Abstract
Background
Within the developing country setting data are few that characterise the disease burden due to RSV and which clearly define the age group to target vaccine intervention.
Methods
Children numbering 635, recruited 2002-03, were intensively monitored until each experienced three RSV epidemics. RSV was diagnosed by use of immunofluorescence on nasal washings collected on occurence of acute respiratory infection. Incidence estimates were adjusted for seasonality in RSV exposure.
Results
From 1187 child years of observation (cyo) a total of 409 RSV episodes were identifed; 365 primary and 82 repeat. Adjusted incidence estimates (per 1000cyo) of lower respiratory tract infection (LRTI), severe LRTI and hospital admission were 90, 43, and 10, respectively, and corresponding estimates in infants were 104, 66 and 13. The proportion of all-cause LRTI, severe-LRTI and hospitalizations in the cohort due to RSV was 13%, 19% and 5%, respectively. 55-65% of RSV LRTI and severe-LRTI occured in children over 6 months old. The risk of RSV disease following primary symptomatic infection remained significant beyond the first year of life and a quarter of all re-infections were associated with LRTI.
Conclusions
RSV accounts for a substantial proportion of the total respiratory disease in this rural population: we estimate 85,000 infant cases of severe LRTI annually in Kenya. The majority of this morbidity occurs in late infancy and early childhood; ages at which the risk of disease following infection remains significant. Disease from re-infection is common. Our results inform the debate on vaccine target age group and effectiveness.
Keywords: respiratory syncytial virus, incidence, burden of disease, vaccination strategy, Kenya
Introduction
Respiratory syncytial virus (RSV) is recognised to be the major viral cause of severe lower respiratory tract infection in infants and young children worldwide. A vaccine by which to lessen this disease burden is evidently needed, but none has gone beyond early stage trials[1, 2]. The presumed vaccine target age group is early infancy which presents a difficulty in achieving adequate immunogenicity without reactogenicity[3]. Further uncertainty over vacine effectiveness arises from the host's inability to prevent reinfection[4], perhaps related to antigenic diversity[5]. The complex interaction between infection and immunity calls for care to be given in vaccination strategy design[6]. In the developing world RSV is a potential candidate for future vaccine intevention. However, there is a marked paucity of data from this setting by which to characterise the disease burden[7-11] and inform on vaccination strategy, in particular, the key age groups for intervention[11, 12]. Detailed epidemiological studies, in particular in the resource poor setting, to accurately define incidence and improve understanding of the relationship between natural immunity to disease and the host age, history of past exposure and maternal antibodies, could significantly contribute to vaccine design and strategy for optimal effectiveness.
Addresing these issues we established a birth cohort in 2002 within a rural Kenyan population to undergo intensive monitoring for RSV infection and disease. A report of findings over the first year of life of 338 children recruited into the first phase of the study has been published[13]. Here we present results from observations of acute respiratory infection in the full birth cohort of 635 children each followed over three RSV epidemics, during the period 2002 to 2005. The results pose fundamental questions on future vaccine use.
Methods
The study was undertaken within a rural district of coastal Kenya which experiences a tropical climate with seasonal rains (roughly March to July and October-December). The population of predominantly subsistence farmers has a growth rate of 3.1% per annum, with 18% under the age of 5 years[14]. Malaria is endemic though highly seasonal[15]. The community is served by a district hospital (KDH) based in the town of Kilifi (2006 population 41,000). The Collaborative Research Programme between the Kenya Medical Research Institute and the Wellcome Trust, which provides the infrastructure for this study, runs the hospital pediatric wards and operates a Demographic Surveillance System (DSS) within an area of ∼900 km2 immediately surrounding KDH[16]. Infant and child under 5 mortality (/1000 per year) in the DSS are estimated at 50 and 74, respectively (Kilifi DSS, unpublished data). Ethical clearance for this study was obtained from the Kenyan National Ethics Review Board and from Coventry Research Ethics Committee, UK. The survey methods have been reported earlier[13], and are presented here in detail where relevent to the analysis.
Study participants were recruited at KDH in the maternity ward and at the maternal child health clinic. Children were eligible if their home was located in the DSS and within easy access to the hospital. Written informed consent was obtained for participation. Recruitment took place over two calendar years in two phases, each spanning approximately half a year and separated by 6 months. The birth cohort was monitored over four calendar years until each phase of recruits had experienced three RSV epidemics. Surveillance was through active household visits by field workers timetabled each week during RSV epidemics and otherwise monthly, and also through presentation (either passively or by referral at a home visit) to the research out-patient clinic at KDH, or for admission to KDH. Children at home with symptoms or signs predominantly reflecting lower respiratory tract involvement.were referred to the clinic. Mothers were encouraged to bring their children to the research clinic if they identified any symptoms of respiratory infection. A blood slide for malaria diagnosis was taken for children with an axillary temperature ≥ 37.5°C or with a history of fever over the previous week, and for all hospital admissions.
Nasal washings[17] were collected at home or at clinic visits if over the preceding week the child had a history of, or was observed to have, a minimum of (i) difficulty in breathing, or (ii) a runny nose and/or nasal congestion, or (iii) an acute cough. Within KDH pediatric ward a nasal specimen (nasal washing or nasal pharyngeal aspirate) was collected if the child presented with a diagnosis of LRTI or bronchiolitis, or severe or very severe pneumonia, or was hypoxic (pO2<90%) as determined by Oximeter (Nelcor®). Samples were examined for RSV antigen by use of direct immunofluorescence test (DFA, Chemicon). Details of collection, cold chain to the laboratory and processing are previously reported.[13] The severity of respiratory disease was ascribed following a clinical review uniform to the research clinic and the pediatric ward. LRTI was assigned to children with acute cough or difficulty in breathing in association with one or more of the following (i) raised respiratory rate for age, (ii) intercostal indrawing, or (iii) inability to feed, reduced conscious level, or hypoxia (O2 saturation <90% by Oximetry), the latter group only if confirmed by the clinician's own diagnosis of LRTI or bronchiolitis. Severe LRTI was assigned to a child with criterion (ii) or (iii), or both, of the above. A hospital admission was assigned only if linked to a KDH in-patient record. For non-admitted children malaria was ascribed using the criteria defined by Mwangi et al[15], and in hospital was assigned to those with a discharge diagnosis including malaria.
Data analysis was performed using Stata (v8.2, STATACorp, Texas). Observation time for each child included all days from date of recruitment until the last visit of the study or until otherwise lost to follow-up (see Table 1), excepting days absent from the District. A discrete RSV episode was defined as a visit resulting in a positive RSV specimen occurring no less than 14 days after a previous RSV episode. An episode of RSV was assigned the maximum severity of LRTI over seven days beginning the day of nasal sampling. A concurrent diagnosis of malaria and RSV was exclusively assigned to RSV, whereas concurrent malaria and non-RSV LRTI was ascribed to malaria. Incidence of infection defined per 1000 child years of observation (cyo) was estimated by Poisson regression, details of which are provided in supplementary material (Annex 1). Observation time was divided into RSV epidemic and non-epidemic periods. Incidence estimates are presented in crude form (ie cases / cyo * 1000) and adjusted to take into acount bias in the ratio of observation time within and between epidemics arising from the discontinuous cohort recruitment and the censoring at the end of the study. Seasonality in total LRTI is markedly less than RSV and is consequently ignored. Proportions were compared using Fisher's Exact test (2 tailed), with binomial exact confidence intervals, and assessment of trend in proportions across ordered groups made using an extension to the Wilcoxon rank-sum test (‘nptrend’ STATA command).
Table 1. Recruitment, surveillance and loss to follow up characteristics for a birth cohort in Kilifi District, Kenya.
| Phase 1 | Phase 2 | Total | |
|---|---|---|---|
| (a) Recruitment | |||
| Number | 338 | 297 | 635 |
| Dates recruited | Jan 31st 2002 to May 30th 2002 |
Dec 3rd 2002 to Jul 3rd 2003 |
|
| Dates born | Jan 21st 2002 to May 5th 2002 |
Dec 2nd 2002 to July 13th 2003 |
|
| Recruited from | |||
| Maternity ward | 249 | 286 | 535 |
| MCHCa (days) | 89 (9) | 11 (8) | 100 (9) |
| Birth weight <2.5kg, % (n)b | 13.9 (317) | 16.7 (246) | 2.96 (563) |
| Male sex (%) | 175 (51.8) | 146 (49.2) | 321 (50.6) |
| (b ) Surveillance | |||
| Follow up time, cyoc | 687.0 | 500.0 | 1187.3 |
| Visits (/cyo) | 19605 (28.5) | 14288 (28.6) | 33893 (28.5) |
| Active | 15574 (22.7) | 10741 (21.5) | 26315 (22.2) |
| Passive | 3910 (5.7) | 3445 (6.9) | 7355 (6.2) |
| Hospital | 121 (0.2) | 102 (0.2) | 223 (0.2) |
| Nasal washing indicated (/cyo) | 4451 (6.5) | 4265 (8.5) | 8716 (7.3) |
| Nasal washing taken (/cyo) | 4364 (6.4) | 4128 (8.3) | 8492 (7.2) |
| (c) Loss to follow-up | |||
| Reason exit (% of total) | |||
| Study end | 248 (73) | 188 (63) | 436 (69) |
| Refused | 20 (6) | 39 (13) | 59 (9) |
| Died | 8 (2) | 8 (3) | 16 (3) |
| Movedd | 43 (13) | 31 (10) | 74 (12) |
| Requestede | 19 (6) | 31 (10) | 50 (8) |
| Median age at exit, m (IQR) | 28 (25-29) | 23 (19-25) | 25 (21-28) |
| Drop out rate , /100cyo | 13.1 | 21.8 | 16.8 |
Maternal and child health clinic (Median age of infant in days at recruitment)
Birth weight available for 563 of 635 children
Child years of observation
Child moved permanently from locality
Asked to leave study because of access difficulties for field teams or inability of the family to keep appointments
Results
Recruitment, surveillance and loss to follow-up characteristics
A total of 635 children (51% male) were enrolled into the study at or near birth from KDH in two phases; January to May 2002, and December 2002 to July 2003 (Table 1a). 535 (84%) of the children were recruited in the maternity ward of KDH. 47% of the cohort resided within Kilifi Township. Follow up over three epidemics for each phase realised a total of 1187 cyo (Fig.1 panels a-b). The mean age of a child at exit from the study was 22 months (median 25 months, Table 1b). Of the recruits 199 (31%) exited prior to the end of the study (a rate of 17% of the cohort per annum). In 16 (8%) of these the cause was death of the child; eleven occurring in the first year of life (562 cyo), ie 20/1000 infant mortality.
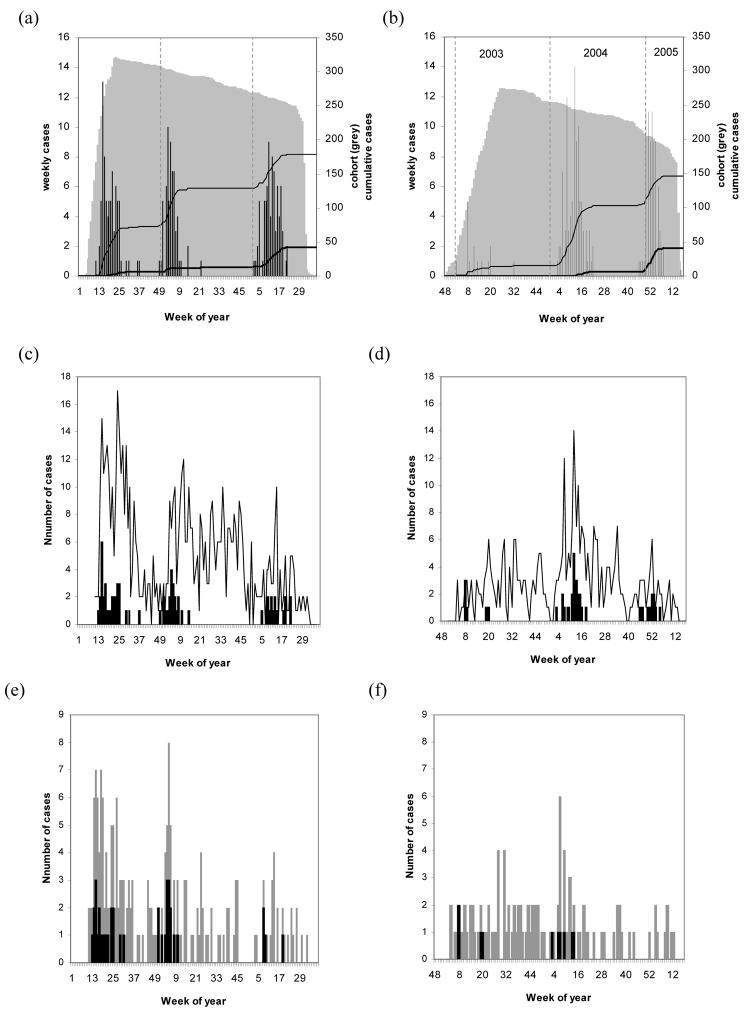
Figure 1.
Infection and disease within a birth cohort from Kilifi District, Kenya. Panels on the left show data for cohort children recruited in the first phase (Jan-May 2002), and on the right second phase recruits (Dec 2002 – July 2003). Panels a-b show, for each week and year of surveillance, numbers of children within the cohort (grey area), numbers of RSV cases (histogram), and cumulative number of primary (thin line) and repeat cases (thick line). Similarly, panels c-d show cases of LRTI (markers) and RSV-LRTI (bars), and panels e-f cases of severe-LRTI (light bars) and RSV severe-LRTI (dark bars). Limits for each RSV epidemic are as follows: epidemic 1 from weeks (inclusive) 11-26 year 2002, epidemic 2 weeks 49-52 2002 and weeks 1-15 2003, epidemic 3 weeks 2-22 2004, epidemic 4 weeks 46-52 2004 and weeks 1-7 2005.
The intensity of surveillance was 29 visits /cyo (1 per 13 days), with an average interval between visits of 9 days within RSV epidemic periods and 18 days between. On average, for each child there were 22 home visits and 6 clinic visits, per year (these rates were highly homogeneous). The rate of clinic presentations within and between epidemics did not change (RR 1.02, 95%CI 0.98-1.07, P=0.324). There were 8716 visits fulfilling the criteria for a nasal specimen, of which 8492 (97%) were collected ( 7 per cyo). Blood slides were indicated in 5113 visits, of which 570 (11.1%) were parasite positive, and 490 (9.6%) had an accompanying diagnosis of malaria, representing 1.4% of all visits. Laboratory IFAT results were obtained for 8471 of the nasal samples, yielding 409 (4.8%) RSV antigen positive episodes, comprising 130 from active visits, 268 from clinic visits and 11 from admissions to KDH. A diagnosis of malaria was made in 86/1008 (8.5%) RSV negative LRTI diagnoses, with 12 severe LRTI and 10 hospitalised.
RSV infections in the birth cohort
Of the 409 separate RSV episodes, 326 were the first observed in a particular child and hereafter denoted as primary cases, and 83 were repeat infections. There were four distinct RSV epidemics (Fig.1 panels a-b) occurring with approximate annual periodicity (intervals between the epidemics were 9, 14 and 10 months) with the majority of cases in the first quarter of the year, and not associated with any meteorological measures. Over the 4 calendar years of observation (2002-2005), there were 70 out of 208 weeks (or 0.34 of the year on average) defined as epidemic (see legend to Fig.1). The proportion of the cohort observation period that was within defined epidemic periods was 0.44 indicating an over-sampling from within epidemics.
Crude incidences (/1000 cyo) for total, primary and re-infections are 345 (cyo 1187), 394 (827), and 230 (361), respectively. Corresponding adjusted incidence estimates (ie weighted for epidemic over-sampling) are 261 (95%CI 236-287), 298 (264-337) and 169 (134-214). There was no statistically significant difference in the incidence of RSV infections by age group (infants 0-11m versus children 12-30m) or by sex. The incidence of re-infections was approximately half that for primary infections, regardless of age.
RSV associated disease in the cohort
Out of the 409 RSV episodes, 275 (67%) appeared confined to the upper respiratory tract and 134 (33%) involved the lower respiratory tract, of which 66 (49%) were assigned severe. Of all RSV cases, 11 (3%), all severe LRTI, were admitted to KDH none of whom had a co-bacterial infection. No RSV infection coincided with the death of the child. A concurrent malaria diagnosis was made in 6 (1.5%) of 409 RSV infections, of which two had LRTI, one of which was severe and none were hospitalised.
The risks of LRTI, severe LRTI and hospital admission following primary RSV infection were 35%, 18% and 3%, respectively, and correspondingly for repeat RSV infection were 24%, 8% and 2%. Stratifying by age group (Table 2), following primary infection the peak disease risk tends to be in children under 6 months of age, followed by a trend for decline with increasing age. For LRTI this trend is gradual with the risk never declining to less than 20% (ie around 40% of the peak risk) even in children aged 24 months or over. The risk of severe LRTI following RSV infection appears to decline only from age 9-11m and most significantly in the age group 18-30m. In relation to repeat infection, the data indicate a substantial risk of LRTI, particularly in children ≥18m, (25.4%, 95%CI 15.3-37.9), and of severe LRTI (9.5%, 95%CI 3.6-19.6), although numbers are too few in the first 18months to identify any trend with age. Two children aged 18-30m were, however, admitted to hospital.
Table 2. Risk of RSV associated LRTI, severe LRTI and hospitalisation, by age group and infection history, in a birth cohort from Kilifi District, Kenya.
| LRTI | Severe-LRTI | Admitted | ||||||
|---|---|---|---|---|---|---|---|---|
|
Age group (months) |
Infections | Number | Risk % | Number | Risk % | Number | Risk % | cyo |
| Primary infection | ||||||||
| 0-2 | 68 | 34 | 50.0 | 24 | 35.3 | 2 | 2.9 | 128 |
| 3-5 | 16 | 9 | 56.3 | 6 | 37.5 | 2 | 12.5 | 121 |
| 6-8 | 19 | 7 | 36.8 | 6 | 31.6 | 1 | 5.3 | 124 |
| 9-11 | 77 | 22 | 28.6 | 13 | 16.9 | 1 | 1.3 | 109 |
| 12-17 | 53 | 21 | 39.6 | 7 | 13.2 | 3 | 5.7 | 158 |
| 18-23 | 60 | 14 | 23.3 | 2 | 3.3 | 0 | 0.0 | 133 |
| 24+ | 33 | 7 | 21.2 | 1 | 3.0 | 0 | 0.0 | 52 |
| total | 326 | 114 | 35.0 | 59 | 18.1 | 9 | 2.8 | 827 |
| Repeat infection | ||||||||
| 0-5 | 6 | 4 | 66.7 | 1 | 16.7 | 0 | 0.0 | 25 |
| 6-11 | 9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 54 |
| 12-17 | 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 98 |
| 18-23 | 39 | 7 | 17.9 | 2 | 5.1 | 1 | 2.6 | 110 |
| 24-30 | 24 | 9 | 37.5 | 4 | 16.7 | 1 | 4.2 | 74 |
| total | 83 | 20 | 24.1 | 7 | 8.4 | 2 | 2.4 | 361 |
Notes.
Cases of severe LRTI are nested within LRTI cases
Fisher's Exact test by age for primary infection : LRTI P=0.002, severe-LRTI P<0.001, admissions P=0.063
Trend with age statistic (z) for primary infection: LRTI −3.68, P<0.001; severe-LRTI −5.72, P<0.001
Fisher's Exact test by age for repeat infection: LRTI P=0.01; severe-LRTI P= 0.410, admissions P=1.00
Trend with age statistic (z) for repeat infection: LRTI −0.3, P<0.761
Fisher's Exact test by infection history (Ha: primary > reinfection; 1 tail): LRTI P=0.038; severe-LRTI P=0.020; admissions P=0.608
Adjusted incidence estimates, by age group, for RSV associated LRTI, severe LRTI and hospitalisation are presented in Table 3. For all disease categories, peak incidence occurred in infants under 6 months of age, and is significantly lower in the older age group (6-30m) for LRTI (IRR 0.494 P=0.002, 95%CI 0.318-0.768) and severe-LRTI (IRR 0.347 P<0.001, 95%CI 0.198-0.610). For infants (0-11m) adjusted incidence (95%CI) of LRTI, severe LRTI and admission is 104 (79-137), 66 (47-91), and 13 (5-34) respectively. Corresponding estimates for children aged 12-30m are 77 (39-151), 22 (9-56), and 7 (1-61) respectively. There was no relationhip between incidence and sex of the child or low birth weight (<2.5kg).
Table 3. Adjusted RSV incidence (/1000 cyo) by age group and severity of associated disease, in a birth cohort from Kilifi District , Kenya.
| LRTI | Severe-LRTI | Admitted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age class (months) |
cyoa | Proportion epidemicb |
Incidence | 95%CLc | Incidence | 95%CL | Incidence | 95%CL | |||
| 0-5 | 274 | 0.40 | 147 | 102 | 212 | 86 | 55 | 132 | 20 | 6 | 64 |
| 6-11 | 288 | 0.51 | 63 | 36 | 111 | 47 | 23 | 93 | 6 | 1 | 45 |
| 12-17 | 256 | 0.31 | 88 | 50 | 156 | 29 | 12 | 70 | 13 | 2 | 64 |
| 18-23 | 244 | 0.49 | 59 | 33 | 104 | 11 | 4 | 33 | 3 | 0 | 27 |
| 24-30 | 126 | 0.48 | 88 | 47 | 165 | 28 | 10 | 80 | 6 | 1 | 23 |
| All age | 1187 | 0.44 | 90 | 74 | 108 | 43 | 32 | 56 | 10 | 5 | 21 |
child years of observation
proportion of observation time during RSV epidemics
Confidence Limits.
RSV disease as a proportion of all-cause LRTI and hospital admissions
The temporal occurrence of RSV associated LRTI and severe LRTI relative to all-cause LRTI and severe LRTI is recorded in Fig.1 (panels c-d and e-f, respectively). RSV was identified in 13% of LRTI, 19% of severe-LRTI and 5% of admissions. Stratified by age (Table 4) the data show a higher risk of RSV in cases of LRTI, severe LRTI and hospitalisations in infants compared with older children, which is statistically significant only for LRTI.
Table 4. Proportion of LRTI, severe-LRTI and hospital admissions associated with RSV in a birth cohort from Kilifi District, Kenya.
| LRTI | severe-LRTI | Admissions | ||||
|---|---|---|---|---|---|---|
| age class | RSV/ LRTI | % (95%CI) |
RSV / s-LRTI | % (95%CI) |
RSV/admit | % (95%CI) |
| < 1 year | 76 / 489 | 15.5 (12.4-19.0) |
50 / 239 | 20.9 (15.9-26.6) |
6 / 105 | 5.7 (2.1-12.0) |
| ≥ 1 year | 58 / 567 | 10.2 (7.9-13.0) |
16 / 114 | 14.0 (8.2-21.8) |
5 / 118 | 4.2 (1.4-9.6) |
| total | 134 / 1056 | 12.7 (10.7-14.8) |
66 / 353 | 18.7 (14.8- 23.2) |
11 / 223 | 4.9 (2.5-8.7) |
Notes. Fisher's Exact test by age: LRTI P=0.012, severe-LRTI P<0.145, admissions P=0.759.
Crude incidences can be estimated for infants, young children and total, given respective denominators of 562, 626, 1187 cyo.
The characteristics of RSV repeat infections
The distribution of total number of clinical RSV infections per individual was 254 with one, 64 with two, 6 with three, and 1 each with four and five. Of the 72 children with more than one RSV episode, 19 (26%) had a repeat infection that involved the lower respiratory tract and 6 (8%) had severe LRTI. In 10 (14%) a repeat infection was more severe than the primary episode. Of the 83 repeat infections 19 (23%) occurred within the same epidemic with median interval between re-infections of 24 days (range 18-66 days). There were five children re-infected within the first 6 months of life (one twice). Of these six repeat infections four resulted in LRTI (one severe), five occured within a single epidemic with an interval between episodes of 19-63 days, and one occurred in the succeeding epidemic (gap 113 days). Of the eight children with 3 or more RSV infections four were admitted at least once, however none had an RSV-associated hospital admission, and none had in-patient reports that would indicate an immunocompromised condition.
Discussion
A birth cohort of 635 children from a rural Kenyan location was intensively monitored for a median of 25 months spanning four calendar years to identify RSV infections and associated disease outcomes. The estimated incidence (per 1000 cyo) of symptomatic RSV episodes was 261, and of RSV associated LRTI, severe LRTI and hospitalisation at KDH was 90, 43 and 10, respectively. These rates represent around 13% of all LRTI, 19% of all severe LRTI and 5% of all hospitalisations experienced by the cohort. Infants had the higher rates of RSV associated disease, with estimates of 104, 66 and 13 for LRTI, severe LRTI and admission, respectively, representing in excess of 16% of total LRTI, 21% of severe LRTI and 6% of admissions in this age group. If we extrapolate this disease burden to the total annual birth cohort in Kenya of 1.3m[14], then RSV is expected to result annually in around 135,000 cases of LRTI, 85,000 cases of severe LRTI and over 17,000 hospitalisations of infants.
The data provided by this study are unusual; a literature review reveals few community-based studies of RSV incidence in resource poor settings[8, 9, 12], with estimates of RSV-LRTI incidence in infants ranging widely from around 40 to over 200 cases per 1000cyo, and only two studies with estimates for severe LRTI, each of around 15 cases/1000cyo[8, 9]. Our study, which involved highly intensive surveillance, provides community incidences at the higher end of or, in the case of severe LRTI, well above those reported elsewhere. Furthermore, we can assume that these are under-estimates, largely because case surveillance would have greater sensitivity using RT-PCR methods and serology.[4, 13, 18, 19] Not surprisingly, insensitivity declines with increasing case severity and our estimates of RSV–associated hospital admission are inline with those from the US[4, 20] and from other developing countries.[12]
In our study the occurrence of RSV associated severe LRTI was four to five times higher than the rate of RSV hospitalisation. WHO guidelines recommend all such cases for admission[21]. A proportion (26%) of the severe cases who did not end up in the ward had been referred from the clinic but an admission could not be verified. The remaining three quarters were not deemed admissible by experienced Clinical Officers. The situation was the same for all-cause severe LRTI. The study may have influenced the decision to refer, since the patients had relatively good hospital access and were able to return free of charge if the child's condition deteriorated. Nevertheless, only half the admissions to KDH were via the research clinic. It is clear from these data, and from the US[22], that studies based on passive hospital surveillance overlook a significant community burden of severe RSV disease.
Although the estimated incidence of RSV LRTI, severe LRTI and hospitalisation was highest in the under 6 month age group, significant disease incidence was observed in older groups continuing into the third year of life. If we assume, relative to children aged <6m, roughly four times the number of children 6-30 months of age, and an incidence that is one third to one half (Table 3), then around 55-65% of significant disease due to RSV in the wider community occurs in ages outside that typically designated as the vaccine target. A delay in vaccination to an age where the child is more immunologically mature and when interference from maternal antibody is less likely, may have potential to prevent a majority of the community burden of RSV.
While the highest risk of lower respiratory tract disease following primary symptomatic RSV was seen in children <6m of age, a substantial risk remained up to 18m of age. Even if scaled for under-estimation of the true denominator of infection these risks would remain substantial. We identify a significant risk of disease following re-infection in children in their second and third years of life, which suggests that potential benefits of delay in first exposure by use of maternal immunization [23] may be less than hoped for, and that severe disease from re-infection should not be ignored when considering the merits of RSV vaccination. Furthermore, our data suggest that immunity to symptomatic re-infection (frequently involving the lower respiratory tract) is short lived in a substantial minority of children, including those infected under 6m. It follows that a candidate vaccine which provides no better protection than natural infection, delivered in the first 2 months of life, might not prevent considerable early re-infection with associated high risk of disease
In summary, an intensively monitored birth cohort of over 600 children within a rural developing country location has provided evidence of a substantial burden of RSV severe disease within the wider community that would not be identified through hospital based surveillance. The majority of this disease occurs in older infants and young children. We report a significant risk of disease following primary infection beyond early infancy and upon re-infection, and rapid loss of immunity to symptomatic re-infection. These findings have a bearing on the potential effectiveness of RSV vaccines. Consideration is required of the merits of broadening the target age range for a live attenuated vaccine in preventing a large community burden of severe RSV.
Acknowledgements
We are indebted to the enrolled children and their caregivers, all staff of the RSV team (field, ward and laboratory), the research out-patients clinic, pediatrics wards, and the DSS team. The study is published with permission of the Director of KEMRI. Financial support was provided by the Wellcome Trust (061584 and 076278). The funding agency had no role in study design, data collection or preparation of this manuscript. The authors declare there to be no conflict of interest in relation to the publication of this work.
Annexe 1 – details of statsitical analysis of incidence
Using Stata (v8.2, STATACorp, Texas) to analyse the data, follow up time was organised into RSV epidemic (at-risk) and non-epidemic periods and into periods within monthly age catgories, using the survival time procedures ‘stset’ and ‘stsplit’. An epidemic was defined as a period of weeks within which at least 3 cases were found in any contiguous 3 week period and delimited by weeks with at least one case. Incidence of infection defined per 1000 child years of observation (cyo) was estimated using Stata survey ‘svy’ Poisson regression procedures for reasons given below. Bias in incidence estimates is likely due the restricted months of recruitment of children in 2002 and 2003 (Table 1) and the periodic risk of RSV. The result is an uneven distribution of observation time for any particular age group within and between RSV epidemics. For some age groups this will result in over observation within epidemic periods, and for others under observation. In addition, even across all ages incidence will be biased, in this case over observation within RSV epidemics, because right censoring at the end of the study ocurred directly after the end of the final (third) epidemic of each phase of the cohort (thus reducuing follow up time in the non-epidemic season). To compute unbiased incidence estimates it is necessary that the fraction of observation time within RSV epidemic periods should conform to that expected from the proportion of each year that was intra-epidemic. We account for sampling bias in RSV epidemic/non-epidemic surveillance by applying probability weights (pweights in Stata), Wki, for each observation in time-period k (k=n,e where n=non-epidemic, e= epidemic), and in specified age-class, i (i=1,m), defined as
which is the ratio of the expected total days at risk for age-class i in time-period k to observed total days at risk in age-class i, time-period k. Here Pk is the average proportion of a year which is epidemic (k=e) or non-epidemic (k=n) and Yki is the number of person days at risk for age-class i in time-period k. For simplicity we ignore the variation between years in the ratio of epidemic/non-epidemic periods, assuming an average over all years of obersvation. Weighted Poisson regression was carried out using STATA survey ‘svy’ procedures setting the child as the primary sampling unit, accounting for clustering of infection within the individual and facilitating tests of signifcance of inclusion of explanatory variables (age, sex, and birth weight), with incidence rate ratios(IRR) and 95% confidence limits (CL) presented where appropriate.
References
- 1.Polack FP, Karron RA. The future of respiratory syncytial virus vaccine development. Pediatr Infect Dis J. 2004;23:S65–73. doi: 10.1097/01.inf.0000108194.71892.95. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh MP, Weisman LE. Prevention and treatment of respiratory syncytial virus infection in infants: an update. Expert Rev Vaccines. 2006;5:261–8. doi: 10.1586/14760584.5.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 4.Glezen W, Taber L, Frank A, Kasel J. Risk of primary infection and reinfection with respiratory syncytial virus. American Journal of Diseases of Children. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 5.Melero JA, Garcia-Barreno B, Martinez I, Pringle CR, Cane PA. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol. 1997;78:2411–8. doi: 10.1099/0022-1317-78-10-2411. [DOI] [PubMed] [Google Scholar]
- 6.Gomes MG, White LJ, Medley GF. Infection, reinfection, and vaccination under suboptimal immune protection: epidemiological perspectives. J Theor Biol. 2004;228:539–49. doi: 10.1016/j.jtbi.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Selwyn B, Coordinated Data Group of BOSTID Researchers The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Reviews of Infectious Diseases. 1990;12(supplement 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 8.Broor S, Parveen S, Bharaj P, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ. 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- 10.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 11.Weber M, Mulholland E, Greenwood B. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine and International Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Nokes DJ. Respiratory syncytial virus disease burden in the developing world. In: Cane PA, editor. Respiratory Syncytial Virus. Vol. 14. Elsevier; 2007. pp. 183–230. (Perspectives in Medical Virology). [Google Scholar]
- 13.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory Syncytial Virus Epidemiology in a Birth Cohort from Kilifi District, Kenya: Infection during the First Year of Life. J Infect Dis. 2004;190:1828–32. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Finance and Planning . Analytical Report on Population Projections. VII. Nairobi: Central Bureau of Statistics, Government of Kenya; 2002. Central Bureau of Statistics. [Google Scholar]
- 15.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowgill KD, Ndiritu M, Nyiro J, et al. Effectiveness of Haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–8. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall C, Douglas RJ. Clinically useful method for the isolation of respiratory syncytial virus. Journal of Infectious Diseases. 1975;131:1–5. doi: 10.1093/infdis/131.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Henderson F, Collier A, Clyde WJ, Denny F. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. New England Journal of Medicine. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 19.Freymuth F, Vabret A, Galateau-Salle F, et al. Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin Diagn Virol. 1997;8:31–40. doi: 10.1016/s0928-0197(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 20.Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven; 2001. pp. 1443–85. [Google Scholar]
- 21.WHO . Pocket book for hospital care of children:Guidelines for the management of common illness with limited resources. 2005. [Google Scholar]
- 22.Fisher RG, Gruber WC, Edwards KM, et al. Twenty years of outpatient respiratory syncytial virus infection: a framework for vaccine efficacy trials. Pediatrics. 1997;99:E7. doi: 10.1542/peds.99.2.e7. [DOI] [PubMed] [Google Scholar]
- 23.Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine. 1998;16:1456–63. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]