporated into the analyses. The contraction with highest level of EMG activity was selected for this purpose. Since we were interested to compare the neural activation during fast voluntary contractions with the neural activation during voluntary contractions where torque is developed more slowly, the contractile and electromyographic data were normalized to the steady-state maximal isometric knee extension condition at the day of testing. These data are also part of another study and are reported elsewhere (Mulder et al. 2007).
Electrically evoked contractions
For each experimental session, the peak torque (T) attained during the 10 Hz (T10) train was expressed relative to the maximal tetanic torque reached during the 150 Hz tetanus (T150), i.e., expressed as a T10/T150 ratio. The force profiles of the 10 Hz tetanus showed clear oscillations (Fig. 2). The force oscillation amplitude (FOA) relative to the mean force was also calculated and used as a measure of the degree of force-fusion (Gerrits et al. 1999; Vøllestad et al. 1997).
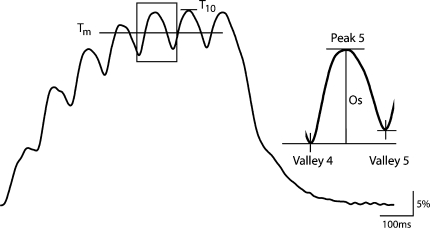
Fig. 2.
Methods used for evaluation of contractile properties of the quadriceps femoris muscle evoked by electrically evoked muscle stimulation at 10 Hz. The torque elicited at 10 Hz stimulation was first expressed as a percentage of the maximal torque evoked at 150 Hz (T150). T10 was the peak value of the 10 Hz torque trace. The force oscillation amplitude (FOA) was determined by expressing the mean amplitude of the torque oscillation (Os) between the fourth and seventh stimulus as a fraction of the mean torque (Tm) during this time
The contractile characteristics at high pulse frequency stimulation (i.e., 300 Hz) were quantified by assessing the time to peak tension from the start of the evoked contraction (TPT300). The start of contraction was defined as the instant the first pulse of the 80 ms train was delivered. Half-relaxation time was determined for each session as the time needed for the elicited torque to decay to half the maximal value following the last pulse of the 150 Hz tetanus (HRT150). Force data were filtered using a fourth-order, 50 Hz low-pass filter. This filter was found not to affect the course of torque development; it only removed high-frequency noise from the signal.
Statistical analysis
Values are expressed as mean ± SE (standard error of the mean). Independent-samples t tests were used to determine whether the absolute values of variables related to muscle strength, neural activation and contractile properties of the quadriceps femoris muscle differed at baseline (BR4). Changes in muscle strength and contractile properties after 56 days of bed rest were assessed by means of linear regression, and expressed as a percentage change with respect to the value at BR4. One-sample t tests were used to determine whether the normalized slope (slope/intercept on y-axis) of the linear regression was significantly different from zero. Independent-sample t tests were used to determine whether the groups differed in their response to bed rest. Pearson’s correlation coefficients were calculated to establish significance of correlation. The level of significance was set at P < 0.05.
Results
Fast voluntary isometric knee extensions
Data of neural activation and steady state isometric strength are presented elsewhere (Mulder et al. 2006). Briefly, whereas neural activation remained unaltered for Ctrl, maximal voluntary isometric steady-state strength significantly declined after 56 days of bed rest by about 17%. For the RVE group neural activation increased by approximately 30%, whereas voluntary steady-state torque was maintained.
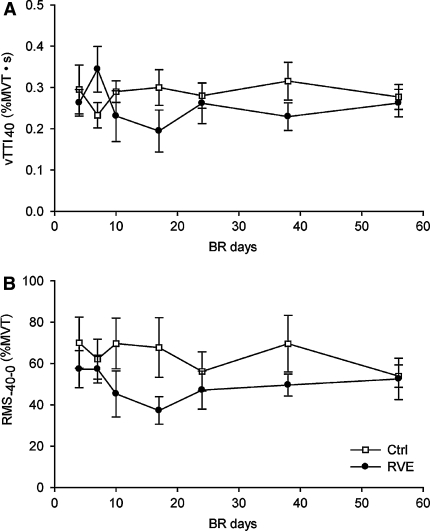
The ability to perform fast voluntary contractions showed a substantial variability during bed rest. There was considerable variation in vTTI40 as well as in RMS−40–0 during these contractions, both within one session as well as across sessions. Nonetheless, significant positive linear relationships (P < 0.01) between RMS−40–0 and the vTTI40 were obtained for 12 of the 16 subjects in the present study (4 RVE subjects and 8 Ctrl subjects). Significant Pearson’s correlation coefficients (r) ranged from 0.52 to 0.90; with a median value of 0.74. When individual data were pooled per group, each group had a significant relationship between normalized EMG amplitude and normalized vTTI40 (RVE, r = 0.572; P < 0.01; Ctrl, r = 0.758; P < 0.01). However, as can be seen in Fig. 3, for neither group did RMS−40–0, or vTTI40 change during the course of the bed rest.
Fig. 3.
Mean values (±SE) of the voluntary time torque integral over the first 40 ms (TTI40) after torque development (vTTI40; a) and the sEMG amplitude 40 ms before the onset of torque development (RMS−40–0; b) obtained during 56 days of bed rest (BR). For each session, both vTTI40 and RMS−40–0 are expressed as a percentage of the corresponding values at the steady-state maximal voluntary torque (MVT) of that session
Intrinsic contractile properties
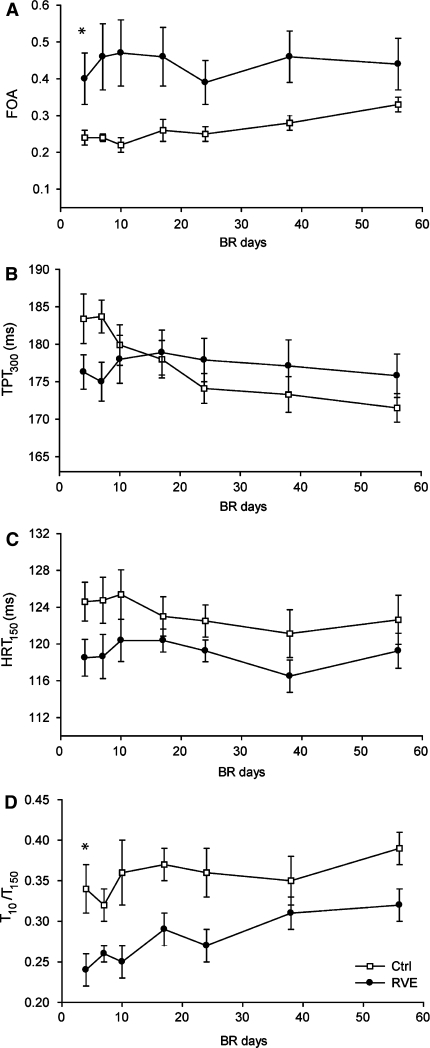
The contractile properties obtained from electrically evoked contractions during bed rest are shown in Fig. 4. The course of FOA during bed rest was significantly different for Ctrl and RVE subjects (P < 0.05). For Ctrl, the FOA increased substantially (by 47.5 ± 10.0%, P < 0.01) during the bed rest study (Fig. 4a). In contrast, no change in the FOA was observed for RVE. The groups also differed in their response to bed rest with respect to contraction time as indicated by TPT300 (P < 0.01). TPT300 declined significantly by 6.8 ± 0.9% (P < 0.001) for Ctrl, whereas no changes were observed for RVE (Fig. 4b). Furthermore, in the Ctrl group the HRT150 tended to be somewhat shortened by 2.5 ± 1.1% (P = 0.056) after 56 days of bed rest, whereas for RVE it again remained unaltered (Fig. 4c). Group differences in HRT150 did, however, not reach significance. Interestingly, the T10/T150 ratio increased for both Ctrl (by 15.0 ± 5.5%; P < 0.05) and RVE (by 40.6 ± 17.1%; P < 0.05) and these changes were not significantly different between groups (Fig. 4d).
Fig. 4.
Mean values (±SE) of the force oscillation amplitude (FOA; a) time to peak tension at 300 Hz stimulation (TPT300; b), half relaxation time at 150 Hz stimulation (HRT150), and peak torque at 10 Hz stimulation expressed as a fraction of the maximal torque obtained during tetanic stimulation at 150 Hz (T10/T150) obtained during 56 days of bed rest (BR)
Discussion
Contractile characteristics of electrically evoked knee extensions
The rate at which muscle force develops during a fast voluntary contraction is influenced by both peripheral factors and neural activation properties. In order to separate these influences, the contractile response of the knee extensor group was also assessed by means of percutaneous sub-maximal muscle stimulation. This is a reliable method, which has been used to assess intrinsic muscle characteristics in various human populations (Binder-Macleod et al. 1995; de Haan et al. 2000; Gerrits et al. 2001; Harridge et al. 1996). Although it is not possible to assess the specific characteristics of the portion of the muscle fibre population that is recruited during the sub-maximal stimulation procedure of the present study, the activation of about 40% of the muscle fibres suffices to represent the global contractile characteristics of the quadriceps muscle. Previous studies have shown that the torque frequency relationship, as well as the contractile properties of the twitch are not intrinsically influenced by the absolute force level, provided that a force between 20 and 50% MVC is reached during maximal tetanic stimulation (Binder-Macleod et al. 1995).
In the absence of the exercise countermeasure, the knee extensors exhibited characteristics of a faster muscle following 56 days of bed rest. The degree of fusion at 10 Hz stimulation was decreased, the time to reach peak torque at 300 Hz stimulation was reduced and relaxation after tetanic stimulation at 150 Hz tended to be faster. The possibility of an error seems unlikely, since we found complementary changes towards enhanced contractile speed at all frequencies studied. In addition, these changes were observed only for the inactive control group.
The stiffness of the series elastic component is amongst the factors known to affect the rate of torque development (Bojsen-Moller et al. 2005). Although not measured in the present study, from previous other studies it appears that with muscle unloading tendon stiffness often decreases (Kubo et al. 2000; Reeves et al. 2005). This would tend to result in a reduced rate of torque development of the whole muscle–tendon complex, opposite to what we observed. The increased rate of torque development, as well as the tendency towards a faster rate of relaxation in the present study could, however, be explained by an elevated rate of cross-bridge cycling (Unsworth et al. 1982). Although at odds with some previous findings (e.g., Davies et al. 1987; Gondin et al. 2004; Narici et al. 2003) such interpretation would be consistent with documented elevations in maximal unloaded shortening velocity (Caiozzo et al. 1994; Widrick et al. 2001; Yamashita-Goto et al. 2001) potentially linked to shifts in muscle fibre phenotype from slow to fast as a direct consequence of muscle unloading (Fitts et al. 2000; Gerrits et al. 2003; Ohira et al. 1999; Talmadge 2000; Trappe et al. 2004). Because the assessed muscle torque in the present study is the resultant of the entire muscle–tendon complex, and given a likely increase in tendon compliance by bed rest, the observed enhanced contractile speed characteristics, might even underestimate the underlying intrinsic changes in the present study.
Despite faster contractile properties, torque production at 10 Hz stimulation relative to maximal tetanic stimulation was increased after bed rest. Although consistent with previous research (Seki et al. 2001), the higher relative torque responses at low frequency stimulation did not result from a significant enhancement of twitch summation, as previously opted (Gondin et al. 2004). In contrast, the level of force fusion substantially decreased during bed rest period in the present study. At present, the exact processes responsible for these anomalous findings remain unclear, but a similar phenomenon was reported in the paralyzed muscles of individuals with spinal cord injury (Gerrits et al. 1999), which may be considered as an extreme model for muscle unloading.
In part, our data supports the hypothesis postulated by Rittweger et al. (2006) that the large number of contraction–relaxation cycles during resistive vibration exercise (Blottner et al. 2006) may be effective in preserving muscle fibre contractile properties. Indeed, no changes in time to reach peak torque at 300 Hz stimulation, the rate of relaxation after tetanic stimulation at 150 Hz, or the level of force fusion at low stimulation frequency (i.e., the FOA) were observed in the exercise trained subjects. The significant difference between some baseline values, e.g., FOA and T10/T150 ratio (Fig. 4a, d), and the tendencies for TPT300 (P = 0.098) and HRT150 (P = 0.057) to be lower in RVE compared to Ctrl at baseline deserves attention, since it may point towards a difference between groups with respect to muscle fibre type at the start of the study, with RVE exhibiting a faster muscle. Nonetheless, at least for the FOA it has been demonstrated that it is still much higher (i.e., 0.65 in Gerrits et al. 1999) in paralyzed muscles of people with spinal cord injury. This makes it unlikely that the preservation of FOA in the present study resulted from a ceiling effect for RVE.
Despite the observation that speed characteristics were unaltered for the exercise-trained group, the relative peak torque at low stimulation frequency also increased for this group. As the level of force fusion remained unaltered during bed rest for RVE, other factors are likely involved. Once possibility is that the peak torque during 10 Hz stimulation increased as a consequence of an enlarged twitch response. Although not directly measured in the present study, the torque developed during the first response of the 10 Hz tetanus increased during the course of the bed rest by about 26%, which was comparable to the elevation in 10 Hz peak torque (∼40%) for RVE. Interestingly, the relative torque production of the first response of the 10 Hz contraction also increased (by about 30%) for the inactive control group. However, the reduced fusion of successive individual twitches diminished the increase in peak torque at 10 Hz stimulation to about 15%. Despite the differences in contractile speed characteristics, the percent change in 10 Hz peak torque after 56 days of bed rest was not different between groups. At present, the selectivity of the exercise countermeasure paradigm to prevent changes in contractile speed properties, but not in the torque response at low frequencies of stimulation, remains difficult to explain. Indeed, more research is needed to determine the effectiveness of resistive vibration exercise as a countermeasure, since the individual merits of resistance training versus vibration training could not be quantified in the present study.
Fast voluntary isometric knee extensions at maximal effort
Adequate preservation of the rate at which muscle torque develops during a forceful volitional contraction is imperative for astronauts, as neuromuscular deconditioning, coupled to a weakened load-bearing skeleton, increases the risk of fall-related bone fractures after prolonged space missions. To add to the concern, compared to steady-state contractions, much higher levels of neural activation are needed for contractions where torque develops as rapidly as possible (de Haan 1998; de Ruiter et al. 1999). As such, we hypothesised that the ability to perform fast and forceful voluntary contractions would be more deteriorated by bed rest confinement than the ability to perform maximal steady-state contractions. Surprisingly, and at odds with the finding of others (di Prampero and Narici 2003; Koryak 1998), we found no evidence for such bed rest induced functional impairment in either group (Fig. 3).
For the same inactive control subjects as used in the present study, we previously reported an absence of neural deconditioning for maximal voluntary steady-state contractions, whether examined by the twitch interpolation technique, or by the assessment of electromyographic activity of the quadriceps femoris muscle (Mulder et al. 2006, 2007). We concluded that the preservation of neural activation of this specific motor task was likely associated with the repeated functional retesting sequence employed during bed rest. The observation that the percent reduction in maximal isometric knee extension strength of the left leg, which was not repeatedly retested during bed rest, exceeded the level of atrophy by a factor of two after 8 weeks of bed rest, strengthened this notion (Mulder et al. 2006). Based on the findings of the present study, we are inclined to suggest that the repeated retesting regime also served as a contributory factor in maintaining neural activation during fast isometric knee extensions. Although such an effect was not intended at the time, this supposition is important since it suggests that neural activation of different isometric motor tasks can be maintained during bed rest without rigorous exercise training regimes.
In part, the absence of loss of muscle functionality following bed rest might have resulted from changes in the contractile properties of unloaded muscles. Widrick et al. (1998) reported significant atrophy of single human soleus muscle fibres after 17 days of spaceflight. Absolute peak power of these fibres was, however, partly or fully preserved by an elevated contraction velocity. In the present study, the examined muscle group in the inactive control group also acquired mechanical characteristics of a faster muscle during the course of the bed rest. As selective neural deconditioning could not be demonstrated for the fast voluntary contractions, the expectation might arise of an increased rate of voluntary torque development for the inactive control group. Such a systematic change was not observed. Although it seems difficult to explain this absence based on the changes in intrinsic contractile characteristics, it is clear that neural activation and the subsequent mechanical response during voluntary contractions are much more variable than the activation and response when contractions are electrically evoked. To overcome this variability, larger alterations than those observed in intrinsic contractile characteristics would have been required to allow for detectable changes for the fast voluntary isometric actions.
Interestingly, an increase in the rate of torque development could have also been expected for the exercise trained RVE group. For this group we previously found that the amplitude of the surface EMG during the maximal steady-state contractions was substantially increased at the end of bed rest (by ∼30%; Mulder et al. 2007). The latter was suggested to result from an increase in the mean motor unit firing rate, due to a change in the excitability of the alpha motoneurons (Mulder et al. 2007). Such modulation has been associated with increased rate of force development after resistance training (Holtermann et al. 2007). However, in the present study the neural activation during the fast voluntary contractions and the subsequent initial rate of torque development remained unaltered for the exercise trained group. The current exercise-training regime consisted mainly of relatively slow dynamic loaded contractions while vibration was simultaneously applied to the feet. Such a motor task is quite different from the isometric contractions performed during the testing of the subjects. Based on the selectivity of training, and the fact that the number of exercise training sessions by far outweighed the number of testing sessions (89 vs. 7), it is likely that neural activation strategies employed by the subjects were more guided towards optimal performance during the training sessions, than optimal performance during the fast voluntary isometric actions during testing.
In conclusion, in the subjects who were confined to 8 weeks of bed rest without preventive measures the knee extensor muscle group acquired intrinsic contractile properties of a faster muscle. Resistive vibration exercise proved effective to counteract these changes at the muscle level. An unexpected finding of the present study was that neither group showed deterioration in the capacity to maximally activate the knee extensors at the very start of a voluntary contraction performed as fast and forcefully as possible. For the RVE group this might indicate an effective countermeasure design. However, considering that neural activation and voluntary muscle function were also maintained in the Ctrl group, it is also conceivable that the multiple retesting of the subjects resulted in or at least contributed to these preservations.
Acknowledgments
The Berlin Bed Rest study was supported by grant 14431/02/NL/SH2 from the European Space Agency. The study was further sponsored by Charité, University Medicine Berlin (Campus Benjamin Franklin), DLR (German AeroSpace), MSD Sharp & Dohme, Lilly Germany, Servier Germany, Hoffmann-LaRoche, Siemens, Novartis, and Seca.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326 [DOI] [PubMed]
- Adams GR, Caiozzo VJ, Baldwin KM (2003) Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95:2185–2201 [DOI] [PubMed]
- Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T (2001) Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand 172:269–278 [DOI] [PubMed]
- Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52 [DOI] [PubMed]
- Berg HE, Tesch PA (1996) Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand 157:63–70 [DOI] [PubMed]
- Berg HE, Larsson L, Tesch PA (1997) Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol 82:182–188 [DOI] [PubMed]
- Binder-Macleod SA, Halden EE, Jungles KA (1995) Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc 27:556–565 [PubMed]
- Blok JH, van Dijk JG, Drost G, Zwarts MJ, Stegeman DF (2002) A high-density multichannel surface electromyography system for the characterization of single motor units. Rev Sci Instrum 73:1887–1897 [DOI]
- Blottner D, Salanova M, Puttmann B, Schiffl G, Felsenberg D, Buehring B, Rittweger J (2006) Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest. Eur J Appl Physiol 97:261–271 [DOI] [PubMed]
- Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P (2005) Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99:986–994 [DOI] [PubMed]
- Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM (1994) Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol 76:1764–1773 [DOI] [PubMed]
- Davies CT, Rutherford IC, Thomas DO (1987) Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. Eur J Appl Physiol Occup Physiol 56:306–312 [DOI] [PubMed]
- de Haan A (1998) The influence of stimulation frequency on force–velocity characteristics of in situ rat medial gastrocnemius muscle. Exp Physiol 83:77–84 [DOI] [PubMed]
- de Haan A, de Ruiter CJ, van der Woude LH, Jongen PJ (2000) Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve 23:1534–1541 [DOI] [PubMed]
- de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A (1999) Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol 84:1137–1150 [DOI] [PubMed]
- de Ruiter CJ, Kooistra RD, Paalman MI, de Haan A (2004) Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol 97:1693–1701 [DOI] [PubMed]
- Deschenes MR, Giles JA, McCoy RW, Volek JS, Gomez AL, Kraemer WJ (2002) Neural factors account for strength decrements observed after short-term muscle unloading. Am J Physiol Regul Integr Comp Physiol 282:R578–R583 [DOI] [PubMed]
- Desplanches D (1997) Structural and functional adaptations of skeletal muscle to weightlessness. Int J Sports Med 18(Suppl 4):S259–S264 [DOI] [PubMed]
- di Prampero PE, Narici MV (2003) Muscles in microgravity: from fibres to human motion. J Biomech 36:403–412 [DOI] [PubMed]
- Edgerton VR, Roy RR (2000) Invited review: gravitational biology of the neuromotor systems: a perspective to the next era. J Appl Physiol 89:1224–1231 [DOI] [PubMed]
- Ferrando AA, Tipton KD, Bamman MM, Wolfe RR (1997) Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 82:807–810 [DOI] [PubMed]
- Fitts RH, Riley DR, Widrick JJ (2000) Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89:823–839 [DOI] [PubMed]
- Gerrits HL, de Haan A., Hopman MT, van der Woude LH, Jones DA, Sargeant AJ (1999) Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve 22:1249–1256 [DOI] [PubMed]
- Gerrits HL, Hopman MT, Sargeant AJ, de Haan A (2001) Reproducibility of contractile properties of the human paralysed and non-paralysed quadriceps muscle. Clin Physiol 21:105–113 [DOI] [PubMed]
- Gerrits HL, Hopman MT, Offringa C, Engelen BG, Sargeant AJ, Jones DA, de Haan A (2003) Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflugers Arch 445:734–740 [DOI] [PubMed]
- Gondin J, Guette M, Maffiuletti NA, Martin A (2004) Neural activation of the triceps surae is impaired following 2 weeks of immobilization. Eur J Appl Physiol 93:359–365 [DOI] [PubMed]
- Harridge SD, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjornsson M, Saltin B (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432:913–920 [DOI] [PubMed]
- Holtermann A, Roeleveld K, Engstrom M, Sand T (2007) Enhanced H-reflex with resistance training is related to increased rate of force development. Eur J Appl Physiol 101:301–312 [DOI] [PubMed]
- Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T (2001) Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol 84:7–12 [DOI] [PubMed]
- Koryak Y (1998) Effect of 120 days of bed-rest with and without countermeasures on the mechanical properties of the triceps surae muscle in young women. Eur J Appl Physiol Occup Physiol 78:128–135 [DOI] [PubMed]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T (2000) Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol 83:463–468 [DOI] [PubMed]
- Mulder ER, Stegeman DF, Gerrits KH, Paalman MI, Rittweger J, Felsenberg D, de Haan A (2006) Strength, size and activation of knee extensors followed during 8 weeks of horizontal bed rest and the influence of a countermeasure. Eur J Appl Physiol 97:706–715 [DOI] [PubMed]
- Mulder ER, Gerrits KH, Kleine BU, Rittweger J, Felsenberg D, de Haan A, Stegeman DF (2007) High-density surface EMG study on the time course of central nervous and peripheral neuromuscular changes during 8 weeks of bed rest with or without resistive vibration exercise. J Electromyogr Kinesiol (in press) [DOI] [PubMed]
- Narici M, Kayser B, Barattini P, Cerretelli P (2003) Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol 90:275–282 [DOI] [PubMed]
- Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR (1999) Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol 87:1776–1785 [DOI] [PubMed]
- Reeves ND, Maganaris CN, Ferretti G, Narici MV (2005) Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98:2278–2286 [DOI] [PubMed]
- Rittweger J, Belavy D, Hunek P, Gast U, Boerst H, Feilcke B, Armbrecht G, Mulder E, Schubert H, Richardson C, de Haan A., Stegeman DF, Schiessl H, Felsenberg D (2006) Highly demanding resistive vibration exercise program is tolerated during 56 days of strict bed-rest. Int J Sports Med 27:553–559 [DOI] [PubMed]
- Schulze K, Gallagher P, Trappe S (2002) Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc 34:303–313 [DOI] [PubMed]
- Seki K, Taniguchi Y, Narusawa M (2001) Alterations in contractile properties of human skeletal muscle induced by joint immobilization. J Physiol 530:521–532 [DOI] [PMC free article] [PubMed]
- Shigematsu R, Rantanen T, Saari P, Sakari-Rantala R, Kauppinen M, Sipila S, Heikkinen E (2006) Motor speed and lower extremity strength as predictors of fall-related bone fractures in elderly individuals. Aging Clin Exp Res 18:320–324 [DOI] [PubMed]
- Smith SM, Heer M (2002) Calcium and bone metabolism during space flight. Nutrition 18:849–852 [DOI] [PubMed]
- Talmadge RJ (2000) Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23:661–679 [DOI] [PubMed]
- Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P (2004) Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557:501–513 [DOI] [PMC free article] [PubMed]
- Unsworth BR, Witzmann FA, Fitts RH (1982) A comparison of rat myosin from fast and slow skeletal muscle and the effect of disuse. J Biol Chem 257:15129–15136 [PubMed]
- Vøllestad NK, Sejersted I, Saugen E (1997) Mechanical behavior of skeletal muscle during intermittent voluntary isometric contractions in humans. J Appl Physiol 83:1557–1565 [DOI] [PubMed]
- Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL, Fitts RH (1998) Force–velocity–power and force–pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol 85:1949–1956 [DOI] [PubMed]
- Widrick JJ, Romatowski JG, Norenberg KM, Knuth ST, Bain JL, Riley DA, Trappe SW, Trappe TA, Costill DL, Fitts RH (2001) Functional properties of slow and fast gastrocnemius muscle fibers after a 17-day spaceflight. J Appl Physiol 90:2203–2211 [DOI] [PubMed]
- Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T (2001) Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol 91:417–424 [DOI] [PubMed]