Abstract
Background:
Intestinal helminth infections may protect against allergen skin test reactivity in endemic areas and it has been suggested that IL-10 may mediate this effect. We investigated if IL-10 and populations of IL-10+ T cells may modulate atopy in children living in an endemic area for intestinal helminth parasites.
Methods:
Ecuadorian school children from rural communities were assessed for allergen skin test reactivity to Periplaneta americana and Dermatophagoides pteronyssinus. Skin test positive (n=39) and skin test negative (n=41) children were bled and peripheral blood leukocytes were cultured in the presence of Ascaris lumbricoides antigen to measure IL-10 protein and the frequencies of T cell populations expressing intracellular IL-10. We investigated also if these immunological factors affected the association between specific IgE and skin test reactivity to aeroallergens.
Results:
There was no evidence of associations between levels of A. lumbricoides-induced IL-10 protein or IL-10+ T cells and allergen skin test reactivity. The association between allergen-specific IgE and skin test reactivity was not affected by levels of IL-10 protein or the frequencies of IL-10+ T cells.
Conclusions:
Our data do not support a role for IL-10 in modulating atopy in children living in an intestinal helminth-endemic area of the tropics.
Keywords: atopy, IgE, allergen skin test reactivity, Ascaris lumbricoides, intestinal helminths, IL-10, regulatory T cells
Introduction
Atopy can be defined as the presence of either a positive allergen skin test or the presence of allergen-specific IgE in serum. Positive allergen skin tests and the presence of allergen-specific IgE are strongly associated in studies conducted in industrialized countries1,2 but are weak or non-existent in tropical rural regions of Africa3,4 and Latin America,5 such that elevated levels of allergen-specific IgE are present in a much higher proportion of individuals than have positive skin tests to the same allergen.
The disassociation between the results of allergen skin testing and specific IgE in tropical regions may be caused by regulation of allergic effector responses. Previous studies that have examined the determinants of allergen skin test reactivity have implicated a modulatory role for the cytokine IL-10.6 IL-10 has important anti-allergic effects,7,8 is able to inhibit IgE-induced activation of human mast cells9 and has been associated with reduced risk of allergen skin test reactivity.6,10
Populations living in rural areas of the tropics where the prevalence of allergic disease tends to be low,11,12 have been hypothesized to have a highly regulated immune system capable of controlling allergic inflammatory responses 13,14 better than those living in urban areas or in industrialized countries where the prevalence of allergic disease may be much greater.12,15 Chronic exposures to infectious diseases, particularly helminth parasites, may contribute to more highly developed immune regulation.13,14
Allergic inflammation may be regulated by regulatory T cells among which IL-10+ T cells are considered to be important. 16 In the current study, we wished to test the hypothesis that intestinal helminth-induced IL-10 and IL-10+ regulatory T cells attenuate allergen skin test responses in children living in an area of high intestinal helminth prevalence. Further, we investigated if helminth-induced IL-10 and IL-10+ regulatory T cells affect the association between allergen skin test responses and levels of allergen-specific IgE. To do this, we examined the relationship between in vivo (allergen skin prick testing) and in vitro (allergen-specific IgE in plasma) atopic responses to aeroallergens and the production and expression of IL-10 by leukocytes stimulated with A.lumbricoides antigen in blood from school children living in an endemic area of Ecuador. The study was designed to investigate possible IL-10-associated immunological mechanisms underpinning the inverse association between allergy and intestinal helminth infection and we recruited an atopy-enriched sample from a population where an inverse association between intestinal helminth infections and allergen skin test reactivity has been demonstrated consistently. 11,14
Methods
Study design and population
Children in this cross-sectional were recruited from 5 small rural schools in a sub-tropical area of Pichincha Province in Ecuador. Children were recruited on the basis of either a positive skin prick test to Dermatophagoides pteronyssinus and/or P. americana or negative skin prick tests to six relevant aeroallergens that included D. pteronyssinus and P. americana. Allergen skin testing was repeated twice separated by 1 month and children with inconsistent allergen skin test results between the two time points were excluded. Skin test reactions to D. pteronyssinus and P. americana account for ∼90% of positive skin test reactions in the study area. 11 The study protocol was approved by the ethics committees of the Hospital Pedro Vicente Maldonado, Ecuador, and St George's Hospital, UK.
Blood and stool collection and analysis
Immediately after the second skin test, blood (10 mL) was collected by venipuncture into plastic tubes (BD Vacutainer, Plymouth, UK) containing Sodium heparin as anticoagulant. White cell and differential counts were calculated for each individual using standard methods. Stool samples were collected on the same day as allergen skin testing, and examined for the presence of helminth eggs and larvae using the modified Kato-Katz method and formol-ethyl acetate concentration methods.17 The sensitivity of the Kato-Katz method for the quantification of infection intensities was 70 eggs per gram.
Allergen skin prick testing
Skin prick testing was done to house dust mite extract (Dermatophagoides pteronyssinus; Greer Laboratories, Lenoir, NC), Alternaria tenuis (Greer), American cockroach (Periplenata americana Greer), cat (Greer), grass pollen mix (Greer), tree pollen (Greer), histamine (ALK-Abello, Horsholm, Denmark), and saline (ALK-Abello) controls. Allergens were pricked onto the volar surface of the forearm and reactions recorded after 15 minutes. Reactions were positive if mean diameter was at least 3 mm greater than saline. Skin testing was performed by a single observer (MC).
Measurement of antibodies
Levels of total IgE and specific IgE for D. pteronyssinus, A. lumbricoides, and P. americana were determined using the CAP system (Pharmacia Diagnostics AB, Uppsala, Sweden). Levels of A.lumbricoides-specific IgG and IgG4 were measured as described previously5 and the cut-off for positivity was defined as greater than 3 standard deviations above the mean values of 10 control sera from subjects living in Quito without a previous history of intestinal helminth infection.
Histamine release
Histamine release by heparinised whole blood incubated with Ascaris antigens at a concentration of 0.03 micrograms/mL (A. lumbricoides adult, A. suum L2/L3 and A. suum L3/L4) was measured in 29 individuals (12 allergen skin test negatives and 17 positives) using a commercial assay (histamine enzyme immunoassay kit, Immunotech, Westbrook, ME) following the manufacturer's instructions.
Whole blood culture
Heparinized venous blood was collected and diluted 1:4 in RPMI 1640 medium (BioWhittaker, Walkersville, MD) containing L-glutamine (BioWhittaker), 80 mg/ml gentamicin and 1% HEPES (GIBCO BRL, Gaithersburg, MD). Diluted whole blood (0.5 mL) was cultured alone or in the presence of A.lumbricoides adult worm antigen (prepared as described previously5) at 10 □icrograms/mL, D.pteronyssinus (Greer Laboratories) at a final concentration of 100 AU/mL, P. Americana allergen extract (Greer Laboratories) at a dilution of 1/50, and Staphylococcus enterotoxin B (SEB; Sigma Aldrich, St Louis, MO) at 10 □icrograms/mL. Cultures were incubated for 24 hours in a humidified atmosphere of 5% CO2 at 37°C. Supernatant fluids were collected and stored in liquid nitrogen.
IL-10 analysis in supernatant fluids
Levels of IL-10 were measured in supernatant fluids from whole blood cultures using a commercially available antibody pair (Pharmingen, San Diego, CA) by sandwich ELISA following the manufacturers instructions. The detection limit for the assay was 19.5 pg/mL. Values below this were assigned the value of the detection limit.
Preparation and preservation of cells for flow cytometry
Blood was diluted with an equal volume of supplemented RPMI 1640 (as for whole blood cultures) and was cultured in microplate wells in the absence of antigen, and with A.lumbricoides adult worm antigen, D.pteronyssinus, P.americana, and SEB at the concentrations described above. Cultures were maintained at 37°C in 5% CO2. After 2 hours, Brefeldin A (Sigma-Aldrich) was added to each culture well (final concentration 20 □icrograms/mL) and the cultures were incubated for a further 12 hours. After incubation, stimulated whole blood was resuspended in BD Lysing solution (Pharmingen) for 10 mins. Cells were then washed once in cold PBS, centrifuged at 1200 rpm for 10 mins and fixed by resuspending the cell pellets in 4% paraformaldehyde (Sigma-Aldrich) in PBS for 5 mins. Cells were washed in 1% BSA/PBS, centrifuged and the pellet resuspended in PBS/10%DMSO, and placed in a −20°C freezer overnight and then stored in liquid nitrogen.
Flow cytometry
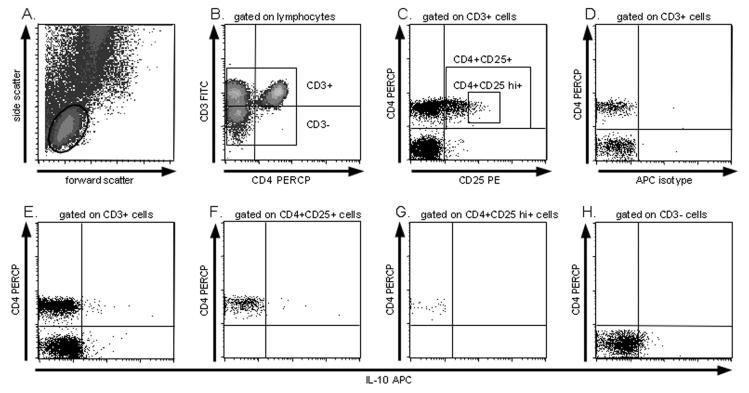
Fixed cells were thawed at room temperature and washed in PBS/0.1% BSA, centrifuged, and the pellet resuspended and permeabilized in a solution of PBS/1%BSA/0.1% saponin (Calbiochem, San Diego, CA) for 1 hour at 4°C. Cells were then stained for 30 min at 4°C with fluorescein isothiocyanate (FITC)–conjugated mouse anti-human CD3 (Immunotech), Peridin Clorophylla Protein-conjugated (PerCP) mouse anti-human CD4 (Pharmingen), R-phycoerythrin (PE)-conjugated mouse anti-human CD25 (Pharmingen) and allophycocyanin (APC)-conjugated rat anti-human IL-10 APC (Pharmingen). FITC-conjugated mouse IgG1 (Pharmingen), PE-conjugated mouse IgG1, PerCP-conjugated mouse IgG1k (Pharmingen) and rat IgG2a APC (Pharmingen) were used as isotype controls. After staining, cells were washed twice in PBS/0.1% saponin and resuspended in PBS. Data were collected on a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using CellQuest software (BD Biosciences). A schematic diagram to illustrating how IL-10+ PBL populations were identified by flow cytometry in a single individual is provided in Figure 1.
Figure 1.
Schematic for IL-10 detection by flow cytometry. A. Lymphocyte identification by forward and side scatter. B. CD3+ and CD3− cells identified by CD3-FITC staining C. CD3+ gated cells are analyzed for CD4 and CD25 positivity, enabling identification of CD4+CD25+ and CD4+CD25hi+ subsets. D. IL-10 positivity was determined by isotype staining. E-H. are plots of IL-10 staining for CD4+ cells (E. upper panels), CD4+CD25+ cells (F.), CD4+CD25hi+ cells (G.), and CD3− cells (H.).
Statistical analysis
Production of IL-10 by and frequencies of stimulated peripheral blood leukocytes in vitro were normalized by subtraction of the relevant medium control values. We wished to test the hypothesis that allergen skin test reactivity is associated with lower levels of IL-10 and with lower frequencies of specific populations of putative regulatory T cells (defined as CD3+CD4IL-10+, CD3+CD4+CD25+IL-10+ and CD3+CD4+CD25highIL-10+) in whole blood stimulated with A. lumbricoides antigen. We examined also the associations between the frequencies of putative regulatory T cells and the presence of specific IgE (≥0.7 IU/mL) for D. pteronyssinus or P. Americana. The associations between atopy and immunological parameters were analysed by logistic regression. The effects of the variables, total IgE, age, sex, crowding (persons per sleeping room) and socioeconomic level on the estimates of effect for atopy were assessed by multiple logistic regression but were not included in the final models because none of these factors were significantly associated with atopy. The interaction between recent anthelmintic treatment and active intestinal helminth infection on skin test responses in these models were assessed by addition of an interaction term to the models. For these analyses, active intestinal helminth infection was defined by the presence of eggs in fecal samples. The presence of antibodies to A. lumbricoides (IgG, IgG4, and IgE) was taken to indicate past infections with or exposures to A. lumbricoides or other intestinal helminth infections with which A. lumbricoides shares extensive immunologic cross-reactivity.18 To assess whether putative regulatory T cells might affect the association between allergen-specific IgE (to P. americana or D. pteronyssinus) and skin test reactivity (to P. americana or D. pteronyssinus), we added the variables for these cell populations to logistic regression models of the association between specific IgE and skin test reactivity for each of P. americana and D. pteronyssinus. Comparisons of data between skin test reactive and non-reactive children, classified as continuous or binary variables, were performed also using rank sum and Chi-squared tests/Fisher's exact test, respectively. Socioeconomic level was calculated as a score based on paternal and maternal education and occupation and material goods in the household. Analyses were performed using Stata 7.0 (Stata Corporation, College Station, TX).
Results
Characteristics of study population
Baseline characteristics of the study children stratified by allergen skin test reactivity are shown in Table 1. Important confounding factors of age, sex, socioeconomic level, and level of household crowding were similar between allergen skin test positive and negative subjects. Although all children had evidence of prior exposure to intestinal helminths as determined by the presence of specific antibodies to A.lumbricoides (IgG, IgG4, and IgE) and evidence of basophil histamine release (>10%) to Ascaris adult and larval stage antigens, the overall prevalence of active intestinal helminth infections measured by stool microscopy was only 36.7%. This was likely due in part to the high percentage of children that had received anthelmintic treatment (42.5%), as a previous survey of the prevalence of active intestinal helminths in this population showed a prevalence of 63.4% for any intestinal helminth infection.11
Table 1. Baseline characteristics of study population.
| Variable | SPT negative (n=41) |
SPT positive (n=39) |
|---|---|---|
| Age (years) | ||
| Mean (range) | 9.2 (7-13) | 9.3 (7-13) |
| Sex (%) | ||
| Male/Female | 61/39 | 54/46 |
| Socioeconomic level | ||
| Mean (range) | 2.1 (1-4) | 2.1 (1-4) |
| Crowding (persons/room) | ||
| Mean (range) | 4.2 (1.3-9.0) | 3.4 (1.3-8.0) |
| Allergen skin test reactivity (%) | ||
| Any‡ | 0 | 100% |
| HDM | 0 | 56.4% |
| P. americana | 0 | 69.2% |
| Recent treatment with albendazole* | ||
| No | 53.7% | 61.5% |
| Yes | 46.3% | 38.4% |
| Intestinal helminths in stools | ||
| Any | 30.0% | 43.4% |
| A.lumbricoides | 15.0% | 23.1% |
| Intensity [GM (range)] | 7,394 (71-162,121) | 822 (71-25,201) |
| T.trichiura | 25.0% | 33.3% |
| Intensity [GM (range)] | 463 (70-2,590) | 296 (70-3,920)2.6% |
| Hookworm | 2.5% | |
| Total IgE (IU/mL) | ||
| GM (range) | 729 (10-5,253) | 1,291 (190-8,898) |
| Anti-A. lumbrocioides IgE | ||
| GM kU/L (range) [%]∫ | 2.8 (<0.35-100) [85.4%] | 4.4 (<0.35-100) [94.9%] |
| Anti-A. lumbricoides IgG (%) | 100% | 100% |
| Anti- A. lumbricoides IgG4 (%) | 100% | 100% |
| Histamine release (>10%) (n=29) | (n=12) | (n=17) |
| A. lumbricoides§ | 100% | 100% |
GM – geometric mean
Epg – eggs per gram of stool
anthelmintic treatment with 400 mg of albendazole within the previous 2 months.
Dermatophagoides pteronyssinus, Alternaria tenuis, P. americana, cat, grass pollen mix, and tree pollen mix.
proportion with levels of A.lumbricoides-specific IgE >0.35 kU/L
Histamine release to any of adult worm and larval antigen preparations (L2/L3 and L3/L4)
Relationship between allergen skin test reactivity and IL-10 protein and intracellular expression by PBLs
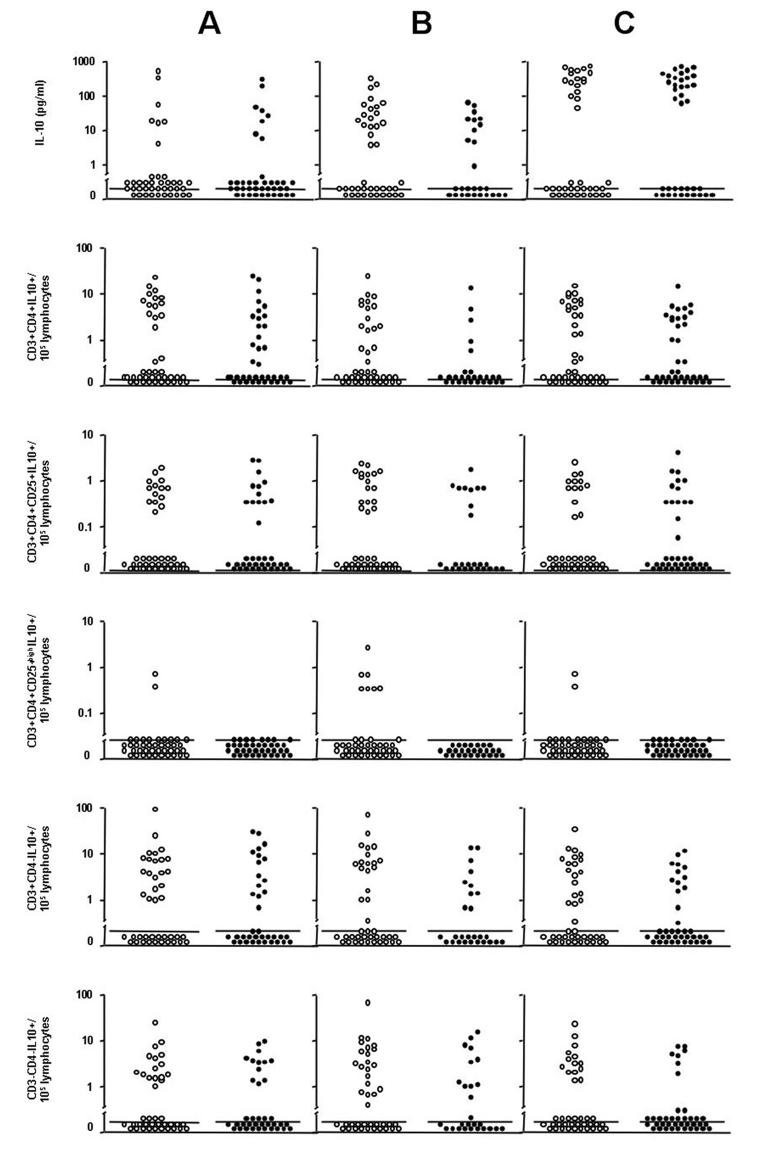
We observed no relationship between allergen skin test reactivity to any allergen and levels of IL-10 protein from whole blood cultures stimulated with A.lumbricoides antigen (Figure 2-A1).
Figure 2.
IL-10 protein and frequencies of IL-10+ PBLs by skin test reactivity. Shown are A. lumbricoides-antigen induced IL-10 (graph A1) and IL-10+ PBLs (graphs A2-A6) by skin test reactivity to any allergen, P. americana-allergen IL-10 protein (graph B1) and IL-10+ PBLs (graphs B2-B6) by reactivity to P. americana, and D. pteronyssinus-allergen IL-10 protein (graph C1) and IL-10+ PBLs (graphs C2-C6) by reactivity to D.pteronyssinus. Skin test positive (closed circles) and negative (open circles). Bars represent median values.
There was no association between allergen skin test reactivity and the frequencies of CD3+CD4+IL-10+ (OR 1.01, 95% CI 0.98-1.05, P=0.48) and CD3+CD4+CD25+IL-10+ (OR 1.06, 95% CI 0.81-1.38, P=0.69) and CD3+CD4+CD25highIL-10+ (OR 0.15, 95% CI 0.02-1.38, P=0.09) T cells from A.lumbricoides antigen-stimulated cultures (Figures 2-A3 and 2-A4). There were no differences in the proportions of subjects with elevated frequencies of IL-10+ PBLs above medium according to allergen skin test reactivity: CD3+CD4+IL-10+ (skin test positives 47.4% [18/38] vs. skin test negatives 39.0% [16/41], P=0.45), CD3+CD4+CD25+IL-10+ (34.2% vs. 34.2%, P=0.96), CD3+CD4+CD25highIL-10+ (0% vs. 4.9%, P=0.17), CD3+CD4−IL-10+ (42.1% vs. 51.2%, P=0.42) and CD3−CD4−IL-10+ (34.2% vs. 41.7%, P=0.51). There were no associations between allergen skin test reactivity to any allergen and the frequencies of other IL-10+ T cell populations (Figures 2-A2, A3, A5). Active intestinal helminth infection was not associated with production of IL-10 by PBLs or the frequencies of putative regulatory T cell populations expressing intracellular IL-10 from whole blood cultures stimulated with A. lumbricoides antigen (data not shown). Levels of IL-10 protein from whole blood cultures (Figures 2-B1) and the frequencies of IL-10+ T cell populations (Figures 2-B2 to 5) from cultures stimulated with P. americana or D.pteronyssinus were not associated with skin test reactivity to the respective allergens.
Relationship between allergen-specific IgE and IL-10 protein and intracellular expression by PBLs
IL-10 protein production by whole blood cultures stimulated with specific allergen extracts (P. americana and D. pteronyssinus) was not associated with the presence of specific IgE (>0.7 kU/L) (data not shown). The frequencies of the IL-10+ T cell populations from cultures stimulated with P. americana and D. pteronyssinus were not associated with the presence of specific IgE (>0.7 kU/L) (data not shown).
Associations between allergen skin test reactivity and allergen-specific IgE
There was evidence of a disassociation between the results of allergen skin testing and levels of specific IgE (Table 2). The relationship between the results of skin prick tests for D. pteronyssinus and P. americana allergen extracts and levels of specific IgE for the same allergens is shown in Table 2. A total of 20 (34.5%) out of 58 skin test negative children for D. pteronyssinus had detectable levels of specific D. pteronyssinus-specific IgE (≥0.35 kU/L) while 15 (28.3%) of the 53 skin test negative children for P. americana had detectable levels of specific IgE. Relatively fewer skin test negatives had significant levels of specific IgE using a more stringent cut-off of specific IgE of ≥0.7 kU/L: 19.0% and 17.0% for D.pteronyssinus and P. americana, respectively.
Table 2. Relationship between allergen-specific responses measured by skin test reactivity and levels of specific IgE for D. pteronyssinus and P. americana.
| Skin test reactivity | Specific IgE (kU/L) |
||
|---|---|---|---|
| <0.35 | 0.35-0.69 | ≥0.70 | |
| D,pteronyssinus | |||
| No (n=58) | 38 (65.5%) | 9(15.5%) | 11 (19.0%) |
| Yes (n=22) | 3 (13.6%) | 1 (4.6%) | 18 (81.8%) |
| P.americana | |||
| No (n=53) | 38 (71.7%) | 6 (11.3%) | 9 (17.0%) |
| Yes (n=27) | 9 (33.3%) | 6 (22.2%) | 12 (44.4%) |
The denominators for the percentages are the number of individuals with negative or positive skin tests for each allergen as appropriate.
Effects of IL-10 and IL-10+ PBLs on the association between specific IgE and skin test reactivity
The independent effects of IL-10 protein and IL-10+ populations of putative regulatory T cells (CD3+CD4+L-10+, CD3+CD4+CD25+IL-10+ and CD3+CD4+CD25highIL-10+) on the associations between allergen-specific IgE and skin test reactivity to each of D.pteronyssinus and P. americana were assessed by adding these variables separately to models of these associations. The findings are shown in Table 3 and indicate that IL-10 and the putative regulatory T populations had no effect on this association for either P. americana or D. pteronyssinus.
Table 3. Associations between allergen-specific IgE and skin test reactivity.
| Models | Allergen skin test reactivity | Putative regulatory T cells | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| P. americana IgE | 1.16 | 0.96-1.41 | 0.12 | |||
| + A. lumbricoides IL-10 protein | 1.15 | 0.95-1.40 | 0.14 | 0.99 | 0.99-1.01 | 0.54 |
| + A. lumbricoides CD3+CD4+IL-10+ | 1.15 | 0.95-1.39 | 0.14 | 1.00 | 0.96-1.03 | 0.83 |
| + A. lumbricoides CD3+CD4+CD25+IL-10+ | 1.18 | 0.97-1.42 | 0.10 | 1.27 | 0.90-1.80 | 0.17 |
| + A. lumbricoides CD3+CD4+CD25highIL-10+ | 1.14 | 0.95-1.37 | 0.16 | 0.35 | 0.05-2.39 | 0.29 |
| + P. americana IL-10 protein | 1.16 | 0.97-1.41 | 0.11 | 0.98 | 0.96-1.01 | 0.19 |
| + P. americana CD3+CD4+IL-10+ | 1.18 | 0.97-1.43 | 0.10 | 1.00 | 1.00-1.00 | 0.44 |
| + P. americana CD3+CD4+CD25+IL-10+ | 1.16 | 0.96-1.41 | 0.13 | 0.95 | 0.71-1.26 | 0.71 |
| + P. americana CD3+CD4+CD25highIL-10+ | 1.15 | 0.95-1.39 | 0.15 | 0.26 | 0.05-1.37 | 0.11 |
| D. pteronyssinus IgE | 2.89 | 1.29-6.49 | 0.01 | |||
| + A. lumbricoides IL-10 protein | 3.01 | 1.31-6.91 | 0.01 | 1.00 | 1.00-1.01 | 0.29 |
| + A. lumbricoides CD3+CD4+IL-10+ | 2.72 | 1.21-6.10 | 0.02 | 1.02 | 0.96-1.08 | 0.54 |
| + A. lumbricoides CD3+CD4+CD25+IL-10+ | 3.02 | 1.31-6.70 | 0.02 | 0.79 | 0.57-1.10 | 0.16 |
| + A. lumbricoides CD3+CD4+CD25highIL-10+ | 2.83 | 1.31-6.70 | 0.01 | 0.79 | 0.57-4.10 | 0.16 |
| + D. pteronyssinus IL-10 protein | 3.01 | 1.29-6.97 | 0.01 | 1.00 | 1.00-1.00 | 0.38 |
| + D. pteronyssinus CD3+CD4+IL-10+ | 2.80 | 1.25-6.28 | 0.01 | 1.02 | 0.96-1.09 | 0.55 |
| + D. pteronyssinus CD3+CD4+CD25+IL-10+ | 3.08 | 1.32-7.15 | 0.01 | 0.85 | 0.63-1.15 | 0.30 |
| + D. pteronyssinus CD3+CD4+CD25highIL-10+ | 2.87 | 1.28-6.43 | 0.01 | 0.74 | 0.14-4.04 | 0.73 |
Shown are odds ratios (OR) and 95% confidence intervals (95% CI) for the effects of simultaneously controlling for putative regulatory T cell populations and allergen-specific IgE on the risk of skin test reactivity in logistic regression models.
Discussion
We have investigated the associations between production of IL-10 protein in supernatant fluids from whole blood peripheral blood leukocytes (PBLs) stimulated with A. lumbricoides antigen or aeroallergen extracts and the expression of intracellular IL-10 by populations of PBLs defined by surface staining for CD3, CD4, and CD25. For the study we investigated a particularly interesting population – school children living in an area of the rural tropics where the prevalence of allergic symptoms is extremely low11 and where we have shown previously strong inverse associations between allergen skin test responses and active intestinal helminth infections.11 In such a population regulatory effects to control allergic inflammation associated with intestinal helminth infection or other potentially protective rural environmental exposures14 are likely to be well developed. The study of such children may provide important insights into the immune regulation of allergic inflammation. Our data do not support the hypothesis that intestinal helminth-induced IL-10-expressing regulatory T cells affect allergen skin test responses or affect the association between allergen-specific IgE in plasma and skin test reactivity to the same allergen.
A strength of the study is the recruitment of children with allergen skin test reactivity that was confirmed by repeated skin testing and the study of a well-characterized study population in whom important confounding factors have been identified previously5 for which skin test reactive and non-reactive children were balanced at baseline. Limitations of the study were - 1) A high proportion of children (42.5%) had received anthelmintic treatment within 2 months of blood sampling that was reflected in a lower than expected prevalence of active intestinal helminth infections in the study sample (36.7%) – however, all had evidence of immune sensitization to A. lumbricoides infection measured by detection of specific antibodies and histamine release to A. lumbricoides antigens, indicating past exposures to or infections with these and other intestinal helminth parasites. Further, there was no evidence of a modification of the effects for the associations between allergen skin test reactivity and IL-10+ PBL populations by anthelmintic treatment. 2) The study had relatively low power to detect small effects particularly for low frequency T cell populations (i.e. CD3+CD4+CD25highIL-10+). The issues of cost and the time required to perform these labor-intensive immunological assays have limited the number of subjects that can be studied and it may be preferable to restrict the number of analyses performed (e.g. focus on specific populations of regulatory T cells) so that a greater number of subjects can be investigated in future studies. An important regulatory marker is the transcription factor forkhead box P3 (FOXP3) that is considered to identify natural regulatory cells19 among CD4+CD25+ T cells. We were unable to assess the expression of FOXP3 in this population because a reagent for the detection of this marker by flow cytometry was not available at the time the analyses were performed.
A previous study has described an inverse association between the production of IL-10 by peripheral blood mononuclear cells (PBMCs) stimulated with Schistosoma mansoni antigen and the risk of allergen skin test reactivity to D.pteronyssinus in an African population living in an area endemic for intestinal helminths and S.mansoni infection6 The same study showed also that increasing levels of IL-10 appeared to reduce the risk of a positive skin test to D.pteronyssinus associated with the presence of a given level of specific IgE.6 Possible explanations for these observations are: 1) peripheral blood responses may reflect a generalized anti-inflammatory systemic effect of infection on host allergic responses including those occurring in the skin; 2) parasite-specific IL-10+ T cells are present in the skin, the site of parasite entry by schistosome cercariae, and affect mast cell function by release of IL-10; 3) parasite antigen-induced IL-10 is a marker for other immunologic factors that directly mediate these effects; and 4) parasite antigen-induced IL-10 is a marker for non-immunologic causal factors (e.g. those associated with poverty and that contribute to the risk of chronic infections with schistosomiasis). In the present study, performed in an area endemic for intestinal helminth parasites, we were unable to show a similar association with IL-10 or the frequencies of putative regulatory T cells. Our data, therefore, may favor a role for non-IL-10-associated factors in mediating the observed effects.
IL-10 does not appear to play a prominent role in the human immune response to A.lumbricoides and T.trichiura infections,20,21 the most prevalent intestinal helminth parasites in the study population (Table 1; reference 11). This is in contrast to tissue helminth infections, such as filarial and schistosome infections, that have appear to have strong regulatory effects in humans that are mediated at least in part by IL-10.22,23 The possible allergy modulating effects of helminth parasites that are largely confined to the intestinal tract (i.e. intestinal helminths) are likely to be subtler than those of helminths parasites that are present in the systemic circulation (e.g. adult schistosomes and some filarial adults and larvae), at least when measuring immune responses in peripheral blood, and it is probable that current assays using peripheral blood are too insensitive to detect such effects. Our data do not support an important role for intestinal helminth infection in inducing populations of IL-10+ putative regulatory T cells that may be capable of regulating allergic inflammatory responses to aeroallergens and that may have a role in suppressing allergic inflammation in experimental murine models of intestinal helminth infection.24
A high proportion of individuals had detectable levels of allergen-specific IgE (≥0.35 kU/L) in the absence of positive skin test to D. pteronyssinus and P. americana but fewer skin test negatives had specific IgE of higher titer (≥ 0.7 kU/L). It is possible that the low prevalence of allergic disease in this population11 may be caused by inhibition of allergic effector responses (e.g. immediate hypersensitivity reactions causing allergen skin test reactivity) rather than by inhibition of allergic sensitization. However, caution should be exercised in the interpretation of low titer IgE - the disassociation could be due also to false-positive serological reactions caused by factors such as immunological cross-reactivity between intestinal helminth antigens and inhalant allergens.25 A previous study in Kenya showed that serum IgE antibodies (≥0.7 kU/L) to cat allergen were present in 47% proportion of the children studied in a rural village even though none had neither a positive skin test nor a significant exposure history to cats.4 Further, the IgE antibodies to cat could not be inhibited by cat extract.4
In conclusion, we have examined the associations between markers of atopy and the production and expression of IL-10 by PBLs in children living in a rural area of the Tropics that is endemic for intestinal helminth parasites. Our data does not support an important regulatory role for intestinal helminth-induced IL-10 or IL-10+ T cell populations in modulating allergen skin test reactivity in this population.
Acknowledgements
We thank the children, parents, and teachers of the study schools for their cooperation during the study; and acknowledge the support of the local Directors of Health in the study Districts, and the foundation SALUDESA.
Funding statement: The study was funded by the Wellcome Trust (grant, 060120/Z/99/C).
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest
References
- 1.Ali M, Ramanarayanan MP. Correlation of a micro-ELISA assays with skin test and the RAST assay in inhalant allergy. Ann Allergy. 1984;53:143. [PubMed] [Google Scholar]
- 2.van der Ree JS, de Groot H, van Swieten P, Jansen HM, Aalberse RC. Discrepancies between the skin test and IgE antibody assays: study of histamine release, complement activation in vitro, and occurrence of allergen-specific IgG. J Allergy Clin Immunol. 1988;82:270–81. doi: 10.1016/0091-6749(88)91011-1. [DOI] [PubMed] [Google Scholar]
- 3.van den Bigelaar AHJ, Lopuhaa C, van Ree R, van der Zee JS, Jans J, Hoek A, et al. The prevalence of parasite infestation and house dust mite sensitisation in Gabonese schoolchildren. Int Arch Allergy Immunol. 2001;126:231–38. doi: 10.1159/000049519. [DOI] [PubMed] [Google Scholar]
- 4.Perzanowski MS, Ng'ang'a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–8. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 5.Cooper PJ, Chico ME, Sandoval C, Nutman TB. Atopic Phenotype is an Important Determinant of IgE-mediated Inflammation and Th2 Cytokine Expression to Ascaris Antigens in Children Exposed to Ascariasis. J Infect Dis. 2004;190:1338–46. doi: 10.1086/423944. [DOI] [PubMed] [Google Scholar]
- 6.van den Bigelaar AHJ, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with Schistosoma hematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–27. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 7.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–96. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, Richmond P, et al. Atopic dermatitis in young children is associated with impaired interleukin-10 and interferon-gamma responses to allergens, vaccines and colonizing skin and gut bacteria. Clin Exp Allergy. 2005;35:1309–17. doi: 10.1111/j.1365-2222.2005.02348.x. [DOI] [PubMed] [Google Scholar]
- 9.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Immunol. 2001;31:694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 10.Macabaus C, Sly PD, Burton P, Tiller KL, Smallacombe TB, Kendall G, et al. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29:1233–31. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper PJ, Chico ME, Griffin GE, Nutman TB. Allergy symptoms, atopy, and geohelminth infections in a rural area of Ecuador. Am J Resp Crit Care Med. 2003;168:313–7. doi: 10.1164/rccm.200211-1320OC. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60:1357–60. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 13.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–94. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 14.Cooper PJ, Barreto ML, Rodrigues LC. Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br Med Bull. 2006;79-80:203–18. doi: 10.1093/bmb/ldl015. [DOI] [PubMed] [Google Scholar]
- 15.Addo-Yobo EOD, Woodcock A, Allotey A, Baffoe-Bonnie B, Strachan D, Custovic A. Exercise-induced bronchospasm and atopy in Ghana: two surveys ten years apart. PLoS Medicine. 2007;4:355–60. doi: 10.1371/journal.pmed.0040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature Reviews Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 17.WHO Diagnostic techniques for intestinal parasitic infections (IPI) applicable to primary health care (PHC) services. 1985 WHO/PDP/85.2. [Google Scholar]
- 18.Bhattacharya T, Santra A, Majumder DNG, Chatterjee BP. Possible Approach for Serodiagnosis of Ascariasis by Evaluation of Immunoglobulin G4 Response Using Ascaris lumbricoides Somatic Antigen. J Clin Microbiol. 2001;39:2991–4. doi: 10.1128/JCM.39.8.2991-2994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 20.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Kennedy MW, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine phenotype. J Infect Dis. 2000;182:1207–13. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner H, Turner J, Kamgno J, Pion SD, Boussinesq M, Bradley JE. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J Infect Dis. 2002;185:665–72. doi: 10.1086/339005. [DOI] [PubMed] [Google Scholar]
- 22.King CL, Medhat A, Malhotra I, Nafeh M, Helmy A, Khaudary J. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–21. [PubMed] [Google Scholar]
- 23.Doetze A, Satoguina J, Burchard G, Rau T, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and TGF-□ but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–30. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 24.Wilson MS, Maizels RM. Regulatory T cells induced by parasites and the modulation of allergic responses. Chem Immunol Allergy. 2006;90:176–95. doi: 10.1159/000088892. [DOI] [PubMed] [Google Scholar]
- 25.Arruda LK, Santos AB. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr Opin Allergy Clin Immunol. 2005;5:399–402. doi: 10.1097/01.all.0000182536.55650.d0. [DOI] [PubMed] [Google Scholar]