Abstract
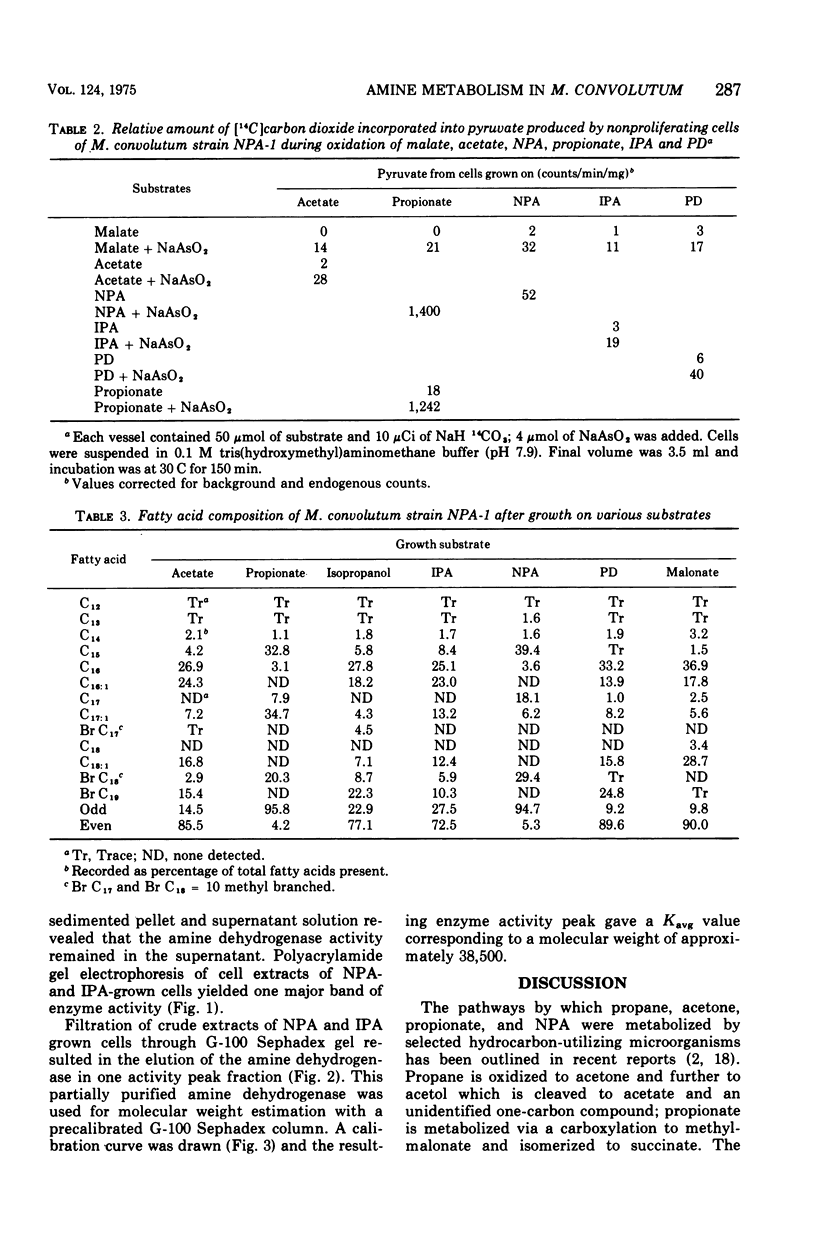
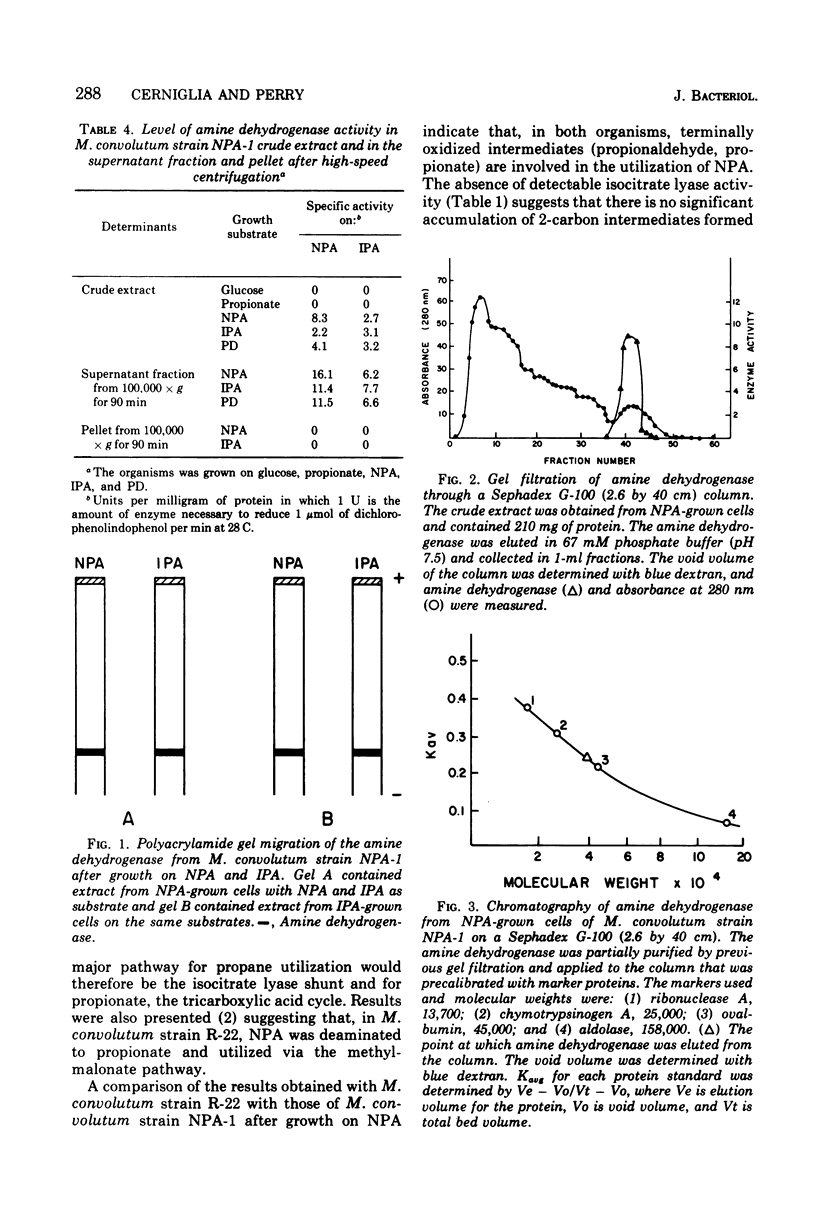
Mycobacterium convolutum strain NPA-1 can utilize n-propylamine (NPA), isopropylamine (IPA), and 1,3-propane diamine (PD) as sole source of carbon, nitrogen, and energy. Enzyme assays, fatty acid profiles, and 14CO2 incorporation experiments indicate that NPA is deaminated to propionate and further metabolized via the methylmalonyl succinate pathway, and IPA and PD were metabolized (after deamination) through a C2 + C1 cleavage. An inducible amine dehydrogenase was present in cell extracts after growth on the three amines. Polyacrylamide gel electrophoresis of cell extracts from NPA- and IPA-grown cells yielded one major band of amine dehydrogenase activity. When extracts of NPA-grown cells were assayed with NPA, IPA, or PD as substrate, the relative position of the major band on gel electrophoresis was equivalent. Similar results were obtained with extracts prepared from IPA-grown cells. Sephadex G-100 chromatography also indicated one major peak of activity. This suggests that one enzyme of broad specificity is involved in deamination of IPA, NPA, and PD. IPA-grown cells utilized NPA readily, whereas NPA-grown cells could not utilize IPA without lag. Since amine dehydrogenase activity was present in extracts of cells after growth on either substrate, this lag was probably due to the inability to transport IPA without an induction period. The molecular weight of the amine dehydrogenase was approximately 38,500 as determined by gel filtration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blevins W. T., Perry J. J. Metabolism of Propane, n-Propylamine, and Propionate by Hydrocarbon-Utilizing Bacteria. J Bacteriol. 1972 Oct;112(1):513–518. doi: 10.1128/jb.112.1.513-518.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton C. A., Crabbe M. J., Large P. J. Microbial oxidation of amines. Partial purification of a trimethylamine mono-oxygenase from Pseudomonas aminovorans and its role in growth on trimethylamine. Biochem J. 1974 May;140(2):253–263. doi: 10.1042/bj1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL K. K. Quantitative estimation of peak areas in gas-liquid chromatography. Nature. 1961 Jul 22;191:377–378. doi: 10.1038/191377a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Microbial oxidation of amines. Spectral and kinetic properties of the primary amine dehydrogenase of Pseudomonas AM1. Biochem J. 1971 Aug;123(5):757–771. doi: 10.1042/bj1230757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- King D. H., Perry J. J. Characterization of branched and unsaturated fatty acids in Mycobacterium vaccae strain JOB5. Can J Microbiol. 1975 Apr;21(4):510–512. doi: 10.1139/m75-072. [DOI] [PubMed] [Google Scholar]
- King D. H., Perry J. J. The origin of fatty acids in the hydrocarbon-utilizing microorganism Mycobacterium vaccae. Can J Microbiol. 1975 Jan;21(1):85–89. doi: 10.1139/m75-012. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Wagner C. Oxidation of C-1 compounds by Pseudomonas sp. MS. Biochem J. 1970 Feb;116(3):357–365. doi: 10.1042/bj1160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perry J. J., Scheld H. W. Oxidation of hydrocarbons by microorganisms isolated from soil. Can J Microbiol. 1968 Apr;14(4):403–407. doi: 10.1139/m68-064. [DOI] [PubMed] [Google Scholar]
- Smith J., Kornberg H. L. The utilization of propionate by Micrococcus denitrificans. J Gen Microbiol. 1967 May;47(2):175–180. doi: 10.1099/00221287-47-2-175. [DOI] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Divergent metabolic pathways for propane and propionate utilization by a soil isolate. J Bacteriol. 1969 Jul;99(1):216–221. doi: 10.1128/jb.99.1.216-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Effect of substrate on the lipids of the hydrocarbon-utilizing Mycobacterium vaccae. Can J Microbiol. 1971 Apr;17(4):445–449. doi: 10.1139/m71-075. [DOI] [PubMed] [Google Scholar]


