Abstract
Many spatial patterns observed in nature emerge from local processes and their interactions with the local environment. The clustering of objects by social insects represents such a pattern formation process that can be observed at both the individual and the collective level. In this paper, we study the interaction between air currents and clustering behaviour in order to address the coordinating mechanisms at the individual level that underlie the spatial pattern formation process in a heterogeneous environment. We choose the corpse clustering behaviour of the ant Messor sanctus as an experimental paradigm. In a specifically designed experimental set-up with a well-controlled laminar air flow (approx. 1 cm s−1), we first quantify the modulation of the individual corpse aggregation behaviour as a function of corpse density, air flow intensity and the ant's position with respect to corpse piles and air flow direction. We then explore by numerical simulation how the forming corpse piles modify the laminar air flow around them and link this result with the individual behaviour modulation. Finally, we demonstrate on the collective level that this laminar air flow leads to an elongation and a slow displacement of the formed corpse piles in the direction of the air current. Both the individual behaviour modulated by air flow and the air flow modulated by the forming corpse piles can explain the pile patterns observed on the collective level as a stigmergic process. We discuss the generality of this coordinating mechanism to explain the clustering phenomena in heterogeneous environments reported in the literature.
Keywords: clustering, Messor sanctus, morphogenesis, interaction with environment, wind sensitivity
1. Introduction
The challenge in the understanding of spatial patterns, on the organismal as well as on the population level (Maynard Smith 1998; Camazine et al. 2001), is the identification of the underlying mechanisms that let molecules, cells or individuals form particular patterns that often fulfil a very specific function. Experimental studies that address these questions usually work in homogeneous environments (constant external factors, see Camazine et al. (2001) for examples), but the coordinating mechanisms may interact with environmental heterogeneities in order to allow emergence of new structures adapted to this environment. The effect of environmental factors on the coordinating mechanisms should therefore be addressed specifically. Social insects are particularly well suited for such studies because both levels of description, the individual level where the interactions between the individuals and their local environment occur, and the collective level where the pattern shows up, are open to observation and quantification. This is particularly true for the clustering of objects into spatial patterns. Social insects are known to aggregate many kinds of objects, e.g. brood (Franks et al. 1992), seeds (Hölldobler & Wilson 1990; Gorb & Gorb 2000), sand pellets (Franks & Deneubourg 1997) or corpses of their dead conspecifics (Howard & Tschinkel 1976; Ataya & Lenoir 1984). In the presence of environmental heterogeneities, the clusters may form at a specific temperature (Ceusters 1986; Roces 1995; Bollazzi & Roces 2003), a particular relative humidity (Cerdan 1989; Tschinkel 1999; Roces & Kleineidam 2000) or low air currents (Scholes et al. 2006). While such performances have been well documented at the collective level, only a few studies have addressed the questions of the underlying mechanisms at the individual level (Franks & Deneubourg 1997; Theraulaz et al. 2002; Challet et al. 2005b). In particular, there is no study on the effects of low-speed air currents on individual behaviour though such air currents represent a key stimulus involved in the maintenance of optimal nest conditions (gas exchange, humidity, temperature). Here, we will first create an experimental set-up that allows us to study the effects of a well-controlled low-speed air flow on object clustering behaviour and then disentangle the coordinating mechanisms at the individual level to explain the patterns observed at the collective level. Numerical simulations based on the physics of fluid mechanics will be of crucial help in both tasks.
The phenomenon of corpse clustering by the ant Messor sanctus was chosen as the experimental paradigm because the clustering process itself is easily observable at both the individual and the collective level, and the pattern formation process in a homogeneous environment (based on the concept of local activation and substrate depletion, Gierer & Meinhardt 1972) is already well understood (Theraulaz et al. 2002). Like many other ants, M. sanctus has a tendency to pick up corpses at low local corpse densities (i.e. the number of corpses within the ant's detection radius of ca 1 cm) and to deposit them at places of high local density (Deneubourg et al. 1991; Chrétien 1996; Theraulaz et al. 2002). If one starts with randomly distributed corpses, this local amplification leads first to the formation of many small corpse piles. Ants then preferentially pick up corpses from small piles and deposit them on large piles. This competition among piles leads to the formation of a few large piles that persist at steady state. We will add a controlled laminar air flow (with speeds ranging from 1 to 6 cm s−1) to this experimental system to investigate how it modifies the individual ant clustering behaviour and the resulting shape of corpse piles. The main objective is to gain a detailed understanding of this interaction between an object clustering process and an environmental factor as a lead to further disentangle the role of air currents in general nest organization.
2. Material and methods
2.1 The ant Messor sanctus
Experiments were performed with a colony of the Mediterranean seed-harvesting ant M. sanctus Emery, Myrmicinae (often grammatically incorrectly called M. sancta). This species has an intermediate polymorphism with body length from 3 to 9 mm. Messor sanctus colonies can reach several thousands individuals. We worked with a queenright colony collected near Narbonne (Languedoc-Roussillon, France). The colony (n≈2000 ants) was maintained under 12 h : 12 h (light : dark) conditions in a 40×30 cm plastic box coated with Fluon to prevent ants from escaping. Ants were fed ad libitum with water, sugar solution and seeds. Twice a week (and a couple of hours before each experiment) they were given maggots and a solid mixture of carbohydrates, vitamins and eggs (Bhatkar & Whitcomb 1970).
Detection of air movement or wind by insects is generally attributed to mechanosensory organs situated on antennae, facial hair plates or cerci (Chapman 1982). There are many reports on anemotactic orientation in insects (Fraenkel & Gunn 1961; Schöne 1984), but the underlying mechanisms are only known for a couple of species: crickets detect wind with their cerci (Ogawa et al. 2001), locusts with antennae and cephalic hairplates (Arbas 1986), while cockroaches use either cerci or antennae (Dagan & Volman 1982; Oswalt et al. 1997). Very little is known about wind detection in ants. It is commonly assumed that they use their antennae (Wehner & Duelli 1971; Wolf & Wehner 2000; Webb et al. 2004).
2.2 The experimental set-up
Creating a stable laminar low-speed air flow is not a simple task (Bejan 1995). One could use forced wind systems such as air tunnels (as used in Bruinsma 1979), but these would be rather large and they would have to be open. It would be impossible to constrain the ants to a particular area; any wall would perturb the laminar air flow. Therefore, we choose to use convective air currents, which have the advantage that ants can be constrained inside a chamber and air flow can be controlled entirely by the outside temperature conditions.
Corpse clustering occurred in a closed chamber (figure 1a) of size 35×35×30 cm (length, width, height). The ant colony was placed beneath the chamber to which ants had access by climbing along a wooden stick and through a hole (diameter 10 mm) in the centre of the chamber floor (subsequently called the arena).
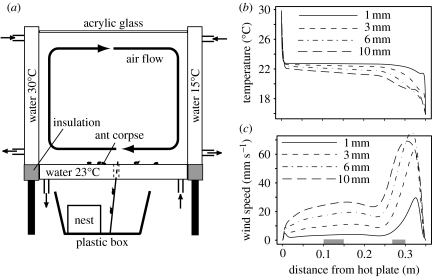
Figure 1.
Experimental set-up and its properties. (a) The experimental set-up used to create a stable laminar air flow; the small arrows indicate the water flow to and from the thermostat or thermokryostat to control temperature in walls and floor. (b) Simulated profile of the temperature and (c) of the horizontal wind speed at different heights above the floor in the experiments with air currents. The x-values represent the distance to the hot plate (on the left). Positive wind speed refers to wind from the right to the left. Ant antenna height is around 3–5 mm. The grey bars indicate the zones where corpse piles were placed for the individual behaviour experiments.
Since the displacement and clustering behaviours of ants are sensitive to temperature (Challet et al. 2005b), we had to control it precisely inside the chamber. The temperature of the floor plate and of each of the four wall plates of the chamber was controlled separately with circulating water coming from a thermostat or a thermokryostat (the latter is equipped with both heating and cooling elements to maintain water temperature at a stable level below ambient temperature). The temperature was monitored every 5 min by 20 thermocouples (eight for the floor plate and three for each wall plate) linked to a data acquisition unit and a computer (HP-BenchLink Data Logger). The relative humidity was maintained at 40–50%. In the experiments without air currents, all the five plates were maintained at 25°C and the room air temperature was stabilized 1–2°C above this level.
In the experiments with a laminar air flow, we kept one side of the chamber at high temperature (30°C) while the opposite side was kept at low temperature (15°C). To control the ant's working temperature, we kept the floor plate (and the other two wall plates) at 23°C (and room temperature at approx. 24°C).
Injecting some smoke into the chamber showed that there is indeed a laminar flow along the floor, but precise measurement of the inside wind and temperature conditions is a difficult task (such low air speeds require optical methods and any introduced thermocouple to measure temperature would modify the air flow). Fortunately, our set-up's Rayleigh number (RaH≈38×106) is well below critical values (RaH≈109) to ensure a laminar flow. The theory of laminar flows is sufficiently well understood to assess temperature and wind conditions inside the chamber by numerical simulations (Bejan 1995). These simulations (using the Fluent software) are based on the well-known physics of convective heat transfer (Navier–Stokes and energy conservation equations, the buoyancy effects associated with air compressibility are represented according to the Boussinesq approximation, see Burmeister 1983). These simulations show that the experimental conditions create a stable laminar air flow of 1–2 cm s−1 at the height of ant antennae (3–5 mm) in about three quarters of the arena (from x=0.01 to 0.25 m in figure 1c), while air flow at this height peaks at 6 cm s−1 close to the cold plate (right side of figure 1c). Such wind speeds may occur inside ant nests (Kleineidam et al. 2001). The simulations also show that ants experience temperatures ranging between 21 and 23°C in ca 90% of the arena, except close to the cold plate where temperature drops to 19°C (figure 1b). In Challet et al. (2005b), behavioural changes could only be detected for higher temperature differences. Thus, we can be quite confident that any change in the aggregation dynamics can be attributed specifically to the presence or absence of air currents.
2.3 Individual corpse clustering behaviour
As mentioned earlier, two wind conditions at ant antenna height (3 mm) can be identified (figure 1c); a large zone towards the hot plate with a stable laminar air flow of 1 cm s−1 (subsequently called the ‘1 cm s−1’ condition) and a smaller zone towards the cold plate where horizontal wind speed ranges from 3 to 6 cm s−1 (‘more than 3 cm s−1’ condition). We took advantage of this heterogeneity in order to study the probabilities to pick up or to drop a corpse at two different wind speeds. Single corpses or corpse piles were placed either at a distance of 5–8 cm from the cold plate (more than 3 cm s−1) or 10–15 cm from the hot plate (1 cm s−1), see the grey bars in figure 1c.
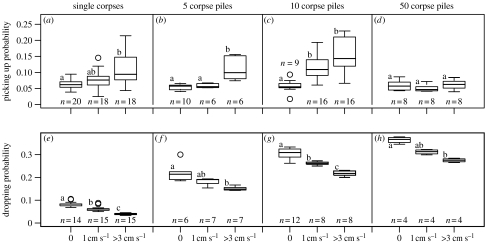
A first series of experiments was designed to estimate the probabilities to pick up a corpse. We arranged corpse piles in both zones (density≈18 cm−2) along a virtual line perpendicular to the wind direction. On each line, we put either six isolated corpses, 14 piles of size 5, 6 piles of size 10 or 3 piles of size 50. Spacing ensures that ants cannot touch and detect two piles at a time and the pile sizes cover a sufficiently wide range of local densities to detect its effect on corpse handling. Approximately 20 ants were then given access to the arena while filming continuously. For each pile, we counted the total number of physical contacts with a roaming ant (one physical contact represents the whole time from the first contact until the pile is out of the ant's detection radius) and the number of such contacts that ended in a corpse being picked up and carried away. Counting on piles of size 1, 5 and 10 was stopped after a picking up event occurred (owing to the pile size change). Experiments took 15–45 min and were repeated at least four times until there were at least 100 picking up events. Each experiment gave an independent estimate of the picking up probability (number of picking up events divided by total number of contacts).
The second series of experiments to estimate the probabilities to drop a corpse followed exactly the same protocol concerning the arrangement of piles and the counting of events, but additional isolated corpses were freely dispersed in the arena to entice ants to pick them up and to drop them on the piles in the two zones. In addition, ants dropped the corpse sometimes spontaneously without any contact with a pile. These events were analysed by measuring the time between the picking up of the corpse and its spontaneous dropping. The survival curve of these times (Haccou & Meelis 1992) was then tested against an exponential distribution. An exponential distribution is the signature of a Markov process, the dropping rate can thus be considered constant in time and be estimated as the slope of this survivor function on log-linear scale, while the mean transport time is the inverse of this slope. The standard errors were estimated by a non-parametric bootstrap (Efron & Tibshirani 1993). Since transporting ants moved freely around in the arena before dropping the corpse, we could not attribute these events to either the 1 cm s−1 or more than 3 cm s−1 condition, these transport times were thus only analysed for a ‘no-wind’ and a ‘with-wind’ condition.
2.4 Effect of corpse piles on the laminar flow
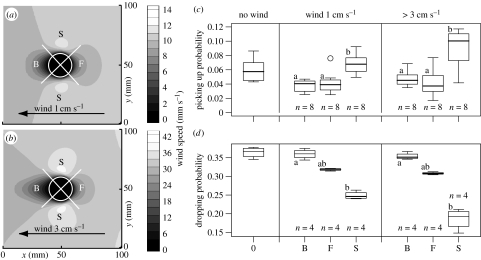
Even small corpse piles modulate the laminar air flow and lead to a considerable variation of the wind speed at ant antenna height (3 mm) around the pile. This might affect corpse-handling behaviour. To visualize how the air flow is modulated by a pile with 50 corpses (20 mm diameter, 4 mm height), we performed simulations by the lattice Boltzmann method (Succi 2001; figure 3a,b). The air flow at antenna height (3 mm) is distinctly accelerated along the pile sides, while there is a rather large zone of low wind speed on the leeside of the pile and a smaller one on the front side. Therefore, we divided the observation zone in the experiments with 50 corpses per pile into four parts, one facing the wind (F), one on the leeside (B) and two on the sides (S; figure 3a,b). Picking up and dropping events were counted separately for each zone and the two wind conditions (1 cm s−1 and more than 3 cm s−1). The picking up or dropping probability was again computed for each experiment and treated as an independent estimate.
Figure 3.
Effect of corpse piles on air flow and clustering behaviour. (a,b) Numerical simulations of the modulation of wind speed at ant antenna height (3 mm) around a corpse pile with diameter 2 cm (white circle) and height 4 mm at a steady laminar flow of 1 cm s−1 (a) and 3 cm s−1 (b). The white crosses delimit the zones (F, front; B, back; S, sides) where picking up (subgraph c) and dropping probabilities (subgraph d) on a real corpse pile were estimated. Statistical results are reported as in figure 2 with letters identifying statistical populations for a given wind condition. The Kruskal–Wallis tests (from c to d, df=2) were (Χ2,p)=(12.7, 0.002); (11.6, 0.003); (9.85, 0.007) and (9.85, 0.007).
2.5 Statistical analysis
To compare the estimated probabilities between the different wind conditions (for each pile size separately) or between event positions on the same pile (for a given wind condition), we use a non-parametric Kruskal–Wallis test (followed by a Nemenyi–Dunn post hoc test, Zar 1999) because the samples were either small or there was a significant heteroscedasticity. Significance level is the usual, α=0.05. All the computations were done with the R statistical software (R Development Core Team 2003).
2.6 Pattern formation on the collective level
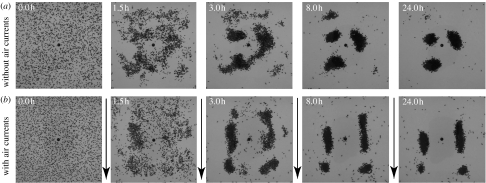
In a third series of experiments, we recorded the overall spatio-temporal dynamics of corpse clustering without and with air currents. Before giving access to the ants, 3270 ant corpses (2.66 corpses cm−2, which is well above the critical density below which no clustering occurs, Theraulaz et al. 2002) were spread as randomly as possible in the arena (figure 4). The ants were then given free access during 24 h and the aggregation process was filmed for 2 s every 10 min through the acrylic glass covering the chamber (Sony digital camera DCR-VX2000E). Nine experiments were made without air currents and 14 experiments with air currents.
Figure 4.
Spatio-temporal dynamics. Typical spatio-temporal dynamics of corpse clustering by ants filmed from the top through the acrylic glass covering the experimental chamber, without (a) and with (b) air currents. Black dots are ant corpses and black arrows indicate the air flow direction.
In the experiments with air currents, there could be a behavioural response to the presence of a hot or cold plate. To ensure that the effects detected were indeed only due to the air currents, we also made a control experiment (four replicates) with the same temperature settings as in the experiments with air currents, but where we lowered the acrylic glass to a height of 3 cm, thus practically stopping convective air currents (confirmed by numerical simulations) while maintaining the same temperature conditions as in the air current experiments.
2.7 Image and data analysis to characterize pattern dynamics
To quantify the spatio-temporal dynamics of the aggregation process, we first analysed a snapshot of the arena (see figure 4 for examples) every 30 min to determine: (i) the number of aggregates; (ii) the surface of the largest aggregate; and (iii) the total surface of aggregates. To identify these aggregates, we proceeded in four steps, using standard smoothing techniques to filter noise in the digital images. First, every pixel below a specific grey level was considered to belong to an aggregate and labelled ‘black’, the others were labelled ‘white’. The threshold grey level was calibrated by eye, comparing the filmed images to the identified clusters. Second, white pixels with more than 75% black neighbours were considered to be black (fusion). Third, black pixels with more than 75% white neighbours were changed to white (erosion). Fourth, the individual aggregates were computed by identifying all the black pixels that can be connected together by passing only through black pixels. The initial random distribution of corpses inevitably created some small aggregates. Thus, we only considered aggregates larger than 87 pixels (which corresponds to an aggregate of approx. 5 corpses) in order to exclude those formed during the random distribution of corpses and those pixels corresponding to moving live ants.
When quantifying the effects of an air current on pile form and position, we selected only the piles that had no contact with arena walls at the end of the experiment in order to avoid any influence of arena walls (figure 4, see Challet et al. (2005a) for the thigmotactic behaviour of M. sanctus). We measured the length (extension in the direction of the air current, y-direction) and the width (extension perpendicular to the air current, x-direction) and calculated the centroid of each pile. These piles were then traced backwards in time using the following procedure: (i) we first located the closest pile centroid 30 min earlier and (ii) if this centroid was larger than 87 pixels and less than 10 pixels (approx. 7 mm) distant from the current centroid then it was considered to represent the same pile 30 min earlier, otherwise the current time was marked as the ‘birth time’ of the pile. This backward tracing was continued until the birth times of all piles were found. We then set all birth times to 0 (what we call relative pile lifetime) and analysed the spatio-temporal evolution of the mean net displacement of the pile's centroid in the direction of the air current (y-coordinate) and perpendicular to it (x-coordinate). Finally, we analysed the influence of air currents on the temporal evolution of the form of the corpse piles by computing the mean ratio of pile length (in the air current direction) to pile width. These ratios were also computed with respect to the relative pile lifetime.
3. Results
3.1 Individual picking up and dropping probabilities
Figure 2 summarizes the individual picking up and dropping probabilities for all conditions (pile size and wind condition). First of all, in the no-wind condition, corpse pile size had the same effect as that already found in Theraulaz et al. (2002) and Challet et al. (2005b): the probability to pick up a corpse from a pile (roughly estimated as the global pile probability divided by the pile size) decreases with pile size while the probability to drop a corpse on a pile increases. Increasing wind speed has a significant effect on both picking up behaviour (probabilities increase except for pile size 50) and on dropping behaviour (probabilities decrease). Messor sanctus ants seem to have a tendency to clear up areas of high wind speed.
Figure 2.
Clustering behaviour as a function of pile size and wind speed. The probability to pick up a corpse from a pile (a–d) and to drop a corpse on a pile (e–h). Each subgraph groups these probabilities for the three different wind conditions (0, no wind; 1 cm s−1, low wind and more than 3 cm s−1, high wind) as a function of pile size (1, 5, 10 or 50). The Kruskal–Wallis tests to detect the effect of wind speed (from a to h, df=2) were: (Χ2,p)=(16.3, <10−3); (12.6, 0.008); (20.9, <10−3); (3.5, 0.178); (35.3, <10−3); (13.0, 0.0015); (22.1, <10−3) and (9.9, 0.007). The statistical populations (found by the Nemenyi–Dunn post hoc test for each pile size) are identified by letters (a–c) and n indicates the sample sizes (number of experiments).
The times before spontaneously dropping a corpse are distributed exponentially in both the no-wind and the with-wind conditions (straight survival curves on log-linear scale, not shown here for space reasons). The mean transport time before a spontaneous dropping increases significantly from 88.3±13.9 s (no-wind) to 127.0±14.0 s (with-wind, Kruskal–Wallis test, Χ12=13.0, df=1, p<10−3). The spontaneous dropping rate (the inverse of the mean transport time) is therefore lower in the with-wind condition than in the no-wind condition (thus the same tendency as for the dropping on corpse piles).
The position around the pile also affects the corpse clustering behaviours (figure 3c,d). The picking up probability is, in both wind conditions, significantly higher on the sides of the pile (S) compared with the front side (F) and leeside (B), but there is no difference between the latter two. The dropping probability shows the opposite tendency: it is significantly lower on the sides of the pile, and this time there is also a significant difference between F and B, the latter being higher. Looking in detail at the wind speeds around the pile (figure 3a,b), we note that high wind speeds are correlated with high picking-up rates and low dropping rates (S), while the opposite occurs at low wind speeds (F and B). Thus, we observe the same pattern as in figure 2, amplification is stronger at low wind speeds.
3.2 Spatio-temporal clustering dynamics
Immediately after placing the wooden stick, ants started climbing into the arena, reaching a stationary number within the first hour. This number is lognormally distributed (11.6×/2.1, i.e. the median times or divided by the multiplicative standard deviation, giving a 95% confidence interval of (2.8, 47.7) ants, see Limpert et al. (2001) for this astute notation that merits wider knowledge).
Typical corpse clustering dynamics with and without air currents are shown in figure 4. Within the first couple of hours, the ants have aggregated the corpses on a few piles. These piles continue to change their shape, becoming more compact and dense. A pile may even disappear by the end of the experiment when its corpses have been transported to other piles (this illustrates the competition between piles).
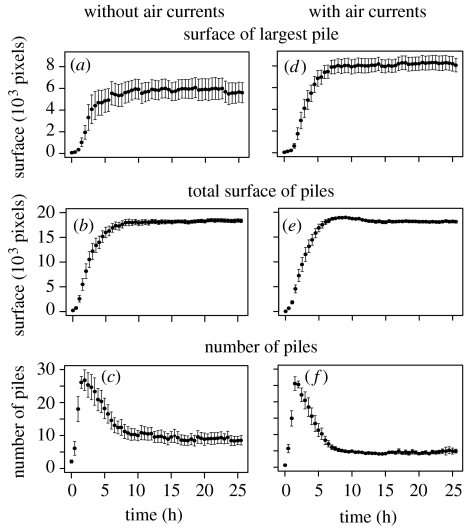
The global statistics of the aggregation dynamics are shown in figure 5. We first note that there are no qualitative differences between the experiments with and without air currents. The slightly higher standard errors in experiments without air currents are due to the lower replication number (9 compared with 14 with air currents). The temporal evolution of the size of the largest pile and the total surface of piles show distinct sigmoid shapes. The number of piles first grows rapidly and then slowly decreases to reach a steady state after 12 h. At that time, 5–8 piles subsisted as a result of competition among the piles, with some of them disappearing while others grew in size. There are slightly fewer, but larger, piles in the with-wind condition, indicating that wind enhances this competition between piles.
Figure 5.
Dynamics of the clustering process. Global statistics (mean±s.e.) of the temporal dynamics of corpse clustering without (left, n=9 experiments) and with air currents (right, n=14 experiments): surface (in pixel) of the largest pile (a, d), total surface of all piles (b, e) and number of piles (c, f).
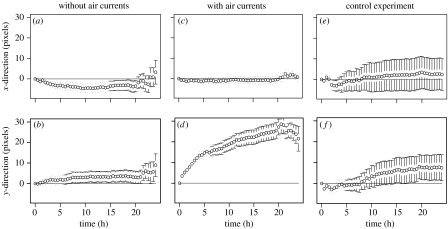
The movement of the piles is strongly influenced by the presence of air currents: while there is no significant displacement of the centroid without air currents in either the x- or y-direction (figure 6a,b) or with air currents perpendicular to the flow direction (figure 6c), there is a significant displacement of the centroid in the direction of the air flow (figure 6d) corresponding to 25 pixels d−1 (approx. 19 mm d−1). In the control experiment with lowered acrylic glass (figure 6e,f), there was no significant displacement in either direction. Therefore, even if ant behaviour is influenced by the presence of a hot and cold plate, pile displacement must be attributed to the presence of the air current. An additional experiment with air currents, but where ants were not given access to the arena, showed no corpse movement at all. The detected displacement is therefore due to the ants' activity.
Figure 6.
Pile displacement. Net displacement of the pile centres (centroids, mean±s.e.) in the direction of the air flow (y, bottom row) and perpendicular to it (x, top row): without air currents (a, b, n=22 piles), with air currents (c, d, n=29) and with air currents in the control experiment (lowered acrylic glass, e, f, n=8).
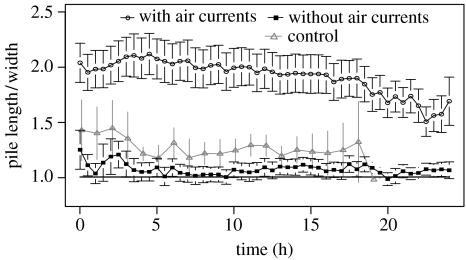
There was also a significant effect of the air current on the pile form (figure 7): while the mean ratio length to width is equal to 1 without air currents, the presence of air currents causes piles to be elongated in the direction of the air flow right from their birth time (where they contain 5 corpses or more) with mean ratios ranging from 1.5 to 2 (figure 4b). In the control experiment with lowered acrylic glass, the piles were slightly elongated, but this elongation was distinctly smaller compared with the one with air currents (grey triangles in figure 7).
Figure 7.
Dynamics of pile form. Temporal dynamics of the ratio length (in direction of the air current) over width of the piles (mean±s.e.), without air currents (filled rectangles, n=22), with air currents (open circles, n=29 piles) and in the control experiment with lowered acrylic glass (open grey triangles, n=8). The grey line indicates the expected ratio of 1 for circular piles.
4. Discussion
With and without air currents, the observed overall dynamics (number of piles and evolution of their size) typically reflect a self-organized process (Camazine et al. 2001). This and previous publications (Theraulaz et al. 2002; Challet et al. 2005b) show that the sigmoid growth of piles is due to a local amplification: the higher the local density of corpses, the higher the probability for a corpse-carrying ant to deposit its corpse and the lower the probability for an ant to pick up a corpse. The decreasing number of isolated corpses or small piles finally brings the global dynamics to a standstill. The individual behaviours (figure 2) and the fact that the global dynamics do not change with the presence of an air current (figure 5) indicate that these mechanisms remain valid.
The individual behaviour experiments reveal that low-speed air currents significantly influence corpse handling. The lower the wind speed, the higher the amplification. On the functional level, this leads ants to clear corpses from areas of high wind speed while clustering them in areas of low wind speed. Such a selective clustering has recently been observed by Scholes et al. (2006): the authors spread brood items (Temnothorax albipennis) in chambers with a heterogeneous air flow situation, and ants reassembled brood preferentially in the nest parts with the lowest wind speeds. Our findings suggest that this selective reassembling is based on a modulation of individual brood picking up and dropping probabilities by the wind speed.
With increasing size, piles begin to modify the laminar flow around them, slowing it down on the side facing the wind and on the leeside, while accelerating it on the other sides. Given the previous results, this suggests that amplification should be higher on the front side and leeside compared with the other sides. This prediction is confirmed by the measured probabilities to pick up and to drop a corpse (figure 3): picking up probability is higher on the sides (S) compared with the front side (F) and leeside (B), while the opposite is true for the dropping probabilities. It should also lead to an elongation of the piles in the direction of the air current. This is indeed observed on the collective level (figure 7). From their birth time (when their size exceeds 5 corpses), the ratio length over width is significantly higher than one and remains so throughout the experiment (though it decreases slightly while the ants ‘compact’ the pile). This elongated pile probably permits an ‘equilibrium’ between the sizes of low wind and high wind zones and their respective amplification strength, thus stabilizing the length over width ratio of the piles. The length over width ratio close to one in the control experiments (lowered acrylic glass to cut the convective air currents) further confirms that it is the air current that modulates clustering behaviour.
The individual clustering behaviours also indicate a stronger amplification on the leeside compared with the front side (same picking up probabilities, but a higher dropping probability on the leeside), predicting a displacement of the pile centroid in the wind direction. This is indeed observed on the collective level (figure 6), with pile centroids moving fast while piles are small and slowing down when the piles grow. The present research does not tell yet whether the larger low-wind zone size on the leeside compared with the front side can explain this asymmetry or whether ants have to ‘know’ on which side they are. In the latter case, we have to ask how ants detect the different sides of a pile. One possibility would be their position with respect to the pile and wind direction (which can be detected by ants, Wolf & Wehner 2000). Bonabeau et al. (1998) suggested another mechanism based on pheromone transport by air currents in the context of termite mound construction. They started with a model of pile formation in termites initially proposed by Deneubourg (1977), in which the termites impregnate the construction material with a volatile pheromone, triggering a positive feedback because termites are attracted by the pheromone and thus deposit subsequent material at places with high pheromone concentration. If the attraction is strong enough and the density of termites is high, then this model predicts the formation of pillars (Deneubourg 1977). Bonabeau et al. (1998) added a constant air flow transporting the pheromone in the model, resulting in the continuous extension of the piles along the wind direction or even the formation of walls along the air flow.
Is this model also relevant for the present work? Clustering of objects is a first step towards construction (Deneubourg et al. 1991; Camazine et al. 2001). Similar mechanisms could therefore be at work. Chemical substances (e.g. oleic acid) also play an important role in the evacuation of dead ants from the nest (Haskins & Haskins 1974; Ataya & Lenoir 1984), though their effect may depend on the social context (Gordon 1983). However, our corpses were at least two weeks old, the effect of oleic acid does not last that long (Ataya & Lenoir 1984). Even if ants applied some volatile marking before dropping a corpse, this could not explain the pattern observed in figure 3c,d (in particular, the same picking up probabilities on the front side and leeside). We also never observed a corpse deposition on the leeside without a physical contact with the pile. The correlation between the modulation of picking up and dropping probabilities with wind speed (figure 2a,e), the modulation of these probabilities around the large piles (figure 3c,d) and the modulation of wind speed by a corpse pile (figure 3a,b) rather suggest that wind speed itself plays the key role.
The influence of air currents on animal behaviour has already been demonstrated in the context of ant displacement (Wehner & Duelli 1971; Curtis 1985; Wolf & Wehner 2000). However, a direct effect on individual clustering or construction behaviours has only been demonstrated for termites (Howse 1966; Bruinsma 1979). Some indirect proof that individual behaviour can be modulated by air currents comes from studies on the relation between nest form and air currents: termite mounds are known to regulate ventilation and temperature (Turner 1994; Korb 2003; Hansell 2005), while the nest form of the ant Atta vollenweideri enhances nest ventilation (Kleineidam & Roces 2000). The present study provides direct and indirect proof that air currents modulate the behaviour of M. sanctus. Since corpses do not move in the absence of ants, it must be their clustering behaviour (picking up, transporting, dropping a corpse) that brings about the detected pile elongation and displacement. Our results show that the probabilities of picking up or dropping a corpse are directly modulated by wind speed and that ants are sensitive to differences as low as 1 cm s−1. Such a behaviour modulation could also be a coordinating factor in nest construction to regulate its microclimate by way of changing the shape of the structures built by ants.
The theoretical concept of spatial pattern formation through local amplification and transport by moving animals (Turing 1952; Gierer & Meinhardt 1972) has been experimentally demonstrated for the clustering phenomena of objects (Deneubourg & Goss 1989; Deneubourg et al. 1991; Hart & Ratnieks 2000) as well as the aggregation of animals (Depickère et al. 2004a,b; Lioni & Deneubourg 2004; Jeanson et al. 2005). It has even been used as a coordinating mechanism for object clustering by a group of robots (Beckers et al. 1994). The present study shows that the coupling of the amplification with an environmental stimulus (wind speed) modifies the spatial pattern through high amplification at low wind speeds. The emerging pile in turn modifies the wind conditions and influences further clustering, leading to a stigmergic (Grassé 1959) pattern formation. In Challet et al. (2005b), we showed that another environmental factor (temperature) modulates not only the amplification but also the transport of corpses, making the modulation of the spatial pattern even more versatile. In particular, this mechanism allows ‘deterministic’ clustering on an environmental template to be reconciled with the probabilistic behaviour that we frequently observe in social insects. These studies suggest that the described interplay between amplifications based on individual behaviour and environmental factors provides a powerful mechanism to create specific patterns in heterogeneous environments. This interplay is probably also a flexible starting point for evolutionary forces (Deneubourg et al. 2002).
In conclusion, we show in a precisely controlled experimental set-up that a laminar low-speed air flow modifies the corpse clustering behaviour in the ant M. sanctus. The corpse piles are elongated and moved in the direction of the air flow. These results can be explained by the negative correlation between the amplification of clustering and wind speed, and the modulation of wind speed by the emerging clusters. In general, individual ant behaviour involved in corpse clustering is governed by local corpse density, wind speed and the ants' position around a corpse pile with respect to the wind direction. We suggest that these mechanisms are generally involved in other clustering activities such as brood assembling, nest building and aeration tunnel construction.
Acknowledgments
We thank the three anonymous referees for their very helpful comments and the members of the EMCC workgroup in Toulouse for helpful and inspiring discussions. This work was supported by a grant from the ACI ‘Physico-chimie de la matière complexe’.
References
- Arbas E.A. Control of hindlimb posture by wind-sensitive hairs and antennae during locust flight. J. Comp. Physiol. A. 1986;159:849–857. doi: 10.1007/BF00603738. [DOI] [PubMed] [Google Scholar]
- Ataya H, Lenoir A. Le comportement nécrophorique chez la fourmi Lasius niger L. Insect. Soc. 1984;31:20–33. [Google Scholar]
- Beckers R, Holland O.E, Deneubourg J.-L. From local action to global tasks: stigmergy and collective robots. In: Brooks R.A, Maes P, editors. Artificial life IV. MIT Press; Cambridge, MA: 1994. pp. 181–189. [Google Scholar]
- Bejan A. Wiley; New York, NY: 1995. Convection heat transfer. [Google Scholar]
- Bhatkar A.W, Whitcomb W. Artificial diet for rearing various species of ants. Fla Entomol. 1970;53:229–232. [Google Scholar]
- Bollazzi M, Roces F. Thermal preference for fungus culturing and brood location by workers of the thatching grass-cutting ant Acromyrmex heyeri. Insect. Soc. 2003;49:153–157. [Google Scholar]
- Bonabeau E, Theraulaz G, Deneubourg J.-L, Franks N.R, Rafelsberger O, Joly J.-L, Blanco S. A model for the emergence of pillars, walls and royal chambers in termite nests. Phil. Trans. R. Soc. B. 1998;353:1561–1576. doi:10.1098/rstb.1998.0310 [Google Scholar]
- Bruinsma, O. H. 1979 An analysis of building behaviour of the termite Macrotermes subhyalinus Ph.D. thesis, Lanbouwhoogeschool te Wageningen, Belgium.
- Burmeister L.C. Wiley; New York, NY: 1983. Convective heat transfer. [Google Scholar]
- Camazine S, Deneubourg J.-L, Franks N.R, Sneyd J, Theraulaz G, Bonabeau E. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Cerdan, P. 1989 Étude de la biologie, de l'écologie et du comportement des fourmis moissoneuses du genre Messor (Hymenoptera, Formicidae) en Crau. Ph.D. thesis, Université de Provence, Aix-Marseille I, France.
- Ceusters R. Simulation du nid naturel des fourmis par des nids artificiels placés sur un gradient de température. Actes Coll. Insect. Soc. 1986;3:235–241. [Google Scholar]
- Challet M, Fourcassié V, Blanco S, Fournier R, Theraulaz G, Jost C. A new test of random walks in heterogeneous environments. Naturwissenschaften. 2005a;92:367–370. doi: 10.1007/s00114-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Challet M, Jost C, Grimal A, Lluc J, Theraulaz G. How temperature influences displacements and corpses aggregation behaviors in the ant Messor sancta. Insect. Soc. 2005b;52:305–315. [Google Scholar]
- Chapman R.F. 3rd edn. Harvard University Press; Cambridge, MA: 1982. The insects: structure and function. [Google Scholar]
- Chrétien, L. 1996 Organisation spatiale du matériel provenant de l'excavation du nid chez Messor barbarus et des cadavres d'ouvrières chez Lasius niger (Hymenoptera: Formicidae). Ph.D. thesis, Université Libre de Bruxelles, Belgium.
- Curtis B.A. Activity of the Namib desert dune ant, Camponotus detritus. S. Afr. J. Zool. 1985;20:41–48. [Google Scholar]
- Dagan D, Volman S. Sensory basis for directional wind detection in first instar cockroaches, Periplaneta americana. J. Comp. Physiol. A. 1982;147:471–478. [Google Scholar]
- Deneubourg J.-L. Application de l'ordre par fluctuations à la description de certaines étapes de la construction du nid chez les termites. Insect. Soc. 1977;24:117–130. [Google Scholar]
- Deneubourg J.-L, Goss S. Collective patterns and decision-making. Ethol. Ecol. Evol. 1989;1:295–311. [Google Scholar]
- Deneubourg J.-L, Goss S, Franks N.R, Sendova-Franks A.B, Detrain C, Chrétien L. The dynamics of collective sorting: robot-like ants and ant-like robots. In: Wilson S.W, Meyer J.-A, editors. Simulation of adaptive behavior: from animals to animats. MIT Press; Cambridge, MA: 1991. pp. 356–365. [Google Scholar]
- Deneubourg J.-L, Lioni A, Detrain C. Dynamics of aggregation and emergence of cooperation. Biol. Bull. 2002;202:262–267. doi: 10.2307/1543477. [DOI] [PubMed] [Google Scholar]
- Depickère S, Fresneau D, Deneubourg J.-L. A basis for spatial and social patterns in ant species: dynamics and mechanisms of aggregation. J. Insect Behav. 2004a;17:81–97. [Google Scholar]
- Depickère S, Fresneau D, Deneubourg J.-L. Dynamics of aggregation in Lasius niger (Formicidae): influence of polyethism. Insect. Soc. 2004b;51:81–90. [Google Scholar]
- Efron B, Tibshirani R.J. Chapman and Hall; London, UK; New York, NY: 1993. An introduction to the bootstrap. [Google Scholar]
- Fraenkel G.S, Gunn D.L. Dover; New York, NY: 1961. The orientation of animals: kineses, taxes and compass reactions. [Google Scholar]
- Franks N.R, Deneubourg J.-L. Self-organizing nest construction in ants: individual worker behaviour and the nest's dynamics. Anim. Behav. 1997;54:779–796. doi: 10.1006/anbe.1996.0496. [DOI] [PubMed] [Google Scholar]
- Franks N.R, Wilby A, Silverman B.W, Tofts C. Self-organizing nest construction in ants: sophisticated building by blind bulldozing. Anim. Behav. 1992;44:357–375. [Google Scholar]
- Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Gorb E, Gorb S. Effects of seed aggregation on the removal rates of elaiosome-bearing Chelidonium majus and Viola odourata seeds carried by Formica polyctena ants. Ecol. Res. 2000;15:187–192. [Google Scholar]
- Gordon D.M. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 1983;9:105–111. doi: 10.1007/BF00987774. [DOI] [PubMed] [Google Scholar]
- Grassé P.-P. La reconstruction du nid et les coordinations interindividuelles chez Bellicositermes natalensis et Cubitermes sp. La théorie de la stigmergie: essai d'interpétation du comportement des termites constructeurs. Insect. Soc. 1959;6:41–83. [Google Scholar]
- Haccou P, Meelis E. Oxford University Press; Oxford, UK: 1992. Statistical analysis of behavioural data: an approach based on time structured models. [Google Scholar]
- Hansell M.H. Oxford University Press; Oxford, UK: 2005. Animal architecture (Oxford animal biology) [Google Scholar]
- Hart A.G, Ratnieks F.L.W. Leaf caching in Atta leafcutting ants: discrete cache formation through positive feedback. Anim. Behav. 2000;59:587–591. doi: 10.1006/anbe.1999.1332. [DOI] [PubMed] [Google Scholar]
- Haskins C.P, Haskins E.F. Notes on necrophoric behavior in the archaic ant Myrmecia vindex (Formicidae: Myrmiciinae) Psyche. 1974;81:258–267. [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Howard D.F, Tschinkel W.R. Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour. 1976;56:157–180. [Google Scholar]
- Howse P.E. Air movement and termite behaviour. Nature. 1966;214:967–968. [Google Scholar]
- Jeanson R, Rivault C, Deneubourg J.-L, Blanco S, Fournier R, Jost C, Theraulaz G. Self-organised aggregation in cockroaches. Anim. Behav. 2005;69:169–180. [Google Scholar]
- Kleineidam C, Roces F. Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insect. Soc. 2000;47:241–248. [Google Scholar]
- Kleineidam C, Ernst R, Roces F. Wind-induced ventilation of the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften. 2001;88:301–305. doi: 10.1007/s001140100235. [DOI] [PubMed] [Google Scholar]
- Korb J. Thermoregulation and ventilation of termite mounds. Naturwissenschaften. 2003;90:212–219. doi: 10.1007/s00114-002-0401-4. [DOI] [PubMed] [Google Scholar]
- Limpert E, Stahel W.A, Abbt M. Log-normal distributions across the sciences: key and clues. BioScience. 2001;51:341–352. [Google Scholar]
- Lioni A, Deneubourg J.-L. Collective decision through self-assembling. Naturwissenschaften. 2004;91:237–241. doi: 10.1007/s00114-004-0519-7. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Weidenfeld & Nicolson; London, UK: 1998. Shaping life: genes, embryos and evolution. [Google Scholar]
- Ogawa H, Baba Y, Oka K. Dendritic calcium accumulation regulates wind sensitivity via short-term depression at cercal sensory-to-giant interneuron synapses in the cricket. J. Neurobiol. 2001;46:301–313. [PubMed] [Google Scholar]
- Oswalt D.A, Appel A.G, Smith L.M. Repellency and perception of moving air by the German cockroach (Dictyoptera: Blattellidae) J. Econ. Entomol. 1997;90:465–472. [Google Scholar]
- R Development Core Team 2003 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing.
- Roces F. Variable thermal sensitivity as output of a circadian clock controlling the bimodal rhythm of temperature choice in the ant Camponotus mus. J. Comp. Physiol. A. 1995;177:637–643. [Google Scholar]
- Roces F, Kleineidam C. Humidity preference for fungus culturing by workers of the leaf-cutting ant Atta sexdens rubropilosa. Insect. Soc. 2000;47:348–350. [Google Scholar]
- Scholes S.R, Sendova-Franks A.B, Swift S.T, Melhuish C. Ants can sort their brood without a gaseous template. Behav. Ecol. Sociobiol. 2006;59:531–540. [Google Scholar]
- Schöne H. Princeton University Press; Princeton, NJ: 1984. Spatial orientation: the spatial control of behavior in animals and man. [Google Scholar]
- Succi S. Clarendon Press; Oxford, UK: 2001. The lattice Boltzmann equation for fluid dynamics and beyond. [Google Scholar]
- Theraulaz G, et al. Spatial patterns in ant colonies. Proc. Natl Acad. Sci. USA. 2002;99:9645–9649. doi: 10.1073/pnas.152302199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel W.R. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: distribution of workers, brood and seeds within the nest in relation to colony size and season. Ecol. Entomol. 1999;24:222–237. [Google Scholar]
- Turing A.M. The chemical basis for morphogenesis. Phil. Trans. R. Soc. B. 1952;237:37–72. [Google Scholar]
- Turner J.S. Ventilation and thermal constancy of a colony of a southern African termite (Odontotermes transvaalensis: Macrotermitinae) J. Arid Environ. 1994;28:231–248. [Google Scholar]
- Webb B, Harrison R.R, Willis M.A. Sensorimotor control of navigation in arthropod and artificial systems. Arthropod Struct. Dev. 2004;33:301–329. doi: 10.1016/j.asd.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Wehner R, Duelli P. The spatial orientation of desert ants, Cataglyphis bicolor, before sunrise and after sunset. Experientia. 1971;27:1364–1366. [Google Scholar]
- Wolf H, Wehner R. Pinpointing food sources: olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J. Exp. Biol. 2000;203:857–868. doi: 10.1242/jeb.203.5.857. [DOI] [PubMed] [Google Scholar]
- Zar J.H. 4th edn. Prentice Hall; Englewood Cliffs, NJ: 1999. Biostatistical analysis. [Google Scholar]