Abstract
Substrate topography plays a vital role in cell and tissue structure and function in situ, where nanometric features, for example, the detail on single collagen fibrils, influence cell behaviour and resultant tissue formation. In vitro investigations demonstrate that nanotopography can be used to control cell reactions to a material surface, indicating its potential application in tissue engineering and implant fabrication. Developments in the catalyst, optical, medical and electronics industries have resulted in the production of nanopatterned surfaces using a variety of methods. The general protocols for nanomanufacturing require high resolution and low cost for fabricating devices. With respect to biological investigations, nanotopographies should occur across a large surface area (ensuring repeatability of experiments and patterning of implant surfaces), be reproducible (allowing for consistency in experiments), and preferably, accessible (limiting the requirement for specialist equipment). Colloidal lithography techniques fit these criteria, where nanoparticles can be utilized in combination with a functionalized substrate to produce in-plane nanotopographies. Subsequent lithographic processing of colloidal substrates utilizing, for example, reactive ion etching allows the production of modified colloidal-derived nanotopographies. In addition to two-dimensional in-plane nanofabrication, functionalized structures can be dip coated in colloidal sols, imparting nanotopographical cues to cells within a three-dimensional environment.
Keywords: colloid, fabrication, lithography, nanotopography, cell biology, tissue engineering
1. Introduction
Nanostructured devices used in biological research have emerged in-line with developments in the electronics industry (Wilkinson 2004), evolving as a result of the ‘silicon road map’; a ‘smaller, faster and cheaper’ technology drive with respect to future global semiconductor fabrication (Mathur 2002). As a result, topographies containing features with dimensions of less than 100 nm, more similar to the dimensions of entities found within cellular environments (Curtis 2004), are now emerging in biological applications. As the required structures and functions of nanopatterned materials differ between electronics and biology, this review focuses on the fabrication of in-plane nanostructures, in use and development, believed to be important in the future investigations and understanding of cell response to nanotopographies. Particular emphasis has been placed on the role of colloidal lithography in producing nanopatterned devices. Advances in tissue engineering and implant design are commanding the development of nanopatterned three-dimensional structures, with few methods existing at present to modify the nanotopography of these devices. The review thus concludes by discussing the use of colloidal sols in a dip-coating capacity to provide nanofeatures to functionalized three-dimensional samples.
2. Current nanofabrication techniques
It is difficult to summarize the limits and qualities of nanofabrication techniques utilized at present, as the field is continually changing, with boundaries constantly being challenged and modified. However, the key methods practiced are outlined within this section, providing the reader with a condensed overview of current procedures. It should be noted that in many instances, more than one fabrication process is implemented to achieve the final production of a device.
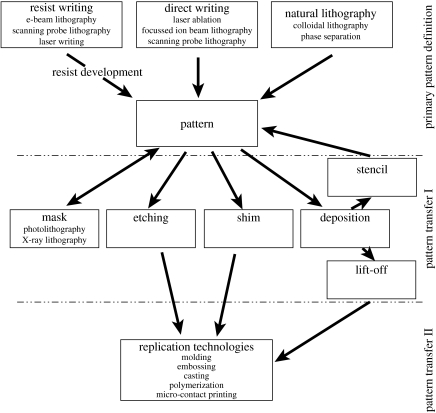
The general protocols for nanomanufacturing require high resolution and low cost for fabricating devices (Xia et al. 1999). This may include the high-cost fabrication of a master, with structures replicated in great quantity in an inexpensive process, resulting in the initial outlay of the master being an insignificant part of the overall cost of the device. The definition of a deliberate pattern is by its very nature a serial process, where every picture element has to be defined one by one. If utilized in the production of replicas, the structures, having undergone the writing process, are referred to as master dies (Wilkinson et al. 1998). The economical and convenient route to multiple copies of a master is termed replication, where information is transferred to a functional material, usually of a polymeric nature, from the master, rapidly, in a single step and with high precision (Xia & Whitesides 1998). Thus, nanopatterning can be separated into three main categories: primary pattern definition or writing, pattern transfer and mechanical transfer techniques (figure 1).
Figure 1.
Overview of nanolithography techniques and processes. ‘Primary pattern definition’ or writing is the first stage in nanofabrication, resulting in patterning of a resist, masking of a base substrate utilizing self-assembly techniques or direct writing on a base substrate via laser ablation, FIB or SPL. Patterns produced during ‘primary pattern definition’ can be transferred to a desired substrate during ‘pattern transfer I’, resulting in the production of a master die. A subsequent pattern transfer step, termed here ‘pattern transfer II’, can be performed using replication technologies, where a master die is utilized to mechanically transfer an inverse representation of the original pattern to a deformable polymer.
At present, the most highly developed and widely used conventional lithography techniques practiced is contact mode photolithography. Photolithography allows master dies to be produced across large areas in a single step inexpensively, which is highly advantageous with respect to biological applications. However, contact lithography requires a mask, the primary pattern of which can be defined during primary pattern definition, using resist writing, e.g. e-beam lithography, scanning probe lithography or laser writing (figure 1). To produce the patterned mask using photolithography, the pattern in the photomask is transferred to a resist and subsequently developed during pattern transfer I (figure 1). At present, photolithography can practically produce below 400 nm features, but in a biological context, this method is more readily used to fabricate 1–2 μm features (Falconnet et al. 2006). In contrast, e-beam lithography has extremely high-resolution capabilities with, practically, 4 nm beam diameters of high current now available (McCord & Rooks 1997). As a result, e-beam is often used to produce nanofeatured masters during primary pattern definition (figure 1), especially in instances where other methods simply fail (Xia et al. 1999). Nevertheless, e-beam defined patterns can only be made accessible for biological research through the use of replication technologies (or mechanical transfer) during pattern transfer II. Hence, it is mainly used to produce master dies, masks for photolithography and small structures for research (pattern transfer I, figure 1). An alternative approach to define high-resolution patterns is X-ray lithography (XRL), used during pattern transfer I (figure 1). XRL is often presented as a possible route to reducing wavelength limitations seen in photon-based lithography, where structures with dimensions of 30 nm have been produced using proximity XRL with wavelengths around 1 nm (Cerrina & Marrian 1996). However, as an emerging technique, much development is still required if it is to become a feasible and accessible means of producing nanostructured substrates for biological applications.
Although photolithography, e-beam and XRL are often used to define primary patterns, replication technologies (pattern transfer II, figure 1) are more readily utilized when investigating the effects of nanotopography on cell behaviour (Flemming et al. 1999). The emergence of polymer replicas with defined topography in biological investigations has ultimately arisen due to the requirement for final biomedical device's to be biocompatible (often biodegradable or bioresorbable (Gogolewski 2002)) following implantation (Simamora & Chern 2006). In vitro examination of a product provides a greater understanding of cell reactions likely to occur in vivo, allowing refinement of the final implant. Replication allows the production of a great number of samples (several hundred (Riehle et al. 2002)) from a single master, inexpensively, reducing the overall fabrication cost for processing nanotopographies, and provides reproducible in-plane patterns with high accuracy for biological applications. As this technique is ‘self-cleaning’ (any dirt on the surface of the master will be removed by the replicating polymer), changes in the pattern due to dust accumulation are unlikely to cause a problem, if the first sets of replicates are discarded. However, care must be taken with respect to the reproducibility of replication technologies concerning pattern distortion and in the case of solvent casting or in situ polymerization with respect to applied solvents. For example, solvent-assisted micromoulding (SAMIM) and casting employ volatile solvents that are required to dissolve the polymer substrate and wet the polydimethylsiloxane (PDMS) elastomer mould without altering or damaging the pattern being transferred (Xia & Whitesides 1998). Shrinkage is often observed in the final SAMIM pattern due to significant swelling of PDMS-based elastomers when exposed to certain solvents, e.g. diisopropylamine, triethylamine, pentane and xylenes (Lee et al. 2003). Further problems associated with using PDMS for SAMIM (including the surface energy not being low enough to produce high-fidelity patterns and the PDMS modulus being too low to maintain structural integrity) have resulted in the development of alternative (more stable) polymeric materials, e.g. photocurable perfluropolyethers (PFPEs; Rolland et al. 2004). Photocurable PFPEs have yet to emerge with respect to nanopatterning in biological applications, although PFPE membranes are currently under investigation as mechanical modulus matched material for implantable corneal lenses (Chan et al. 2006), indicating their biocompatibility.
Alternative replication technologies must also be used with care to prevent distortion of nanopatterns during transfer. For example, during replica moulding, casting and curing steps may cause distortion of pattern dimensions in the final polymer substrate (Zhao et al. 1997). Similarly, when embossing with a rigid master, release of the polymer being embossed can prove problematic. Although many mould release agents may be incompatible with respect to biological applications, suitable methods are emerging to encourage polymer release from the master die, for example, the pattern being replicated can be coated with Teflon (Schulz et al. 2000). Embossing may also damage the master due to the pressure used when transferring the pattern to the replica, and there is also the possibility that the replicas may include distortions in the pattern due to: (i) thermal cycling during the process and (ii) the differential thermal expansion coefficients of the die and the polymer. Although these problems do exist, if carefully monitored, replication technologies continue to be the most widely used techniques when producing nanotopographies for biological applications. It should be noted, however, that distortions in the replicated nanopattern might alter cell response, as observed when comparing regularity effects of nanofeatures on cell behaviour (Curtis et al. 2001, 2004), thus optimization of replication techniques is essential. Replication technologies also require a master die, which is usually produced using e-beam or photolithography (see figure 1), techniques which are often expensive and inaccessible to biologists. Owing to the serial writing processes used to produce masters, current nanolithography methods are also limited to the types of spatial arrangement of features they can produce, with irregular patterns proving difficult to fabricate. Yet, irregular patterns continue to be important in elucidating the role of nanofeature regularity and symmetry on cell-substrate response (Curtis et al. 2001, 2004). Furthermore, complex nanotopographies presented to cells in situ, for example, in the corneal epithelial basement membrane, are often irregularly distributed with deviations in the pattern occurring in the x-, y- and z-planes (Abrams et al. 2000). Bearing in mind the present drive towards biomimetic structures with respect to tissue engineering, the ability to produce irregular nanotopographies similar to those observed in situ is paramount in the rational design and fabrication of scaffolds (Brody et al. 2006).
Self-assembly technologies, e.g. phase-separated block copolymer, polymer demixing and colloidal lithography offer a set of effective methods to define regular and irregular primary patterns with sub-100 nm features for biological investigations. Periodic arrays have been fabricated using block copolymer lithography (Park et al. 2001; Kim et al. 2003; Cheng et al. 2004). However, when considering that regularity and symmetry of substratum nanotopography may greatly influence the elicited cell response (Curtis et al. 2001, 2004), block copolymers may not offer the accuracy required in regular patterns for biological experiments, as explained later. For example, the specification for the deviation from the true position is 10 nm in a 0.5×0.5 mm area when an electron beam is used to produce nanometric dots at a pitch similar to patterns produced using colloidal lithography (with 20 nm diameter particles, 60 nm mean interparticle spacing (Wood et al. 2006)). Furthermore, the deviation from perfect positioning between each 0.5×0.5 mm area is 80 nm at mean±s.d. In the case of block copolymers, misplacement by an amount of 1 dot in good small arrays will result in marked deviation on a larger scale where one square area will differ in orientation by a few degrees from its neighbour. Moreover, surface contamination, regardless of however minimal, will disturb the arrangement locally and cause such a twinning of the pattern. However, block copolymers are often used to produce nanotopographies for biological investigations, having been utilized to date in the production of nanoparticle drug carriers (Sasatsu et al. 2006), films with selectable pore sizes used as scaffolds for cells (Beattie et al. 2006) and artificial extracellular matrix, where electrospun diblock copolymer nanofibres can be functionalized with argeinine–glycine–aspartic acid (RGD) peptides (Kim & Park 2006).
Binary polymer blends have also been utilized to produce topographies for biological investigations. For example, polystyrene–poly(bromostyrene) demixing, where composite films are annealed resulting in the mobilization of polystyrene (PS) to the surface, have been used in the production of in-plane nanometric islands with heights ranging between 13 and 95 nm (Dalby et al. 2004a). Similarly, demixing of poly(n-butyl methacrylate)-polystyrene has been utilized to produce substrates expressing closely packed, 50 nm high islands, which are non-adhesive to fibroblasts (Dalby et al. 2003). Polymer demixing offers a method of producing nanometric structures in the out-of-plane z-direction. However, features produced using polymer demixing techniques tend to exhibit significantly larger micrometric structures in one or more planes, thus do not produce strictly nanometric structures.
Originally developed as a method of replicating sub-macroscopic patterns by Fischer & Zingsheim (1981), and pioneered as a ‘natural lithography’ technique by Deckman & Dunsmuir (1982, 1983), colloidal lithography emerged in the electronics field to produce single-electron device structures (Sato et al. 1997a,b) and emerged in a biological capacity as a method of fabricating model nanostructured biomaterial surfaces (Hanarp et al. 1999). Colloids have since been utilized to produce nanopattern definition for a variety of biological investigations (Wood et al. 2002a, 2005, 2006; Andersson et al. 2003a,b; Dalby et al. 2004b, 2005). When employing natural lithography techniques, colloids tend to be composed of materials deviating from those of the base substrate. As a result, surfaces expressing heterogeneous chemistry are often produced, resulting in chemical patterning as well as topographical patterning of a device (Wood et al. 2002a, 2005). This in itself is often advantageous, allowing the production of well-defined chemical patterns where differences in colloidal and substrate chemistry can be used as a template for further chemical modifications (Michel et al. 2002; Denis et al. 2004). Chemical patterns produced by colloidal lithography can also be used to produce topographical patterns via additional fabrication procedures, allowing the production of surfaces with homogeneous chemistry. For example, colloids can be employed as a mask during dry etching (Wood et al. 2002b; Agheli 2006) or a template, where a homogeneous coating can be applied atop a colloidal pattern (Denis et al. 2002a,b). This is discussed in §4.
Colloidal lithography offers an extremely simple and accessible approach to defining primary patterns (figure 2), varying from irregular or ‘random’ (Wood et al. 2006) through to pseudo-regular (Michel et al. 2002). In combination with reactive ion or chemical etching, colloids can be employed as a mask to produce a master die with nanometric features, allowing the fabrication of polymer replicas (Wood et al. 2002b; figure 3). Moreover, we have developed methods of functionalizing and dip-coating three-dimensional structures, e.g. tissue engineering scaffolds or orthopaedic implants, in a colloidal sol, imparting nanotopographical cues to cells and tissues within a three-dimensional environment; a novel three-dimensional patterning method not easily attainable using conventional lithography techniques.
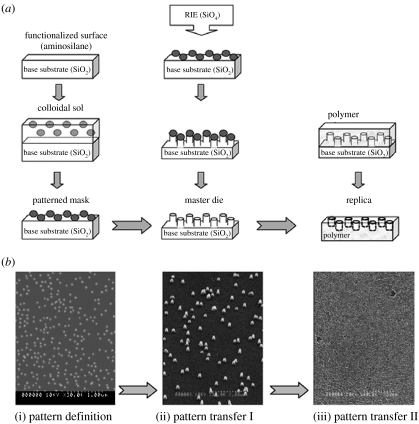
Figure 2.
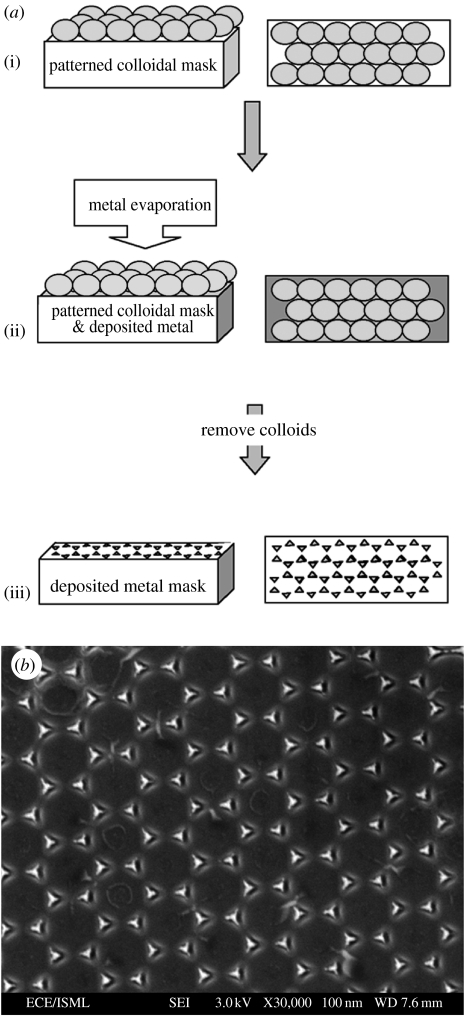
Overview of the main nanolithography steps used during fabrication, where colloids are utilized as a model system. A schematic overview is provided in (a), with scanning electron microscope (Hitachi S-900) images of the final patterns provided in (b). Pattern definition (i) requires a mask to be produced, in this instance by functionalizing a base substrate of silica and immersing in a colloidal sol, producing a patterned mask following sol evaporation (50 nm diameter colloidal gold particles in (b)). Pattern transfer I (ii) uses additive or subtractive techniques to produce a master die, for example, colloids can be used as a mask during reactive ion etching (RIE), where ions etch around the mask, producing, in this instance, a pillared topography (50 nm diameter, 200 nm high pillars, (b) with colloids removed post-etch). Colloids can subsequently be removed using a gold etch, resulting in a pillared master die with uniform chemistry. Pattern transfer II (iii), a process often termed replication technologies, uses a polymeric material to produce many copies of the master die topography, inversely (20 nm diameter, 200 nm deep nanopits in polystyrene, (b)).
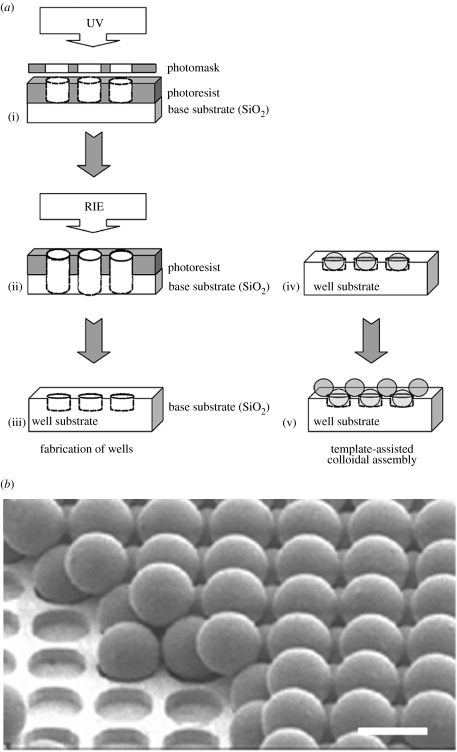
Figure 3.
Schematic overview of template-directed assembly on an ordered microsphere array, (a), as developed by Grego et al. (2005), (b) (image reproduced with kind permission of S. Grego the American Chemical Society). Photolithography (a(i)) is used to produce a patterned resist. RIE, (a(ii)), etches the base substrate exposed by the resist, producing wells of carefully selected dimensions in the substrate following resist removal, (a(iii)). Microspheres with dimensions corresponding to those of the wells, fill individual wells, (a(iv)), providing a template to assist in the ordering of a second set of microspheres, (a(v)). The final device and underlying sequential structures can be seen in the SEM image in (b). Scale bar=5 μm.
3. Colloidal lithography
Nanoparticles offer suitably sized, functional components for developing in-plane patterns (figure 2), and is an extremely convenient route to developing nanofeatures over large areas (Willner et al. 2000). Thus, colloidal gold particles, previously utilized in the development of single-electron devices (Lee et al. 2000; Shipway & Willner 2001), have emerged as a versatile method of producing nanometric features for biological investigations (Riehle et al. 2002; Wilkinson et al. 2002; Wood et al. 2006) and can be used in combination with chemical patterning to produce nanotopographies with homogeneous chemistry for biological applications (Wood et al. 2002b). Colloids can also be used to produce surfaces with heterogeneous chemistry. For example, particles can be adhered to a titanium-coated silica base substrate and used as an etch mask to produce titanium pillars on a silica substrate, allowing the selective adsorption of proteins relative to the underlying template (Michel et al. 2002).
Every aspect of nanopatterning can be altered with respect to colloidal fabrication techniques, e.g. colloidal and substrate materials, colloidal shape, size and monolayer distribution which is reflected in feature pitch (Willner et al. 2000). Furthermore, electrostatic repulsion occurring between individual colloids results in irregular monolayer patterning in the absence of charge shielding materials (Hanarp et al. 2003). The irregular patterning of substrates utilizing alternative nanofabrication techniques, e.g. e-beam lithography, is time-consuming and difficult, thus colloidal lithography is greatly advantageous for irregular in-plane nanopattern production.
3.1 Colloid shape
Colloidal particles are often spherical or quasi-spherical in shape (Xia et al. 1999), with controllable colloidal shapes, which can be favoured for dimensions within the nanometric range in a variety of materials (Ahmadi et al. 1996; Schlotterbeck et al. 2004; Yang et al. 2005). For instance, monodispersed colloidal gold particle dimensions can be selected by changing the molar ratio of trisodium citrate to HAuCl4 during suspension preparation (He et al. 2000), while nanoparticles composed of different materials can be similarly synthesized using other reducing agents (Willner et al. 2000) or a hot-injection procedure where organometallic precursors are rapidly injected into a vigorously stirred hot co-ordinating solvent composed of surfactant molecules (Vanmaekelbergh & Liljeroth 2005). Recent advances in polymer science have led to the development of a number of shape-controlled colloidal particles, including main-chain polyether, poly(9,9-dioctylfluorene-co-benzothiadiazole) and poly(9,9-dioctylfluorene) ellipsoids (Yang et al. 2005) and hexagonal palladium platelets stabilized using amphiphilic hyper-branched polymers (Schlotterbeck et al. 2004). Colloids with such strict specifications often require specialist equipment and knowledge and are frequently produced using elaborate multi-step procedures, although these issues are still being addressed (Schlotterbeck et al. 2004; Yang et al. 2005). Non-spherical polymer colloids must generally be stored in their non-equilibrium shapes under very specific and often impractical conditions with respect to their use as a nanotopography for biological applications, and shape-memory often occurs across a very specific range of particle sizes (Yang et al. 2005) restricting their universal appeal. However, readily accessible off-the-shelf colloidal suspensions are inexpensive and available to purchase in a number of shapes, sizes and materials due to their use in electron microscopy (e.g. British Biocell International, Cardiff, UK), further simplifying the nanofabrication process.
3.2 Colloid materials
Colloids can be fabricated in a number of materials including polymers (Ng et al. 2002; Ray et al. 2005; Yang et al. 2005) and metals (Sonvico et al. 2005), e.g. gold (Eastham et al. 2002; Laurent et al. 2005), palladium (Schlotterbeck et al. 2004) and platinum (Gu et al. 2005). Magnetic (Zhong et al. 2000; Berry et al. 2004) and semiconductor nanoparticles, termed ‘Q particles’ due to their quantum properties (Alivisatos 1996; Vanmaekelbergh & Liljeroth 2005) have also been produced. Quantum dots can be assembled from a variety of materials, including semiconductor nanocrystals of the groups II, III, IV, V and VI elements, e.g. cadmium selenide (CdSe), lead telluride (PbTe), indium phosphide (InP) and silicon (Si; Vanmaekelbergh & Liljeroth 2005), other metals, including gold, silver and cobalt and insulators, e.g. iron oxide or titanium oxide (Parak et al. 2003).
3.3 Colloid immobilization
Colloidal nanoparticles can be used to assemble in-plane sub-monolayers topographies. When nanometric colloidal dimensions are selected to produce topographies, adsorption techniques are required to adhere nanoparticles to a base substrate where nanoscale control over morphology of packing is available (Aizenberg et al. 1998; Pohl et al. 1999). Many methods exist to produce surface functionality at this level (Hunter 1987; Cass & Ligler 1998; Willner et al. 2000). Possibly, the most simple approach for producing colloidal monolayers is drop-casting where the sol is simply added to the base substrate in question and the solvent left to evaporate, with the colloids remaining on the substrate surface (Vanmaekelbergh & Liljeroth 2005). The degree of order in the pattern is primarily determined by the rate of sol evaporation. However, with respect to the use of drop-cast colloidal substrates as a nanotopography for biological applications, the stability of the nanofeatures is brought into question. Indeed, Albrecht-Buehler utilized such a substrate to monitor cell movement across a surface, where fibroblasts left a path, which he termed ‘phagokinetic tracks’, through the unfixed colloids indicative of their journey across the surface (Albrecht-Buehler 1977a,b). However, stable colloidal nanotopographies can be produced utilizing amino-functional silanes as adhesion agents for gold particles on hydroxy-group terminated substrates (Wood et al. 2002a, 2006). The aminosilanes couple with the hydroxyl group sites forming siloxane bonds with the substrate following immersion in water. Molecules are oriented with the free amino groups directed away from the substrate surface. The affinity of the amino groups to gold results in the immobilization of Au colloids on the silane-treated surface (Sato et al. 1997b).
Other methods of functionalized surface production for colloid immobilization producing in-plane nanotopographies are practiced, including avidin–biotin systems (Cass & Ligler 1998), spin-coating (Hong et al. 2002), chemically grown hydrophilic oxide layers (Seeger & Palmer 1999), electrophoretic deposition of colloids (Giersig & Mulvaney 1993), poly(ethylenimine)- (Peschel & Schmid 1995) and poly-l-lysine-coating (Wood et al. 2002b), triple layer positively charged precursor films (Andersson et al. 2003a,b; Hanarp et al. 2003), Langmuir–Blodgett technique (Huang et al. 2001) and DNA-directed colloidal immobilization (Niemeyer et al. 2001). Furthermore, a number of methods have recently emerged allowing the production of linear patterns formed by colloids. For example, a dynamic self-assembly technique, where positively charged poly(styrene-co-vinylimidazole)-latex particles were drop-cast onto a negatively charged silicon wafer, has been employed to produce lines of particles parallel to the air-substrate–liquid interface, where lines are regularly spaced following tilting of the substrate to a small angle relative to the horizontal (Ray et al. 2005). It should be noted that a concentric circle pattern resulted if the wafer remained horizontal and the sol was left to evaporate from the surface. Lines, termed in this next instance ‘microwires’, composed of colloidal gold nanoparticles can also be produced by altering an electric field between two electrodes (Lumsdon & Scott 2005). Possibly requiring refinement if they were to be considered for biological nanotopographies, linear colloidal arrangements may help to elucidate findings regarding cell reactions to discontinuous groove edges (Andersson et al. 2003a).
3.4 Colloid packing
Colloidal density on functionalized surfaces is also controllable (Hunter 1987; Willner et al. 2000). It should be noted, however, that nanoparticle lattices fabricated in this manner have a configurational disorder and can never be identically reproduced (Remacle et al. 1998). Configurational disorder occurs due to the non-identical nature of the nanoparticles, resulting in colloids of varying size and shape. Thus, alterations in electrostatic repulsion, which is dependent upon the size and shape of each colloid, affect the spacing between individual nanoparticles and, subsequently, pattern configuration.
The development of ordered-templates for controlled microsphere patterning offers one method of combating this problem (Grego et al. 2005). This technique utilizes photolithography and dry etching to produce ordered wells into which polystyrene beads are positioned. Through the careful selection of surface functionalizations (of either the substrate or the beads) and initial pitch of bead comprising the monolayer (and thus wells), an ordered second layer of microspheres can be assembled atop the existing template (figure 3). This new lithography technique may crossover to nanopatterning for biological purposes, offering an alternative method of producing nanotopographies. The production of regular arrays of nanoparticles in such a manner further increases the potential of colloids in device fabrication, particularly when considering cell reactions to ordered nanotopographies, where the majority of investigations to date utilize e-beam lithography. Furthermore, the accuracy achievable using template-assisted colloidal fabrication limits possible irregularities occurring in in-plane nanotopographies, believed to alter cell response to the pattern under investigation (Curtis et al. 2001, 2004).
Similarly, block copolymer micelle nanolithography can be used to overcome configurational disorders when producing nanoparticle monolayers and also allows controllable spacing of individual colloids. This is achieved by depositing block micelles of copolymers containing gold nanoparticles on a base substrate, e.g. glass, following which the device is exposed to hydrogen gas plasma, removing the polymer from the surface, but not the gold colloids. The gold particles remain on the surface of the base substrate in hexagonal patterns, where spacing between individual particles is determined by the molecular weight of the diblock copolymer used (Glass et al. 2003). To date, this nanolithography technique has been used to produce chemical nanopatterns using polyethylene glycol (PEG) to passivate the exposed base substrate and the cyclic peptide c-RGDfK (a selective ligand for the αvβ3 integrin required for cell adhesion) adhered to the colloidal particles (Arnold et al. 2004; Cavalcanti-Adam et al. 2006), although, in theory, nanoparticles could be employed as an etch mask (if mask integrity and a suitable base substrate were selected for) or coated with a material to provide uniform chemistry across the device.
When a functionalized substrate is immersed in a colloidal sol, surface assembly is under kinetic control at early time. Following colloidal immobilization at later time, colloidal coverage is limited by interparticle repulsion (figure 4; Grabar et al. 1996). These factors, and for example, citrate ions adsorbed on particle surfaces, limiting colloidal coverage, can be modified, resulting in controlled packing of nanoparticles producing in-plane nanotopography. Salt stabilization is one approach to counteracting interparticle repulsion (Reetz et al. 1997; Andersson et al. 2003a,b; Hanarp et al. 2003), whereby sodium acts as a counter ion in relation to the negative citrate coating of colloids, decreasing the electrostatic repulsion decay length, termed the Debye length. The polyelectrolyte coating of metal colloids can also be modified, where colloidal charge is immobilized, limiting ionic and hydrophobic polyanion and polycation interactions (Gittin & Caruso 2001). Furthermore, synthesis of ligand-stabilized transition metal nanoparticles results in uniform particle size, colloidal stabilization by inert ligand shells, equidistant particle arrangements due to defined ligand shells and the ability to vary feature pitch via chemical modification of ligand shells (Peschel & Schmid 1995).
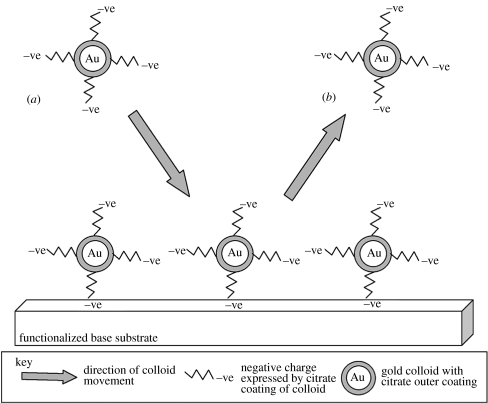
Figure 4.
Transition from kinetic adsorption to electrostatic repulsion of gold citrate-coated colloids as discussed by Grabar et al. (1996). The simple t1/2 no longer applies to the colloidal system. As the functionalized surface is covered with gold colloids, subsequent colloidal attachment is prevented. In (a), a colloid approaches the surface. However, due to the adhesion of particles that have undergone kinetic adsorption, no free NH2 groups are available on the functionalized substrate. Any amino groups that are not directly attached to a colloid are unable to attach subsequent colloids due to the negative citrate charge of previously adhered colloids. Thus, (b), colloids are prevented from attaching to the surface or other colloids.
Substrates coated with poly-l-lysine (Wood et al. 2002b) and bifunctional aminosilanes (Sato et al. 1997a,b) have also been utilized to increase colloidal density during monolayer surface patterning. With respect to bifunctional aminosilanes, treated surfaces are immersed in a colloidal sol and subsequently subjected to an alkanethiol solution. This treatment replaces citrate adsorbates with the alkanethiol molecules due to the strong affinity of sulphur to gold. Furthermore, this bonding is capable of displacing the amino group, present on the substrate and gold bonds, resulting in the mobilization of colloids on the surface. The occurrence of colloidal mobility results in grain formation, and repeated treatments allow for high-density packing of the nanoparticles (Sato et al. 1997a,b). Temperature-controlled self-assembly techniques have also emerged (Han & Grier 2005), producing high-density colloidal monolayer patterning often with very accurate, periodic in-plane topographies (Ng et al. 2002). This method is discussed further in §4. However, as discussed earlier, nanoparticle lattice production, in this instance using temperature-controlled techniques, is susceptible to configurational disorder (Remacle et al. 1998), detracting from the overall regularity of the nanotopography.
4. Colloidal masks
Following fabrication of a colloidal monolayer with selected dimensions, density and materials, nanopatterns can either be used in the current form for biological investigations (Wood et al. 2002a, 2005, 2006) or as masks with respect to pattern transfer (Wood et al. 2002b; figure 2; Dalby et al. 2005) or templates (Denis et al. 2004). It should be noted that patterns composed of colloids adhered to a base substrate are likely to express heterogeneous surface chemistry (e.g. gold colloids attached to a quartz substrate). Further processing of the sample is required (for instance, in the form of etching or coating) if a nanotopography with homogeneous surface chemistry is to be produced. Substrates patterned with colloids can be exposed to reactive ion etching (RIE), often referred to as dry etching, resulting in nanopillar patterning of the base substrate, where pillar diameter reflects colloidal diameter present in the original etch mask (Lewis et al. 1998; Lewis & Ahmed 1999; Wood et al. 2002b; Dalby et al. 2005), and pillar profile is determined by etch time (Tsutsui et al. 1993; Kuo et al. 2003; Wilkinson & Rahman 2004). Furthermore, metals, e.g. nickel, silver, aluminium or chromium can be evaporated onto the colloidal monolayer following which, nanoparticles producing either single- or double-layer colloidal templates can be removed using lift-off techniques, resulting in metal masks reflecting the interstices of the close-packed colloids producing hexagonal or triangular lattice arrangements (Ng et al. 2002; figure 5; Kuo et al. 2003). Samples fabricated in this manner can be exposed to dry etching techniques resulting in nanopillar patterning of the base substrate, where pillar shape and diameter correlate with the original colloidal mask (Hulteen & Van Duyne 1995; Seeger & Palmer 1999). Materials, e.g. polymers and metals, can also be used to coat colloidal surfaces resulting in homogenous surface chemistry across the nanotopography (Andersson et al. 2003b; Denis et al. 2004, respectively).
Figure 5.
Schematic overview of a technique used to produce nanotopographies reflecting the triangular lattice arrangements (or hexagonal lattice patterns) within a colloidal mask, (a) as developed by Ng et al. (2002), (b), (image reproduced with kind permission of V. Ng and IOP Publishing Limited). A closely packed in-plane colloidal mask is produced, (a(i)), and metal is deposited atop this, coating the interstices between the particles, (a(ii)). Colloids are removed from the surface resulting in triangular (or hexagonal) lattice arrangements of deposited metal, (a(iii)), (b). The pattern can then be used as a mask during RIE, where ions only etch the exposed material, in this instance the area where the colloids were originally located.
Pillar height and the final in-plane nanotopography are ultimately determined by etching parameters (when RIE is utilized), including mask integrity, gas, pressure and time (Tsutsui et al. 1993; Kuo et al. 2003). Following exposure to RIE, the original mask can be removed, for example, using potassium iodide and iodine (KI+I2) as an etch for gold colloids (Wood et al. 2002b), or other wet chemical etching methods where particles are removed from a surface due to the combined effects of chemical etch and a net repulsive interaction between the particle and the surface (Qin & Li 2003). Removal of colloids from the final structure results in an in-plane colloidal-based nanotopography expressing a uniform surface chemistry. This is of particular importance when investigating cell reactions to nanopatterned substrates, allowing the reactions to be directly attributed to surface topography rather than surface chemistry. Wet etching can also be employed with respect to colloidal-based nanotopographies having undergone RIE to produce nanofeatures with alternative profiles, a method previously utilized with respect to ‘silicon grass’ (Turner et al. 1997).
Pillar diameter, shape and pitch can be altered via selection of appropriate colloids composing the mask. It should be noted that in comparison to direct etching of the base substrate surrounding the colloids, where cylindrical pillars evolve underneath the areas masked by particles, etching of metals initially evaporated onto colloidal-patterned substrates followed by colloidal lift-off (figure 5) generally results in prism-shaped pillars (Kuo et al. 2003). In this instance, etch parameters can be altered allowing the control of nanopillar arrays with less undercut producing alternative in-plane patterns. Ordered non-spherical polymeric nanostructures can also be achieved utilizing temperature-controlled sintering techniques, which produce a variety of ordered trigonal nanometric features in colloidal interstices following selective dissolution of polydivinyl benzene (PDVB)–PS nanoparticles (Yi & Kim 2003). Nanopillared topographies fabricated on a mechanically stable base substrate, e.g. fused silica, using these techniques, can be utilized during mechanical transfer, e.g. embossing or casting, resulting in inverse patterning of deformable polymers, e.g. polycaprolactone and polystyrene (figure 2; Wood et al. 2002b).
5. Colloidal nanotopographies currently utilized in biological applications
Colloidal-based fabrication techniques have proposed roles in nanostructured model biomaterial surfaces (Hanarp et al. 1999) and model nanotopographies (Wood et al. 2002a) due to their alterable and definable characteristics. Colloidal-patterned substrates have been utilized in a variety of capacities to date with respect to biological applications. Before discussing these, the reader's attention is drawn towards the interface processes associated with surfaces utilized in biological investigations, where, upon reaching a surface, cells are presented with an ionically screened and protein-coated substrate, which, in turn, has been influenced by the features, e.g. topography, of the material (Kasemo & Gold 1999). The effects of surface characteristics on the proteins interacting at this boundary will determine the effectiveness of cell interactions. Thus, when considering biological investigations relative to colloidal-based topographies, the interaction of proteins is paramount in our understanding of cell behaviour relative to the nanofeatured substrate.
Colloidal topographies have been used to investigate protein adsorption on nanopatterned surfaces (Sutherland et al. 2001; Denis et al. 2002a,b). Denis et al. (2002a,b) investigated the effects of surface chemistry (hydrophobic versus hydrophilic) and topography (‘rough’ 23 nm diameter colloids versus smooth substrata) on collagen adsorption. Chemistry was identified as the factor controlling protein adsorption levels, with larger adsorbed amounts and collagen aggregates noted on the hydrophobic smooth substrata. Similar amounts of protein were observed on the ‘rough’ colloidal substrata, however collagen molecules no longer formed aggregates on nanotopographies expressing hydrophobic surface chemistries. Thus, surface chemistry was found to control protein adsorption, while chemistry and topography control protein conformation. Denis et al. (2002a,b) proposed this effect was due to nanotopography and hydrophobicity inhibiting protein mobility. Sutherland et al. (2001) similarly investigated protein adsorption relative to colloidal-derived surfaces. In this instance, colloidal lithography was used as a mask to produce 40 nm diameter and 10 nm deep nanopits. Once again, the effects of surface chemistry (hydrophobic versus hydrophilic) and topography (nanopits versus planar) were investigated for their effects on fibrinogen adsorption and subsequent platelet adhesion. At early times (up to 10 min), the rate of platelet binding was controlled by surface topography, with fibrinogen-coated nanopits most adhesive regardless of surface chemistry. Sutherland et al. (2001) suggested this was due to alterations in protein orientation and/or confirmation resulting in specific binding sites being accessible to platelets, possibly as a result of increased mobility of fibrinogen on the nanotopography. In later phases (up to 1 h), surface chemistry was believed to play an increasing role in determining platelet binding, with a hydrophobic chemistry being the primary determinant, not fibrinogen. These studies indicate the ability to control protein confirmation and orientation at the implant interface using colloidal-derived nanotopographies, ultimately determining cell fate.
Cell-substrate adhesion establishment and composition is paramount to the effects of a surface on cell reactions and, ultimately, cell fate (Galbraith & Sheetz 1998). Focal adhesions are generally accepted as the anchorage points of the plasma membrane of cells to the substratum with which it is in contact (Geiger et al. 2001). Adhesions are composed of a number of proteins, including vinculin, where antibodies specifically raised against the protein in question can be used to identify the location and level of adhesion (Owen et al. 2005). Initial fibroblast adhesion is observed to be greater on substrates patterned with 20 or 50 nm diameter irregularly distributed colloidal gold particles following 20 min and 1 h of seeding in comparison to planar controls (Wood et al. 2006). In contrast, small vinculin accumulations are observed on 160 nm high cylindrical nanocolumns produced in poly(methyl methacrylate) (PMMA) using colloidal lithography, indicating reduced adhesion on this surface in comparison to fibroblasts on planar controls, believed to be a result of reduced surface area presented by the columnar features (Dalby et al. 2004b). However, in bladder epithelial cells (T24 cells), no difference was found in adhesion on 167 nm diameter, 100 nm high titanium pillars, determined by measuring total protein and cell number at 4 and 8 h post-seeding (Andersson et al. 2003c). These difference between cell adhesion on different nanofeatured substrates may occur as a result of cell type used, surface chemistry or nanotopography, although within the results of the individual studies, nanotopography appears to be the dependent variable responsible for observed cell behaviour.
The work by Spatz and co-workers (Arnold et al. 2004; Cavalcanti-Adam et al. 2006; Walter et al. 2006) has resulted in the development of novel substrates using block copolymer micelle nanolithography (Glass et al. 2003), where differences in colloid and base substrate chemistry are exploited. This technique allows the modification of surface properties, where the base substrate limits cell adhesion via PEG and colloidal regions are labelled with RGD, promoting cell attachment. Colloidal spacing, and thus areas expressing RGD-binding sites small enough to support a single integrin (≤8 nm), can be controlled. RGD-functionalized colloidal islands were found to support integrin clustering when pitched between 58 and 73 nm. Local dot-to-dot separation was believed to be most important for cell adhesion in comparison to the total number of dots within a given area. Furthermore, integrin, vinculin, focal adhesion kinase and actin were observed to co-localize on patterns with a pitch of less than 58 nm, indicating that RGD peptides at this spacing was capable of supporting the development of focal adhesions and their associated proteins (Arnold et al. 2004). Similarly, co-localization of vinculin and zyxin was observed on RGD-functionalized colloidal patterns with 58 nm spacing in comparison to surfaces with colloidal pitch of 110 nm (Cavalcanti-Adam et al. 2006). Cellular-unbinding forces using magnetic tweezers further substantiates these finding, where an increase in unbinding forces are observed for decreasing RGD-derived colloidal pitch from 145 to 58 nm 5 min after cell seeding. Furthermore, these results were observed to be time dependent with respect to RGD spacing of 58 and 69 nm (but not 93 and 110 nm), suggesting development of focal adhesions over this period (Walter et al. 2006). These findings may help in elucidating the adhesive mechanisms, specifically integrin spacing, involved in cellular response to topographies composed of nanofeatures with various dimensions and pitch, specifically in relation to work by Andersson et al. (2003a), where a significantly greater number of cells were found to align to continuous groove edges (of the sort produced by photolithography) in comparison to discontinuous groove edges (produced using photolithography and colloidal lithography composed of 107 nm diameter particles).
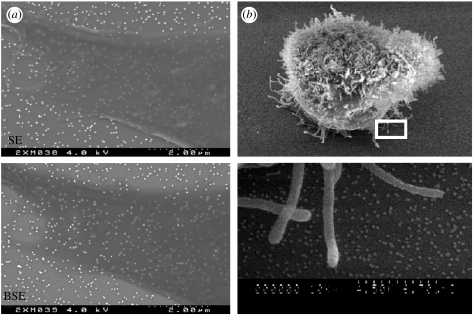
Nanotopographies composed of colloidal gold particles have also been utilized as a method of imaging nanofeatures underlying biological cells, allowing the interactions occurring at the basement membrane between cells and nanofeatures to be examined (figure 6a; Wood et al. 2002b, 2005). In brief, the complementary characteristics of secondary electron (SE) and backscattered electron (BSE) imaging are used to provide information on surface topography and atomic number of the materials, respectively. Through the selection of a colloidal material with a high atomic number (high contrast), in this instance gold (Z=79), colloids can be identified relative to overlying low-contrast biological materials, e.g. cells. Furthermore, BSE detection can be utilized as an electron optical-sectioning tool, allowing the information to be gathered from increasing depths within a sample by collecting images over a number of accelerating voltages. This is invaluable when imaging dense filamentous cytoskeletal structures relative to low-density cell membranes and high-density colloids allowing the interactions between fibrous projections and cell peripheries to be identified in relation to the underlying nanotopography (Wood et al. 2005).
Figure 6.
SEM imaging allows for interactions between cells and colloidal nanotopographies to be investigated. For example, complementary SE and BSE detection can be utilized to image colloidal nanotopographies underlying cells, (a), with imaging in SE mode allowing for direct interactions between the peripheral extensions of cells, for instance lamellapodia, and the surrounding colloids to be identified, (b). (Boxed area in upper half of (b) is shown at higher magnification in the lower half.)
A variety of different (cytoskeletal) morphologies have been observed on colloidal-derived topographies, with increased membrane protrusions and filopoodia or microspikes also noted (figure 6b; Andersson et al. 2003b; Dalby et al. 2004b; Wood et al. 2006). Increased frequency of cell–cell contacts have also been recorded on colloidal topographies in comparison to planar controls (Wood et al. 2006). Other alterations in cell behaviour include: (i) a reduction in pro-inflammatory cytokine IL-6 and chemokine IL-8 in uroepithelial cells recorded on 110 nm colloids coated with titanium, implying this nanotopography may be beneficial to implant coating due to suppressed immune response activators (Andersson et al. 2003b); (ii) significantly reduced nuclear area (but thicker nuclei) on nanocolumns produced in PMMA using a 110 nm diameter colloidal mask, with distance between chromosome 3 centromeres on the nanotopography being shorter for interphase cells (Dalby et al. 2004b). Furthermore, a number of shifts in gene expressions, predominantly repression of genes were also recorded on the colloidal-based topography (Dalby et al. 2004b). Dalby and co-workers suggest these observations may occur as a result of ‘self-induced’ mechanotransduction, where nanotopography alters nuclear morphology and chromosome positioning leading to alterations in the probability of gene transcription (Dalby 2005).
6. Nanopatterning of three-dimensional structures for biological applications
Tissue engineering is emerging as a forerunner in the realization of biologically functional implants, offering great potential in the future treatment and replacement of diseased and defective tissue. As a result, fabrication of three-dimensional scaffolds is constantly under review allowing for the optimization of tissue production (Boccaccini & Blaker 2005). Although a variety of factors are known to influence cell behaviour in a two-dimensional environment, including nanotopography produced using conventional techniques and colloidal lithography (§5), the move from two to three dimensions is not a transitional step, often proving extremely difficult. The majority of fabrication methods employed to produce nanotopographies on a planar substrate cannot be used to pattern a three-dimensional structure. For example, photo-, e-beam and X-ray lithography work by focusing particles in a single plane, thus true three-dimensional patterning is not possible using these methods. Replication technologies require the development of registration for multilevel fabrication (Xia et al. 1999), although two-dimensional structures can be sutured together providing three-dimensional tubes with controlled topography (Curtis et al. 2005). Polymer blends have also been used to pattern the interior of tubes, but in this instance with topographies comprising features with nanometric height (Berry et al. 2005). However, these technologies are unlikely to be capable of providing controlled topographical cues to cells seeded within three-dimensional scaffolds generally utilized in tissue engineering applications or structures used in implants, e.g. orthopaedic screws or plates.
An alternative approach to providing nanotopographical cues to cells in tissue engineering applications is to use nanometric building blocks to fabricate scaffolds. Nanofibrous scaffolds have been produced with synthetic and natural polymers using electrospinning, thermally induced phase separation and self-assembling technologies (Stevens & George 2005). Scaffolds produced by electrospinning can provide topographical cues to cells in the form of polymer nanofibres, are capable of mimicking the size and scale of natural collagen fibres, and can fabricate continuous fibres with controllable alignment (Nair et al. 2004). Similarly, phase separation can be used to provide scaffolds composed of fibrous collagen-like polymer (Yang et al. 2004). Self-assembly techniques are also proposed as a method of producing peptide-based scaffolds (Ryadnov & Woolfson 2003), and can be used to produce temperature-sensitive hydrogels with respect to polymer nanoparticles (Ramanan et al. 2006). These methods provide an alternative approach to providing nanotopographical cues to cells within an artificial three-dimensional environment. However, these techniques cannot simply be used to ‘add’ nanotopography to structures, thus existing scaffolds and implants could not readily be modified.
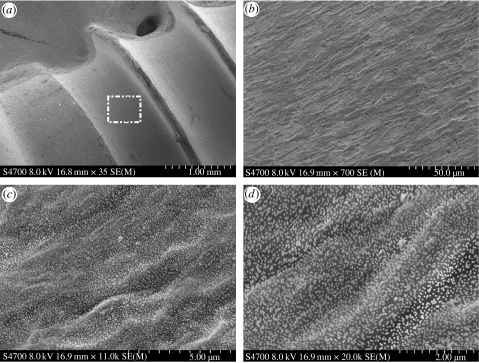
A dip-coating method of applying nanofeatures to existing structures would address this issue. By functionalizing a surface with poly-l-lysine and dip-coating it in a colloidal sol, we can impart nanotopography to a three-dimensional structure, in this instance, an orthopaedic titanium screw (figure 7). This method overcomes the previous constraints of applying nanotopography to three-dimensional devices utilizing a simple, accessible and inexpensive method. The characteristics of the colloids, including chemistry, size and shape, can be altered as discussed earlier, allowing a variety of nanotopographies to be fabricated. Furthermore, the dip-coating method results in colloidal coverage across the surface of the three-dimensional structure, where the resultant nanotopography follows the macro- and micro-contours of the original device. Colloids could act as a mask when modifying the topography using, e.g. a chemical etch. This technique could be modified to take into consideration temporal requirements of the surface, for example, biodegradable polymer colloids could be used to produce an initial nanotopography, which would eventually disappear exposing the device material. Drugs could also be delivered directly to cells at specific sites via modified colloids presented as a nanotopography.
Fig. 7.
Scanning electron micrographs (Hitachi S-4700) of titanium screw (TiO2) dip coated with 50 nm diameter gold colloids. At low magnification (×35), (a), the threads of the screw are visible. An area (white box in (a)) is investigated at sequentially increasing magnifications, (b), (c) and (d). At ×700, (b), the microtopography of the surface of the screw is prominent, but as the magnification is increased from ×11K, (c), to ×30K, (d), the colloidal nanotopography becomes visible. It should be noted that the colloids adhere to the pattern of the underlying titanium microtopography.
7. Conclusion
The versatility and simplicity of colloidal fabrication has resulted in its application in producing in-plane nanotopographies for biological research (Wood et al. 2002a,b, 2005, 2006; Andersson et al. 2003a,b; Dalby et al. 2004b). Properties of a nanopattern believed to affect cell behaviour, e.g. regularity and symmetry (Curtis et al. 2001, 2004), feature height (Dalby et al. 2004a), diameter (Wood et al. 2006) and pitch (Wilkinson et al. 2002), are easily controlled utilizing colloidal fabrication techniques. Colloids are readily available and do not generally require specialized techniques or equipment, proving accessible to biologists. Moreover, colloidal deposition can be controlled, resulting in patterning of specific areas of a substrate (Laibinis et al. 1992; Sato et al. 1996; He et al. 2000). As a result, cells on a control, planar and experimental nanotopography may be viewed simultaneously under constant culture conditions and their preferences and interactions at planar-nanopillared boundaries can be observed (Wood et al. 2002b).
Colloidal-derived nanotopographies currently under investigation in a biological context show great promise in both the control and our understanding of fundamental cell behaviour. The ability to increase or reduce cell adhesion relative to a given surface (Curtis et al. 2001; Arnold et al. 2004; Cavalcanti-Adam et al. 2006; Walter et al. 2006; Wood et al. 2006), alter gene regulation (Dalby et al. 2005) or inflammatory response (Andersson et al. 2003) through the use of colloidal patterning, indicates the potential of this method in controlling responses at the biological interface, specifically in relation to implants and scaffolds. Furthermore, through the selection of colloids with high atomic number, topographies (and filamentous cytoskeletal processes) underlying the cell body can be imaged relative to individual nanofeatures (Wood et al. 2002b; Wood et al. 2005). Although processes, e.g. dry etching and lift-off, are often employed to produce the final device, colloids can be used to produce surfaces without the requirement for specialist equipment, further supporting their relevance in the production of nanotopographies for biological applications.
Nanopatterning of functional medical devices, e.g. implants or tissue engineered constructs, although not yet truly realized, offers great potential in the future treatment or replacement of diseased or defective tissues (Curtis 2005). In vitro studies are emerging to support the use of colloids in clinical applications. For instance, amino acid-coated gold colloids have been successfully used as a template to produce quasi-spherical, self-assembled hydroxyapatite crystals (Rautaray et al. 2005), which could be utilized in bone tissue engineering applications and initial fibroblast adhesion is increased on 20 and 50 nm diameter colloids within the first hour of contact with the substrates in comparison to control, planar surfaces (Wood et al. 2006). Results emerging from such experiments indicate nanotopographies composed of colloids, or colloidal-based nanopatterning of substrates influence cell behaviour as a result of their size, shape, pitch and regularity, properties that can be selected in the final device. Nanofeature patterning of three-dimensional structures using a colloidal dip-coating technique will enable cells and tissues to be influenced and controlled topographically. Thus, colloids offer a real alternative to expensive, time-consuming, low-throughput conventional fabrication methods restricted to producing regular patterns, and can be employed to produce a wide variety of in-plane nanotopographies, with irregular or lightly determinate patterns, across the large areas required for biological and clinical applications.
Acknowledgments
This work was supported by the EPSRC, EU framework V grant QLK 3-CT-2000-01500 (NANOMED) and EU framework VI grant NMP4-CT-2003-505868 (NANOCUES). The author would like to thank Dr D. O. Meredith for his SEM expertise, Dr G. Richards, for his kind donation of orthopaedic screws, Dr G. Rh. Owen for his help with SE and BSE imaging and Profs A. S. G. Curtis, Dr M. O. Riehle and C. D. W. Wilkinson for their support and guidance over the years.
References
- Abrams G.A, Goodman S.L, Nealey P.F, Franco M, Murphy C.J. Nanoscale topography of the basement membrane underlying the corneal epithelium of the Rhesus Macaque. Cell Tissue Res. 2000;1:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- Agheli H. Nanofabrication of polymer surfaces utilising colloidal lithography and ion etching. IEEE Trans. Nanobiosci. 2006;5:9–14. doi: 10.1109/TNB.2005.864013. [DOI] [PubMed] [Google Scholar]
- Ahmadi T.S, Wang Z.L, Green T.C, Henglein A, El-Sayed M.A. Shape-controlled synthesis of colloidal platinum nanoparticles. Science. 1996;272:1924–1926. doi: 10.1126/science.272.5270.1924. [DOI] [PubMed] [Google Scholar]
- Aizenberg J, Black A.J, Whitesides G.M. Controlling local disorder in self-assembled monolayers by patterning the topography of their metallic supports. Nature. 1998;394:868–871. doi: 10.1038/29730. [DOI] [Google Scholar]
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain either actin or tubulin. Cell. 1977a;12:333–339. doi: 10.1016/0092-8674(77)90109-X. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells. Cell. 1977b;11:395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Alivisatos A.P. Semiconductor clusters, nanocrystals and quantum dots. Science. 1996;271:933–937. [Google Scholar]
- Andersson A.-S, Olsson P, Lidberg U, Sutherland D. The effects of continuous and discontinuous groove edges on cell shape and alignment. Exp. Cell Res. 2003a;288:177–188. doi: 10.1016/S0014-4827(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Andersson A.-S, Backhed F, von Euler A, Richter-Dahlfors A, Sutherland D, Kasemo B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials. 2003b;24:3427–3436. doi: 10.1016/S0142-9612(03)00208-4. [DOI] [PubMed] [Google Scholar]
- Andersson A.-S, Brink J, Lidberg U, Sutherland D.S. Influence of systematically varied nanoscale topography on the morphology of epithelial cells. IEEE Trans. Nanobiosci. 2003c;2:49–57. doi: 10.1109/tnb.2003.813934. [DOI] [PubMed] [Google Scholar]
- Arnold M, Cavalcanti-Adam E, Glass R, Blummel J, Eck W, Kantlehner M, Kessler H, Spatz J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chem. Phys. Chem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- Beattie D, Wong K.H, Williams C, Poole-Warren L.A, Davis T.P, Barner-Kowollik C, Stenzel M.H. Honeycomb-structured porous films from polypyrrole-containing block copolymers prepared via RAFT polymerization as a scaffold for cell growth. Biomacromolecules. 2006;7:1072–1082. doi: 10.1021/bm050858m. [DOI] [PubMed] [Google Scholar]
- Berry C.C, Charles S, Wells S, Dalby M.J, Curtis A.S.G. The influence of transferrin stabilised magnetic nanoparticles on human dermal fibroblasts in culture. Int. J. Pharm. 2004;269:211–225. doi: 10.1016/j.ijpharm.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Berry C.C, Dalby M.J, McCloy D, Affrossman S. The fibroblast response to tubes exhibiting internal nanotopography. Biomaterials. 2005;26:4985–4992. doi: 10.1016/j.biomaterials.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Boccaccini A.R, Blaker J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Dev. 2005;2:303–317. doi: 10.1586/17434440.2.3.303. [DOI] [PubMed] [Google Scholar]
- Brody S, Anilkumar T, Liliensiek S, Last J.A, Murphy C.J, Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12:413–421. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass T, Ligler F.S. Oxford University Press; Oxford, UK: 1998. Immobilised biomolecules in analysis: a practical approach. [Google Scholar]
- Cavalcanti-Adam E, Micoulet A, Blummel J, Aurenheimer J, Kessler H, Spatz J.P. Lateral spacing of integrin ligands influences cell spreading and focal adhesion assembly. Eur. J. Cell Biol. 2006;85:219–224. doi: 10.1016/j.ejcb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cerrina F, Marrian C. A path to nanolithography. Mat. Res. Soc. Bull. 1996:56–62. [Google Scholar]
- Chan G.Y, Hughes T.C, McLean K.M, McFarland G.A, Nguyen X, Wilkie J.S, Johnson G. Approaches to improving the biocompatibility of porous perfluoropolyethers for ophthalmic applications. Biomaterials. 2006;27(8):1287–1295. doi: 10.1016/j.biomaterials.2005.08.005. doi:10.10262j.biomaterials.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Cheng J.Y, Mayes A.M, Ross C.A. Nanostructure engineering by templated self-assembly of block copolymers. Nat. Mater. 2004;3:823–828. doi: 10.1038/nmat1211. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G. Small is beautiful but smaller is the aim: review of a life of research. Eur. Cells Mater. 2004;8:27–36. doi: 10.22203/ecm.v008a04. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G. The potential for the use of nanofeaturing in medical devices. Expert Rev. Med. Dev. 2005;2:293–301. doi: 10.1586/17434440.2.3.293. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Gallagher J.O, Pasqui D, Wood M.A, Wilkinson C.D.W. Substratum nanotechnology and the adhesion of biological cells. Are symmetry or regularity of nanotopography important? Biophys. Chem. 2001;94:275–283. doi: 10.1016/S0301-4622(01)00247-2. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Gadegaard N, Dalby M.J, Riehle M.O, Wilkinson C.D.W, Aitchison G. Cells react to nanoscale order and symmetry in their surroundings. IEEE TNS. 2004;3:61–65. doi: 10.1109/tnb.2004.824276. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Wilkinson C.D.W, Crossan J, Broadley J, Joha D.H, Jorgense K.K, Monaghan W. An in vivo microfabricated scaffold for tendon repair. Euro. Cells Mater. 2005;9:50–57. doi: 10.22203/ecm.v009a07. [DOI] [PubMed] [Google Scholar]
- Dalby M.J. Topographically induced direct cell mechanotransduction. Med. Eng. Phys. 2005;27:730–742. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Johnstone H.J, Affrossman S, Curtis A.S. Nonadhesive nanotopography: fibroblast response to poly(n-butyl methacrylate)-poly(styrene) demixed surface features. J. Biomed. Mater. Res. A. 2003;67:1025–1032. doi: 10.1002/jbm.a.10139. [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Pasqui D, Affrossman S. Cell response to nano-islands produced by polymer demixing: a brief review. IEE Proc. Nanobiotechnol. 2004a;151:53–61. doi: 10.1049/ip-nbt:20040534. [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Sutherland D.S, Agheli H, Curtis A.S.G. Use of nanotopography to study mechanotransduction in fibroblasts—methods and perspectives. Eur. J. Cell Biol. 2004b;83:159–169. doi: 10.1078/0171-9335-00369. [DOI] [PubMed] [Google Scholar]
- Dalby M.J, Riehle M.O, Sutherland D.S, Agheli H, Curtis A.S.G. Morphological and microarray analysis of human fibroblasts cultured on nanocolumns produced by colloidal lithography. Eur. Cells Mater. 2005;9:1–8. doi: 10.22203/ecm.v009a01. [DOI] [PubMed] [Google Scholar]
- Deckman H.W.D, Dunsmuir J.H. Natural lithography. Appl. Phys. Lett. 1982;41:377–379. doi: 10.1063/1.93501. [DOI] [Google Scholar]
- Deckman H, Dunsmuir J.H. Applications of surface textures produced with natural lithography. J. Vac. Sci. Technol. B. 1983;1:1109–1112. doi: 10.1116/1.582644. [DOI] [Google Scholar]
- Denis F.A, Hanarp P, Sutherland D.S, Dufrene Y.F. Fabrication of nanostructured polymer surfaces using colloidal lithography and spin-coating. Nano Lett. 2002a;2:1419–1425. doi: 10.1021/nl025750g. [DOI] [Google Scholar]
- Denis F.A, Hanarp P, Sutherland D.S, Gold J, Mustin C, Rouxhet P.G, Dufrene Y.F. Protein adsorption on model surfaces with controlled nanotopography and chemistry. Langmuir. 2002b;18:891–828. doi: 10.1021/la011011o. [DOI] [Google Scholar]
- Denis F.A, Hanarp P, Sutherland D.S, Dufrene Y.F. Nanoscale chemical patterns fabricated by using colloidal lithography and self-assembled monolayers. Langmuir. 2004;20:9335–9339. doi: 10.1021/la049188g. [DOI] [PubMed] [Google Scholar]
- Eastham D.A, Hamilton B, Denby P.M. Formation of ordered assemblies from deposited gold clusters'. Nanotechnology. 2002;13:51–54. doi: 10.1088/0957-4484/13/1/311. [DOI] [Google Scholar]
- Falconnet D, Csucs G, Grandin H.M, Textor M. Review: surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials. 2006;27:3044–3063. doi: 10.1016/j.biomaterials.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Fischer U.Ch, Zingsheim H.P. Submicroscopic pattern replication with visible light. J. Vac. Sci. Technol. 1981;9:881–885. doi: 10.1116/1.571227. [DOI] [Google Scholar]
- Flemming R.G, Murphy C.J, Abrams G.A, Goodman S.L, Nealey P.F. Effects of synthetic micro- and nano-structured surfaces on cell behaviour. Biomaterials. 1999;20:573–588. doi: 10.1016/S0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- Galbraith C.G, Sheetz M.P. Forces on adhesive contacts affect cell function. Curr. Opin. Cell Biol. 1998;10:556–571. doi: 10.1016/S0955-0674(98)80030-6. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada K.M. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Giersig M, Mulvaney P. Formation of ordered two-dimensional gold colloid lattices by electrophoretic deposition. J. Phys. Chem. 1993;97:6334–6336. doi: 10.1021/j100126a003. [DOI] [Google Scholar]
- Gittin D.I, Caruso F. Tailoring the polyelectrolyte coating of metal nanoparticles. J. Phys. Chem. B. 2001;105:6846–6852. doi: 10.1021/jp0111665. [DOI] [Google Scholar]
- Glass R, Moller M, Spatz J.P. Block copolymer micelle nanolithography. Nanotechnology. 2003;14:1153–1160. doi: 10.1088/0957-4484/14/10/314. [DOI] [Google Scholar]
- Gogolewski S. Introduction: editorial. Injury. 2002;33(Suppl. 2):1–5. doi: 10.1016/S0020-1383(02)00125-0. [DOI] [Google Scholar]
- Grabar K.C, Smith P.C, Musick M.D, Davis J.A, Walter D.G, Jackson M.A, Guthrie A.P, Natan M.J. Kinetic control of interparticle spacing in au colloid-based surfaces: rational nanometer-scale architecture. J. Am. Chem. Soc. 1996;118:1148–1153. doi: 10.1021/ja952233+. [DOI] [Google Scholar]
- Grego S, Jarvis T.W, Stoner B.R, Lewis J.S. Template-directed assembly on an ordered microsphere array. Langmuir. 2005;21:4971–4975. doi: 10.1021/la0468850. [DOI] [PubMed] [Google Scholar]
- Gu Y, Xie H, Gao J, Liu D, Williams C.T, Murphy C.J, Ploehn H.J. AFM characterization of dendrimer-stabilized platinum nanoparticles. Langmuir. 2005;21:3122–3131. doi: 10.1021/la047843e. [DOI] [PubMed] [Google Scholar]
- Han Y, Grier D.G. Configurational temperatures and interactions in charge-stabilized colloid. J. Chem. Phys. 2005;122:1–15. doi: 10.1063/1.1844351. [DOI] [PubMed] [Google Scholar]
- Hanarp P, Sutherland D.S, Gold J, Kasemo B. Nanostructured biomaterials surfaces prepared by colloidal lithography. Nanostruct. Mater. 1999;12:429–432. doi: 10.1016/S0965-9773(99)00151-8. [DOI] [Google Scholar]
- Hanarp P, Sutherland D.S, Gold J, Kasemo B. Control of nanoparticle film structure for colloidal lithography. Colloids Surf. A. 2003;214:23–36. doi: 10.1016/S0927-7757(02)00367-9. [DOI] [Google Scholar]
- He H.X, Zhang H, Li Q.G, Zhu T, Li S.F.Y, Liu Z.F. Fabrication of designed archietectures of Au nanoparticles on solid substrate with printed self-assembled monolayers as templates. Langmuir. 2000;16:3846–3851. doi: 10.1021/la991356v. [DOI] [Google Scholar]
- Hong Y.-K, Kim H, Lee G, Kim W, Park J.-I, Cheon J, Koo J.-Y. Controlled two-dimensional distribution of nanoparticles by spin-coating method. Appl. Phys. Lett. 2002;80:844–846. doi: 10.1063/1.1445811. [DOI] [Google Scholar]
- Huang S, Tsutsui G, Sakaue H, Shingubara S, Takahagi T. Experimental conditions for a highly ordered monolayer of gold nanoparticles fabricated by the Langmuir–Blodgett method. J. Vac. Sci. Technol. 2001;B19:2045–2049. [Google Scholar]
- Hulteen J.C, Van Duyne R.P. Nanosphere lithography: a materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Tech. A. 1995;13:1553–1558. doi: 10.1116/1.579726. [DOI] [Google Scholar]
- Hunter R.J. Foundations of colloidal science. vol. 1. Oxford Science Publications, Oxford University Press; Oxford, UK: 1987. [Google Scholar]
- Kasemo B, Gold J. Implant surfaces and interface processes. Adv. Dent. Res. 1999;13:8–20. doi: 10.1177/08959374990130011901. [DOI] [PubMed] [Google Scholar]
- Kim T.G, Park T.G. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(d-l-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221–233. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- Kim S.O, Solak H.H, Stoykovich M.P, Ferrier N.J, De Pablo J.J, Nealey P.F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature. 2003;424:411–414. doi: 10.1038/nature01775. [DOI] [PubMed] [Google Scholar]
- Kuo C.-W, Shiu J.-Y, Chen P. Size- and shape-controlled fabrication of large-area periodic nanopillar arrays. Chem. Mater. 2003;15:2917–2920. doi: 10.1021/cm0343249. [DOI] [Google Scholar]
- Laibinis P.E, Nuzzo R.G, Whitesides G.M. Structure of monolayers formed by coadsorption of two n-Alkanethiols of different chain lengths on gold and its relation to wetting. J. Phys. Chem. 1992;96:5097–5105. doi: 10.1021/j100191a065. [DOI] [Google Scholar]
- Laurent G, Felidj N, Lau Truong S, Aubard J, Levi G, Krenn J.R, Hohenau A, Leitner A, Aussenegg F.R. Imaging surface plasmon of gold nanoparticle arrays by far-field raman scattering. Nano Lett. 2005;5:253–258. doi: 10.1021/nl048234u. [DOI] [PubMed] [Google Scholar]
- Lee T, Liu J, Chen N-P, Andres R.P, Janes D.B, Reifenberger R. Electronic properties of metallic nanoclusters on semiconductor surfaces: implications for nanoelectronic device applications. J. Nanoparticle Res. 2000;2:345–362. doi: 10.1023/A:1010053303142. [DOI] [Google Scholar]
- Lee J.N, Park C, Whitesides G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- Lewis P.A, Ahmed H. Nanopillars formed with a colloidal gold etch mask. J. Vac. Sci. Tech. B. 1999;17:3239–3234. doi: 10.1116/1.590988. [DOI] [Google Scholar]
- Lewis P.A, Ahmed H, Sato T. Silicon nanopillars formed with colloidal particle masking. J. Vac. Sci. Tech. B. 1998;16:2938–2941. doi: 10.1116/1.590322. [DOI] [Google Scholar]
- Lumsdon S.O, Scott D.M. Assembly of colloidal particles into microwires using an alternating electric field. Langmuir. 2005;21:4874–4880. doi: 10.1021/la0472697. [DOI] [PubMed] [Google Scholar]
- Mathur N. Beyond the silicon roadmap. Nature. 2002;419:573–574. doi: 10.1038/419573a. [DOI] [PubMed] [Google Scholar]
- McCord M.A, Rooks M.J. Electron beam lithography. In: Rai-Choudhury P, editor. SPIE handbook of microlithography, micromachining and microfabrication. Microlithography. vol. 1. SPIE Publishing; Washington, DC: 1997. p. 768. [Google Scholar]
- Michel R, Reviakine I, Sutherland D, Fokas C, Csucs G, Danuser G, Spencer N.D, Textor M. A novel approach to produce biologically relevant chemical patterns at the nanometer scale: selective molecular assembly patterning combined with colloidal assembly. Langmuir. 2002;18:8580–8586. doi: 10.1021/la0258244. [DOI] [Google Scholar]
- Nair L.S, Bhattacharyya S, Laurencin C.T. Development of novel tissue engineering scaffolds via electrospinning. Exp. Opin. Biol. Ther. 2004;4:659–668. doi: 10.1517/14712598.4.5.659. [DOI] [PubMed] [Google Scholar]
- Ng V, Lee Y.V, Chen B.T, Adeyeye A.O. Nanostructure array fabrication with temperature-controlled self-assembly techniques. Nanotechnology. 2002;13:554–558. doi: 10.1088/0957-4484/13/5/302. [DOI] [Google Scholar]
- Niemeyer C.M, Ceyhan B, Gao S, Chi L, Peschel S, Simon U. Site-selective immobilisation of gold nanoparticles functionalised with DNA oligomers. Colloid Polym. Sci. 2001;279:68–72. doi: 10.1007/s003960000429. [DOI] [Google Scholar]
- Owen G.Rh, Meredith D.O, ap Gwynn I, Richards R.G. Focal adhesion quantification—a new assay of material biocompatibility? Review. Eur. Cells Mater. 2005;9:85–96. doi: 10.22203/ecm.v009a10. [DOI] [PubMed] [Google Scholar]
- Parak W.J, et al. Biological applications of colloidal nanocrystals: topical review. Nanotechnology. 2003;14:R15–R27. doi: 10.1088/0957-4484/14/7/201. [DOI] [Google Scholar]
- Park M.C, Chaikin P.M, Register R.A, Adamson D.H. Large area dense nanoscale patterning of arbitrary surfaces. Appl. Phys. Lett. 2001;79:257–259. doi: 10.1063/1.1378046. [DOI] [Google Scholar]
- Peschel S, Schmid G. First steps towards ordered monolayers of ligand-stabilised gold clusters. Angew. Chem. Int. Ed. Engl. 1995;34:1442–1443. doi: 10.1002/anie.199514421. [DOI] [Google Scholar]
- Pohl K, Bartelt M.C, de la Figuera J, Bartelt N.C, Hrbek J, Hwang R.Q. Identifying the forces responsible for self-organisation of nanostructures at crystal surfaces. Nature. 1999;397:238–241. doi: 10.1038/16667. [DOI] [Google Scholar]
- Qin K, Li Y. Mechanisms of particle removal from silicon wafer surface in wet chemical cleaning process. J. Colloid Interface Sci. 2003;261:569–574. doi: 10.1016/S0021-9797(03)00053-5. [DOI] [PubMed] [Google Scholar]
- Ramanan R.M, Chellamuthu P, Tang L, Nguyen K.T. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnol. Prog. 2006;22:118–125. doi: 10.1021/bp0501367. [DOI] [PubMed] [Google Scholar]
- Rautaray D, Mandal S, Sastry M. Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir. 2005;21:5185–5191. doi: 10.1021/la048541f. [DOI] [PubMed] [Google Scholar]
- Ray M.A, Kim H, Jia L. Dynamic self-assembly of polymer colloids to form linear patterns. Langmuir. 2005;21:4786–4789. doi: 10.1021/la050165r. [DOI] [PubMed] [Google Scholar]
- Reetz M.T, Winter M, Tesche B. Self-assembly of tetraalkylammonium salt-stabilised giant palladium clusters on surfaces. Chem. Commun. 1997:147–148. doi: 10.1039/a606490f. [DOI] [Google Scholar]
- Remacle F, Collier C.P, Markovich G, Heath J.R, Banin U, Levine R.D. Networks of quantum dots: the role of disorder in modifying electronic and optical properties. J. Phys. Chem. B. 1998;102:7727–7734. doi: 10.1021/jp9813948. [DOI] [Google Scholar]
- Riehle M, Dalby M, Johnstone H, Gallagher J, Wood M.A, Casey B, McGhee K. Nanometric surface patterns for tissue engineering: fabrication and biocompatibility in vitro. Mat. Res. Soc. Symp. Proc. 2002;705:Y5.1.1–Y5.1.11. [Google Scholar]
- Rolland J.P, Hagberg E.C, Denison G.M, Carter K.R, De Simone J.M. High-resolution soft lithography: enabling materials for nanotechnologies. Angew. Chem. Int. Ed. 2004;43:5796–5799. doi: 10.1002/anie.200461122. [DOI] [PubMed] [Google Scholar]
- Ryadnov M.G, Woolfson D.N. Engineering the morphology of a self-assembling protein fibre. Nat. Mater. 2003;2:329–332. doi: 10.1038/nmat885. [DOI] [PubMed] [Google Scholar]
- Sasatsu M, Onishi H, Machida Y. In vitro and in vivo characterization of nanoparticles made of MeO–PEG amine/PLA block copolymer and PLA. Int. J. Pharm. 2006;317:167–174. doi: 10.1016/j.ijpharm.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Sato T, Brown D, Johnson B.F.G. Nucleation and growth of nano-gold colloidal lattices. Chem. Commun. 1997a:1007–1008. [Google Scholar]
- Sato T, Hasko D.G, Ahmed H. Nanoscale colloidal particles: monolayer organisation and patterning. J. Vac. Sci. Tech. B. 1997b;15:45–48. doi: 10.1116/1.589253. [DOI] [Google Scholar]
- Schlotterbeck U, Aymonier C, Thomann R, Hofmeister H, Tromp M, Richtering W, Mecking S. Shape-selective synthesis of palladium nanoparticles stabilized by highly branched amphiphilic polymers. Adv. Funct. Mater. 2004;14:999–1004. doi: 10.1002/adfm.200400053. [DOI] [Google Scholar]
- Seeger K, Palmer R.E. Fabrication of Ordered Arrays of Silicon Nanopillars. J. Phys. D: Appl. Phys. 1999;32:129–132. doi: 10.1088/0022-3727/32/24/102. [DOI] [Google Scholar]
- Shipway A.N, Willner I. Nanoparticles as structural and functional units in surface-confined architectures. Chem. Commun. 2001:2035–2045. doi: 10.1039/b105164b. [DOI] [PubMed] [Google Scholar]
- Schulz H, et al. New polymer materials for nanoimprinting. J. Vac. Sci. Technol. B. 2000;18:1861–1865. doi: 10.1116/1.1305331. [DOI] [Google Scholar]
- Simamora P, Chern W. Poly-l-lactic acid: an overview. J. Drugs Dermatol. 2006;5:436–440. [PubMed] [Google Scholar]
- Sonvico F, Dubernet C, Colombo P, Couvreur P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr. Pharm. Des. 2005;11:2095–2105. doi: 10.2174/1381612054065738. [DOI] [PubMed] [Google Scholar]
- Stevens M.M, George J.H. Exploring and engineering the cell surface interface: review. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Sutherland D.S, Broberg M, Nygren H, Kasemo B. Influence of nanoscale surface topography and chemistry on the functional behaviour of an adsorbed model macromolecule. Macromol. Biosci. 2001;1:270–273. doi: 10.1002/1616-5195(20010801)1:6%3C270::AID-MABI270%3E3.0.CO;2-3. [DOI] [Google Scholar]
- Tsutsui K, Hu E.L, Wilkinson C.D.W. Controlling the profile of nanostructures. J. Vac. Sci. Tech. B. 1993;11:2233–2236. doi: 10.1116/1.586463. [DOI] [Google Scholar]
- Turner S.K, Isaacson M, Craighead H.G, Shain W, Turner J. Cell attachment to silicon nanostructures. J. Vac. Sci. Tech. B. 1997;15:2848–2854. doi: 10.1116/1.589742. [DOI] [Google Scholar]
- Vanmaekelbergh D, Liljeroth P. Electron-conducting quantum dot solids: novel materials based on colloidal semiconductor nanocrystals. Chem. Soc. Rev. 2005;34:299–312. doi: 10.1039/b314945p. [DOI] [PubMed] [Google Scholar]
- Walter N, Selhuber C, Kessler H, Spatz J. Cellular unbinding forces of initial adhesion processes on nanopatterned surfaces probed with magnetic tweezers. Nano Lett. 2006;6:398–402. doi: 10.1021/nl052168u. [DOI] [PubMed] [Google Scholar]
- Wilkinson C.D.W. Making structures for cell engineering. Eur. Cells Mater. 2004;8:21–26. doi: 10.22203/ecm.v008a03. [DOI] [PubMed] [Google Scholar]
- Wilkinson C.D.W, Rahman M. Dry etching and sputtering. Phil. Trans. R. Soc. A. 2004;362:125–138. doi: 10.1098/rsta.2003.1307. [DOI] [PubMed] [Google Scholar]
- Wilkinson C.D.W, Curtis A.S.G, Crossan J. Nanofabrication in cellular engineering. J. Vac. Sci. Technol. B. 1998;16:3132–3136. doi: 10.1116/1.590451. [DOI] [Google Scholar]
- Wilkinson C.D.W, Riehle M.O, Wood M.A, Gallagher J.O, Curtis A.S.G. The use of materials patterned on a nano- and micro-metric scale in cellular engineering. Mater. Sci. Eng. C. 2002;19:263–269. doi: 10.1016/S0928-4931(01)00396-4. [DOI] [Google Scholar]
- Willner I, Shipway A.N, Katz E. Nanoparticle arrays on surfaces for electronic, optical and sensor applications. Chem. Phys. Chem. 2000;1:18–52. doi: 10.1002/1439-7641(20000804)1:1<18::AID-CPHC18>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wood M.A, Meredith D.O, Owen G.Rh. Steps towards a model nanotopography. IEEE Trans. Nanobiosci. 2002a;1:133–140. doi: 10.1109/TNB.2003.809460. [DOI] [PubMed] [Google Scholar]
- Wood M.A, Riehle M.O, Wilkinson C.D.W. Patterning colloidal nanotopographies. Nanotechnology. 2002b;13:605–609. doi: 10.1088/0957-4484/13/5/312. [DOI] [Google Scholar]
- Wood M.A, Meredith D.O, Owen G.Rh, Richards R.G, Riehle M.O. Utilising atomic number contrast for FESEM imaging of colloidal nanotopography underlying biological cells. Nanotechnology. 2005;16:1433–1439. doi: 10.1088/0957-4484/16/9/002. [DOI] [Google Scholar]
- Wood M.A, Wilkinson C.D.W, Curtis A.S.G. The effects of colloidal nanotopography on initial fibroblast adhesion and morphology. IEEE Trans. Nanobiosci. 2006;5:1. doi: 10.1109/TNB.2005.864015. [DOI] [PubMed] [Google Scholar]
- Xia Y, Whitesides G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998;28:153–184. doi: 10.1146/annurev.matsci.28.1.153. [DOI] [Google Scholar]
- Xia Y, Rogers J.A, Paul K.E, Whitesides G.M. Unconventional methods for fabricating and patterning nanostructures. Chem. Rev. 1999;99:1823–1848. doi: 10.1021/cr980002q. [DOI] [PubMed] [Google Scholar]
- Yang F, Murugan R, Ramakrishna S, Wang X, Ma Y.-X, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Yang Z, Huck W.T, Clarke S.M, Tajbakhsh A.R, Terentjev E.M. Shape-memory nanoparticles from inherently non-spherical polymer colloids. Nat. Mater. 2005;4:486–490. doi: 10.1038/nmat1389. [DOI] [PubMed] [Google Scholar]
- Yi D.K, Kim D.-Y. Polymer nanosphere lithography: fabrication of an ordered trigonal polymeric nanostructure. Chem. Commun. 2003:982–983. doi: 10.1039/b300638g. [DOI] [PubMed] [Google Scholar]
- Zhao X.-M, Xia Y, Whitesides G.M. Soft lithographic methods for nano-fabrication. J. Mater. Chem. 1997;7:1069–1074. doi: 10.1039/a700145b. [DOI] [Google Scholar]
- Zhong Z, Gates B, Xia Y, Qin D. Soft lithographic approach to the fabrication of highly ordered 2D arrays of magnetic nanoparticles on the surface of silicon substrates. Langmuir. 2000;16:10 369–10 375. doi: 10.1021/la001211k. [DOI] [Google Scholar]