Abstract
We used an in vitro model of differentiated tracheobronchial epithelium to analyze the susceptibility of different cell types to infection with rhinoviruses (RVs). Primary cells from control subjects were cultured in an air–liquid interface to form differentiated epithelia. Suprabasal and basal fractions were separated after trypsin digestion, and cell suspensions were infected with serotypes RV16 and RV1A. These cell fractions were analyzed for expression of viral capsid protein VP2 (flow cytometry), viral replication (real-time PCR), cytokeratin-14, and intercellular adhesion molecule–1 (ICAM-1). Compared with suprabasal fraction, basal cells had increased percentages of cells staining positive for VP2 (RV1A: 37.8% versus 9.1%, P < 0.01; RV16: 12.0 versus 3.0%, P < 0.05). The average number of viral RNA copies per cell was also higher in basal cells (2.2- and 2.4-fold increase in RV1A- and RV16-infected cells, respectively) compared with suprabasal cells. Furthermore, ICAM-1 was expressed by 33.3% of basal cells, compared with 8.1% of suprabasal cells (P < 0.05). Finally, in culture models of epithelial injury (detached suprabasal cells or scratched surface), there was significantly greater replication of RV1A compared with intact cell layer. These findings demonstrate that basal cells are more susceptible to RV infection than suprabasal cells. For major group RV, this may be in part due to increased expression of ICAM-1; however, minor group RV also replicated more effectively in basal cells. These results suggest the possibility that epithelial cell differentiation is associated with the maturation of antiviral defense mechanisms.
Keywords: airway epithelium, basal cells, rhinovirus, ICAM-1 expression
CLINICAL RELEVANCE
Basal cells in mature airway epithelium are very sensitive to rhinoviruses (RVs). In the case of increased epithelial permeability (diseased airways), RV infection is more severe. Our data in part explain the cause of RV-induced exacerbations of airway diseases.
The tracheobronchial epithelium is a specialized tissue composed of several cell types forming a barrier between the external environment and lung. The functions of airway epithelium include not only the clearance of inhaled air pollutants and microorganisms (1) but also the production of cytokines and mediators that can recruit and activate inflammatory cells (2, 3) and the secretion of factors regulating fibroblast and smooth muscle function (4). The proper structure of airway epithelium is critical for maintaining normal physiology, and epithelial disruption is believed to contribute in the pathogenesis of several lung diseases, including bronchial asthma and chronic obstructive pulmonary disease (5, 6). It is well established that many factors that exacerbate asthma (e.g., allergens, air pollutants, and respiratory viruses) can impair epithelial barrier function and induce cell injury. In addition, cell-derived enzymes and cytokines related to airway inflammation can increase permeability of the epithelial layer and damage cells. Indeed, marked signs of epithelial activation and injury can be found in bronchial biopsies of patients with asthma (7, 8).
Human rhinoviruses (RVs) are important agents responsible for airway infections and exacerbations in the course of chronic obstructive pulmonary disease or bronchial asthma (9, 10). Major group RVs (90% of serotypes) use intercellular adhesion molecule–1 (ICAM-1) receptor to infect target cells. The remaining serotypes belong to the minor group and enter host cells via low-density lipoprotein receptor (LDLR). RVs replicate in epithelial cells of the upper and proximal lower respiratory tracts (11, 12). It was found that antiviral immune responses of bronchial epithelial cells are defective or not optimal in patients with asthma (13, 14). Furthermore, RV infection can diminish the self-repairing capacities of bronchial epithelial cells in culture models of epithelial damage (15). Finally, in contrast to undifferentiated cells, cultured epithelia that are differentiated in vitro are more resistant to RV infection (16). This could be a result of more efficient antiviral responses in apical cells, restriction of the expression of rhinoviral receptors to basal cells, or both.
The main purpose of this study was to determine whether specific subpopulations of cells from differentiated airway epithelium differ in susceptibility to RV infection. To test this hypothesis, airway epithelia were differentiated in vitro, separated into basal and suprabasal fractions, infected, and analyzed for expression of cell surface markers, intracellular viral protein, and RNA. We also tested whether exposure of basal cells in models of epithelial injury leads to more pronounced replication of RV. Our results demonstrate that basal cells from complex airway epithelia are more susceptible to infection with major and minor group RVs.
MATERIALS AND METHODS
Viruses, Antibodies, and Other Reagents
RV16 and RV1A were grown and titered in HeLa cells as previously described (11). The R16-7 monoclonal antibody recognizes capsid protein VP2 and its precursor VP2-3 of RV16 and RV1A (11). Monoclonal antibodies to cytokeratin-14 (CK14), β-tubulin IV (ONS.1A6), goat anti-mouse IgM and IgG secondary antibodies conjugated with FITC and phycoerythrin (PE), and corresponding isotype controls were purchased from Sigma-Aldrich (St. Louis, MO). PE-conjugated anti–ICAM-1 (LB2) and corresponding mouse IgG2b isotype control were purchased from Becton Dickinson and Co. (Franklin Lakes, NJ). Rabbit anti-mouse IgG antibodies conjugated with Alexa fluor-488 and -568 and wheat germ agglutinin (WGA)-Alexa fluor-633 conjugate were purchased from Molecular Probes (Carlsbad, CA). WGA was used to identify Golgi, nuclear, and cellular membranes in confocal microscopy.
Cell Cultures
Epithelial cells were isolated from postmortem lung transplant tracheal and bronchial tissues of four subjects by pronase (Roche, Basel, Switzerland) and DNase (Sigma-Aldrich) digestion (17). Frozen stocks of passage 0 tracheobronchial epithelial cells were thawed and seeded onto 75-cm2 flasks that were coated with human placental collagen (Sigma-Aldrich). When passage 1 cells reached 90% confluence, they were treated with trypsin, resuspended in supplemented bronchial epithelial growth medium (BEGM) (Cambrex, Walkersville, MD), and seeded onto collagen-coated 1.13-cm2 Transwell polycarbonate inserts (7- to 10-μm-thick membranes, 0.4-μm pore size) (Costar; Corning Inc., Corning, NY) at 1.3 × 105 cells/cm2. The next day, medium from upper inserts was removed, and the medium in the bottom well was replaced with a 1:1 mixture of BEGM and Dulbecco's modified Eagle's medium (DMEM) containing supplements (final concentration: insulin, 5 μg/ml; transferrin, 10 μg/ml; rhEGF, 0.5 ng/ml; hydrocortisone, 1.4 μM; epinephrine, 2.7 μM; triiodothyronine, 10 nM; all-trans-retinoic acid, 50 nM; bovine pituitary extract, 4 μl/ml; gentamycin, 50 μg/ml; amphotericin, 50 ng/ml) (18). Cells in inserts were cultured at an air–liquid interface with media changed every other day. Daily measurements of transepithelial resistance (TER) were made using a chopstick EVOM voltohmmeter (World Precision Instruments, Sarasota, FL) after temporary addition of 0.5 ml of culture medium to the upper insert. Only mature, fully differentiated epithelia between Days 30 and 50 of culture were used for the experiments.
Histology
Cultures were fixed for 24 hours at room temperature with 10% formalin, paraffin embedded, and cross sectioned. Slides were stained using hematoxylin-eosin, alcian blue, and Periodic Acid Schiff methods.
Isolation of Apical and Basal Cells
To isolate suprabasal cells, the insert cultures were washed twice with calcium-free PBS and incubated for 30 to 40 minutes in calcium-free minimum essential medium to break intercellular junctions. When TER reached the background level, medium was removed, and 0.7 ml of trypsin/EDTA solution (2.5 mg/ml, 37°C) (Gibco Invitrogen, Carlsbad, CA) was added. After 7 to 10 minutes of incubation at room temperature, the suprabasal cells started to detach and could be easily separated from the basal layer. The detached cells were pipetted several times to make a single-cell suspension and washed with 10% FCS-DMEM. The layer of adherent basal cells that remained in inserts was washed twice in BEGM and incubated for 30 minutes at 37°C. Remaining patches of superficial cells were removed by gentle rinsing with a 200-μl pipette tip, after which trypsin/EDTA solution was added to detach basal cell fraction. Single-cell suspensions of suprabasal and basal cells were washed once in 10% FCS-DMEM and resuspended in BEGM at final concentrations of 0.4 × 106 cells/ml for further experiments.
Infection with RV
Tracheobronchial cell suspensions in BEGM and supplements were transferred into 4.5-ml polypropylene tubes (0.4 × 106 cells per sample) and incubated for 3 to 5 hours (5% CO2, 34°C). Cell suspensions were centrifuged (200 × g for 10 min) and resuspended in 50 μL of PBS containing calcium, magnesium, and RV (multiplicity of infection [MOI], 2.5 or 20 plaque-forming units [PFU] per cell; MOI 10 in three experiments for RT-PCR). After 30 minutes of incubation at room temperature to allow for viral attachment, 0.75 ml of BEGM medium with reduced hydrocortisone (1 × 10−8 M) was added to cell suspensions containing RV, and cells were incubated for an additional 8 hours (34°C, 5% CO2) for viral replication. As a control for these experiments, HeLa cell suspensions in DMEM were processed with the same procedures.
Flow Cytometry
Bronchial epithelial or HeLa cells were washed once in cold PBS, fixed for 30 minutes in 1% paraformaldehyde PBS, and permeabilized for 10 minutes in PBS with 0.12% Triton-X100. Fixed cells were incubated with goat-IgG for 30 minutes to block nonspecific binding and stained for 1 hour with saturating concentrations of appropriate monoclonal antibodies. Samples were washed once with 0.02% Triton-X100 PBS and incubated for 30 minutes with secondary goat anti-mouse conjugates. Stained cells were analyzed with a Becton Dickinson FacsCalibur flow cytometer equipped with a 488-nm argon laser and instrument settings optimized for the acquisition of epithelial cells. At least 5,000 events in forward scatter/side scatter gate were counted. Results are presented as the percentage of cells staining positive, set on uninfected cells or isotype controls. For ICAM-1 expression analysis, cells were stained with PE-conjugated anti–ICAM-1 antibody for 30 minutes and fixed and permeabilized for cytokeratin detection.
Analysis of RV RNA
For experiments with cell suspensions, total RNA was extracted from infected cells with Trizol reagent (Invitrogen) and reverse transcribed. Real-time PCR was performed as described by Mosser and colleagues (19). The RV RNA detected in the reaction was normalized to the total number of cells in each sample.
RV Infection in Models of Epithelial Damage
To analyze the influence of epithelial structure on RV penetration to basal cells and their susceptibility for infection in situ, we introduced three models of epithelial damage. (1) Model of increased permeability. Mature epithelia were washed intensively with calcium-free PBS and incubated in calcium-free minimum essential medium for approximately 40 minutes to dissociate intercellular junctions. Epithelia with decreased TER (range, 200–300 ohms/cm2) were incubated in supplemented BEGM medium (calcium shift) for 15 minutes before infection. (2) The model of suprabasal cell detachment was prepared as described previously. Single-cell layers of basal cells attached to a membrane were left in supplemented BEGM medium for at least 8 hours or overnight to form a confluent monolayer. (3) Model of epithelial scratch. The surface of submerged epithelium was injured by three full-thickness scratches using a 200-μl pipette tip. Inserts were washed with BEGM to remove the debris, and injured epithelia were cultured submerged (250 μl of BEGM to upper well) for 24 hours to induce basal cell migration and regeneration of epithelial wounds. At the time of infection, intact or damaged epithelia were washed with PBS containing calcium and magnesium and incubated with 100 μl of PBS and 0.1% BSA containing RV1A at a concentration of 1 × 107 PFU/ml. After 1 hour of incubation at room temperature to allow for viral attachment, epithelial surfaces were washed three times with PBS to remove excess RVs and incubated at air–liquid interface with low-hydrocortisone BEGM in a bottom well for 12 hours at 34°C. Total RNA was extracted from epithelial cells with an RNeasy mini kit (Qiagen, Inc., Valencia, CA) and reverse transcribed. The amount of RV RNA copies was measured with real-time PCR and calculated as a number of PFU equivalents (standardized to cell number and compared with samples with known RV concentration) in total RNA extracted from each insert (19).
Confocal Microscopy
Monolayers of basal cells were fixed in cold PBS containing 4% paraformaldehyde, permeabilized with 0.3% Triton-X100, incubated overnight with PBS supplemented with 10% FBS, and blocked for an additional hour with 5% rabbit serum in PBS. Membranes were then stained for 1 hour with mouse primary antibodies, washed with cold PBS, and stained for 1 hour with secondary rabbit anti-mouse conjugates and WGA-Alexa fluor-633. The membranes were excised from inserts and mounted inverted onto slides. The MCR-0124 system (BioRad, Hemel Hempstead, UK) with a krypton/argon mixed-gas laser (producing three laser light lines: 488, 568, and 647 nm) combined with a Nikon inverted microscope (Nikon Diaphot 200; Nikon, Tokyo, Japan) was used for confocal microscopy. Image processing was done using LaserSharp 5.2 software (Carl Zeiss, Jena, Germany).
Statistical Analysis
The data are presented as means ± SD in the text or SEM in the figures (GraphPad Software, Inc., San Diego, CA). One-way repeated measures ANOVA with Bonferroni's post-test or nonparametric Mann-Whitney and Wilcoxon tests were used to examine the significance of differences.
RESULTS
Establishment of Air–Liquid Interface Epithelial Cell Cultures
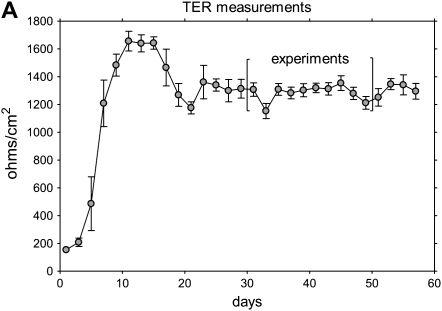
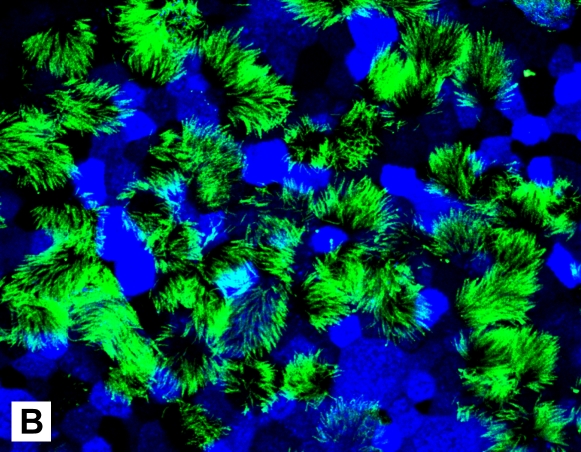
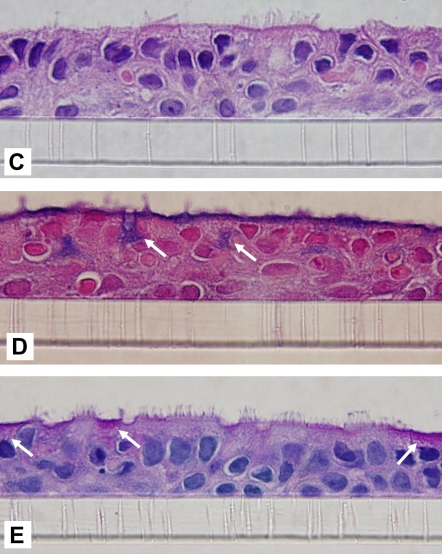
After cell seeding (1.3 × 105 cells/cm2) to obtain confluent monolayers, the epithelium was composed of undifferentiated cells that proliferated rapidly. Visual inspection and measurement of transepithelial resistance (Figure 1A) revealed several distinct phases of growth: formation of tight junctions (increasing TER), formation of multilayered epithelium (Days 7–14, peak TER), appearance of differentiated cells (Days 16–18, gradual reduction in TER), and formation of mature epithelium (∼ 28 d, TER 1,100–1,500 ohms/cm2 and stable). The number of differentiated ciliary cells gradually increased, and, in the majority of inserts, 30 to 50% of the surface was ciliated after 30 days of culture (Figure 1B). Histologic examination of 30-day-old cultures revealed pseudostratified epithelium with two to three cell layers; included in the apical cells were many mucin-producing and ciliated cells (Figure 1C–1E). After 30 days in culture, the epithelial morphology resembled that of native bronchial epithelium, and these conditions were used in subsequent experiments.
Figure 1.
Characteristics of differentiated epithelium. (A) Transepithelial resistance (TER) measurements. Data collected in two successive days from six different cultures are pooled and shown as an average ±SEM. (B) Ciliated cells in the apical layer were demonstrated in 35-day culture (original magnification: ×600). Membranes were fixed, permeabilized, and stained with anti–β-tubulin IV antibody and secondary rabbit–anti-mouse Alexa fluor-488 (green). Cells are counterstained with wheat germ agglutinin (WGA)-Alexa fluor-633 (pseudocolor blue). (C–E) Histologic properties. Cross-sections of paraffin-embedded insert membranes (Day 30 culture; original magnification: ×1,000) were stained with hematoxylin-eosin (C), alcian blue (D), and periodic acid Schiff (PAS) (E). Arrows point to mucin-producing cells (blue in alcian and magenta in PAS-stained samples).
Separation and Analysis of Basal and Suprabasal Cells
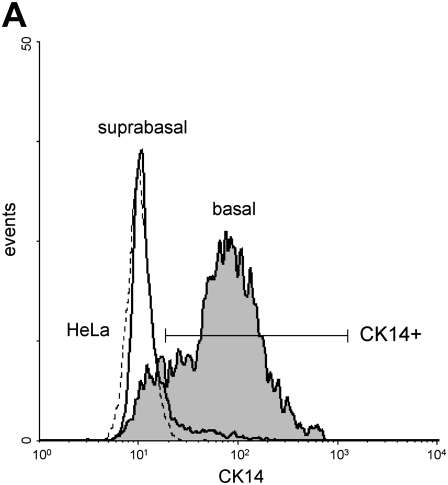
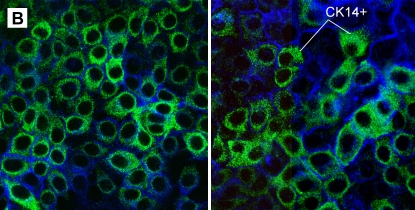
To prepare separate suspensions of suprabasal and basal cells, we developed a technique using incubation with calcium-free medium to disrupt tight junctions followed by treatment with trypsin to detach the upper cell layer. The remaining adherent single cell layer was composed of basal cells firmly attached to the collagen-coated polycarbonate membrane. These cells were removed using trypsin digestion to produce a single-cell suspension. This technique enabled separation of both cell fractions with greater than 90% viability. By flow cytometry, basal cells were 61.0 ± 8.5% CK14+ (Figure 2A); this was in agreement with heterogeneous staining of basal cells for CK14 by confocal microscopy (Figure 2B). We also observed a small percentage (15.3 ± 8.0%) of CK14+ cells in the suprabasal fraction. This may be in part due to the presence of a small number of contaminating basal cells and intermediate cells because in vivo data indicate that a fraction of these cells express CK5/14 markers (20).
Figure 2.
Analysis of CD14 expression in basal cells. (A) Representative flow cytometric histograms of cytokeratin-14 (CK14) fluorescence in suprabasal (bold line), basal (shaded area), and HeLa cells (CK14− control; broken line). (B) Single-cell basal layers obtained after suprabasal cell detachment were stained with anti-CK14 antibody (anti-mouse–IgM-FITC, green) and WGA-Alexa fluor-633 (pseudocolor blue). Two representative photographs of selected cultures are shown (original magnification: ×600).
Higher Expression of ICAM-1 in Basal Cells
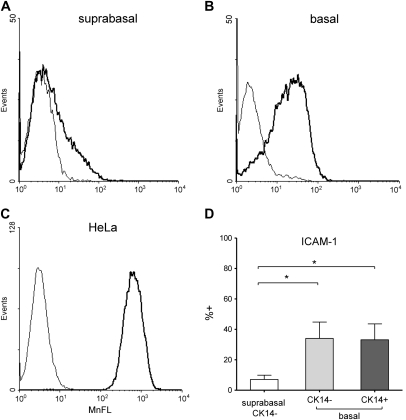
To analyze expression of ICAM-1, a receptor for major group RVs, suspensions of basal and suprabasal cells were double stained with anti–ICAM-1 and anti-CK14 antibody immediately after cell separation. HeLa cells express high levels of ICAM-1 and served as a positive control (Figure 3). Relatively few suprabasal cells expressed ICAM-1 (8.1 ± 7.8% positive), and the fraction of cells expressing this receptor was significantly (P < 0.05) higher in cells from the basal compartment (33.3 ± 22.9%) in CK14+ and CK14− fractions (33.2% and 34.0% positive, respectively).
Figure 3.
Comparison of ICAM-1 expression in epithelial suprabasal (A), basal (B), and HeLa (C) cells. Representative histograms show ICAM-1 fluorescence (bold line) and matched isotype control. (D) ICAM-1 expression was significantly greater on basal cells, including CK14+ and CK14− cell populations. Data are presented as mean ± SEM (n = 5). *P < 0.05.
Comparison of Rhinovirus Infectivity
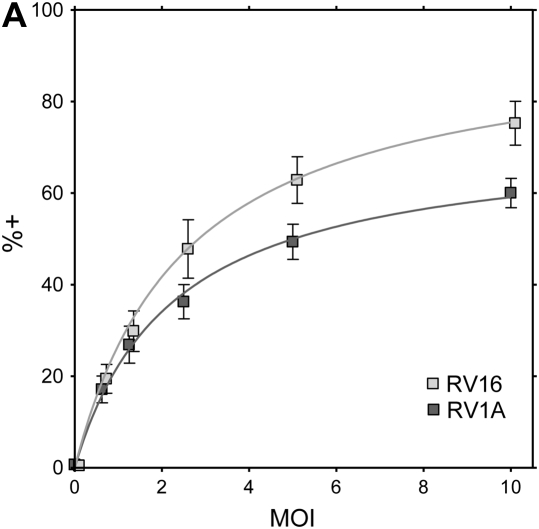
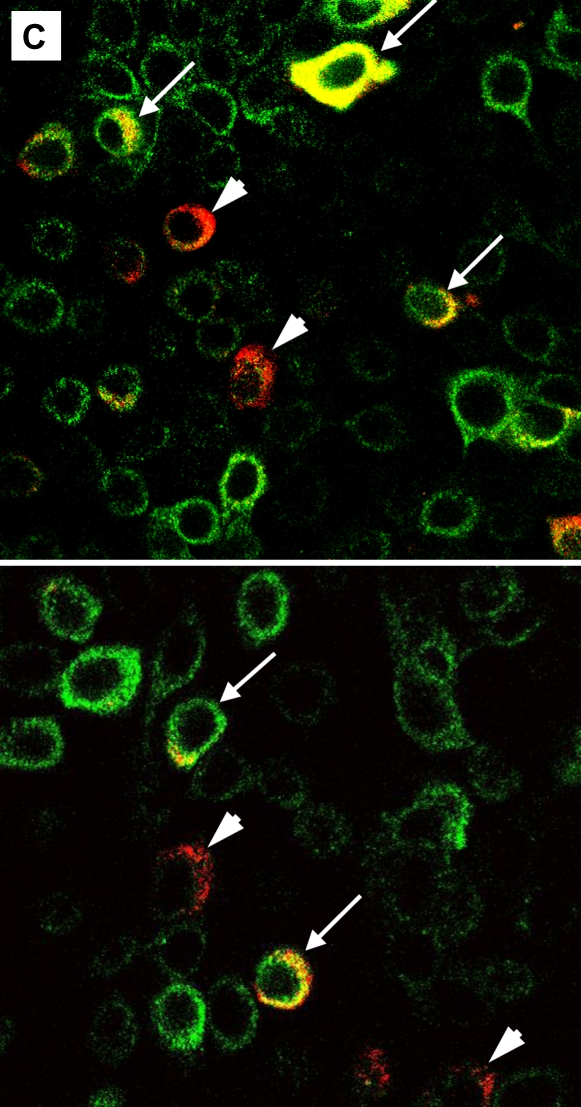
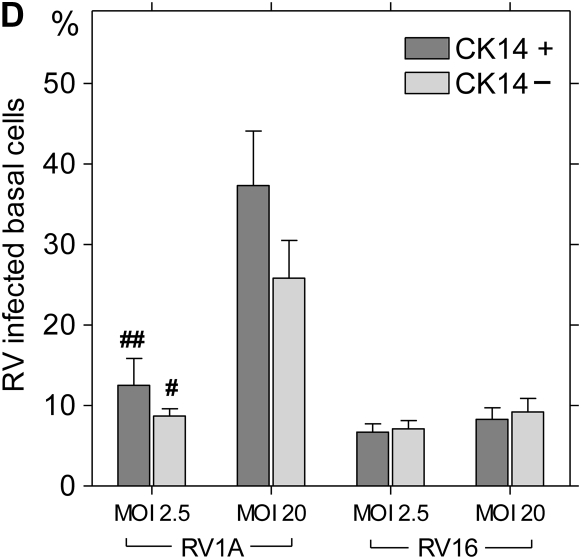
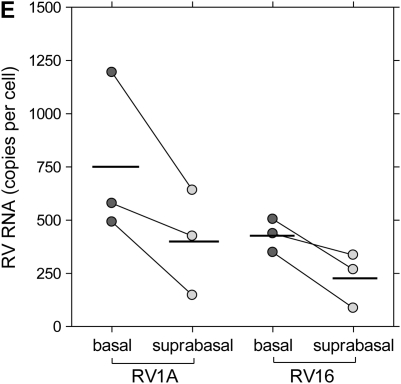
To characterize the infection rates of epithelial cell subpopulations, single-cell suspensions of suprabasal cells, basal cells, and HeLa cells (positive control) were infected with RV16 or RV1A and incubated for 8 hours to allow for viral replication. In preliminary experiments, the expression of VP2 protein in HeLa cells increased with higher MOI (Figure 4A). For bronchial epithelial cells, rates of infection were significantly higher in the basal fraction compared with suprabasal cells (Figure 4B). In RV1A-infected cells (MOI 20), 37.8 ± 10.8% of basal cells versus 9.1 ± 3.5% of apical cells were VP2 positive (P < 0.01). Significant differences were also observed for RV16 infection (12.0 ± 5.1% versus 3.0 ± 1.3%, respectively; P < 0.05). Consistent with these results, the amount of RV1A or RV16 RNA copies per cell was higher in infected basal fraction compared with suprabasal cells (mean, 2.2- and 2.4-fold increase, respectively) (Figure 4E). There was no significant difference in the rates of RV protein detection related to CK14 expression, and this was confirmed by immunofluorescent staining and confocal microscopy (Figures 4C and 4D).
Figure 4.
Replication of human rhinovirus (RV) in differentiated bronchial epithelial cells. (A) Detection of capsid protein after infection of HeLa cells with RV1A and RV16. The percentage of infected cells staining positive for VP2 are plotted in comparison to uninfected control cells (means ± SEM; n = 6). (B) Differences in infection rates in RV-infected basal versus suprabasal epithelial cells. Data represent mean values ± SEM (n = 5). #P < 0.05 for 2.5 versus 20 multiplicity of infection (MOI) comparison. *P < 0.05; **P < 0.01 for suprabasal versus basal comparison. (C) Colocalization of CK14 antigen and RV structural proteins in RV1A-infected monolayers of basal cells obtained after suprabasal cell detachment. Cells were double stained with anti-CK14 antibody (anti-mouse–IgM FITC, green) and anti-VP2 R16-7 antibody (anti-mouse–IgG Alexa fluor-568, red). Examples of infected CK14− (red, arrowheads) or CK14+ cells (yellow, arrows) are shown (original magnification: ×600). (D) RV-infected cells from basal fraction were double stained with anti-VP2 and anti-CK14 antibodies. Flow cytometric data are presented as mean values ± SEM (n = 5). No significant difference was seen when comparing CK14+ and CK14− cells. #P < 0.05; ##P < 0.01 in 2.5 versus 20 MOI comparison. (E) Detection of RV RNA in RV-infected basal versus suprabasal cells. Results of three independent experiments are presented as connected data points (representing samples from the same preparations) and geomeans (horizontal lines).
Infection of Damaged Epithelia
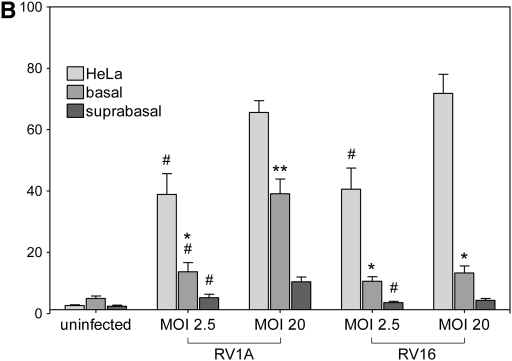
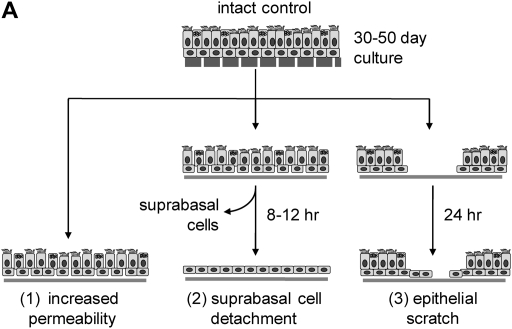
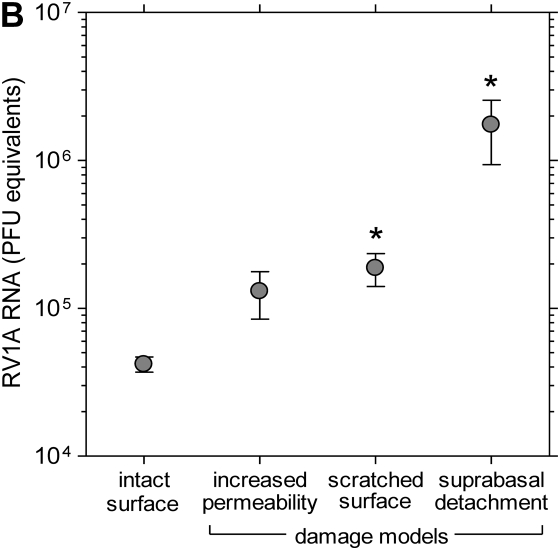
To reproduce structural changes in mature bronchial epithelium similar to those observed in vivo, we used three different culture models of epithelial damage: incubation in the absence of Ca and Mg to increase transepithelial permeability, enzymatic detachment of suprabasal cells, and scratching the differentiated epithelia with a pipette tip (Figure 5A). Samples of damaged and intact epithelial cells were incubated for 10 hours with RV1A, and viral replication was analyzed in cellular lysates. The infection rate was ∼50-fold higher in the model of suprabasal cells detachment and almost 5-fold higher in epithelial wound model (P < 0.05 for each comparison; Figure 5B). There was a trend toward greater infection rates in cultures with increased permeability (low-TER model).
Figure 5.
RV1A infection in a culture model of epithelial damage. (A) Schematic diagram showing models of epithelial injury used in the study (details in text). (B) Replication of RV1A RNA analyzed with real-time PCR and expressed as plaque-forming unit equivalents in lysates extracted from RV-infected control or injured epithelia. Data are presented as means ± SEM (n = 4 independent experiments). *P < 0.05.
DISCUSSION
We used an in vitro model of differentiated tracheobronchial epithelium to analyze the susceptibility of particular cell types to RV infection. Primary airway epithelial cells were differentiated by culturing at the air–liquid interface system to form polarized mucociliary epithelium with structural characteristics that resemble native tissue (18, 21, 22). To separate fractions of basal and suprabasal cells, we developed an easy method to disrupt tight junctions and detach superficial cells. Evaluation of basal versus suprabasal cells revealed differences, including increases in ICAM-1 and CK14 expression. Furthermore, the percentage of RV-infected basal cells was substantially higher, and viral RNA was increased when compared with cells from suprabasal layers. These findings were confirmed using in vitro models of epithelial damage where exposure of basal cells, after detachment of upper cell layers or surface scratch, caused significantly greater RV1A replication. Collectively, these results indicate that, in these in vitro epithelial cell models, cells in the superficial layer are relatively resistant and basal cells are more susceptible to RV.
These results have important implications for mature bronchial epithelium in vivo and potential consequences in the case of epithelial injury. During growth and differentiation, epithelial cells form specific intercellular junctions that contribute to epithelial integrity and spatial localization of the cell layers (23). The differentiated columnar cells form a complex system of tight and adherent junctions on their apical pole. The basal cells are firmly attached to the basal lamina via hemidesmosomes and thus serve to anchor more superficial cells. Inhaled substances and mediators produced in inflamed mucosa can disrupt epithelial structure and increase permeability (24). Some allergens (e.g., Der p 1) are proteases that can cleave cell adhesion proteins and disrupt tight junctions (25, 26). In addition, proinflammatory Th2 (IL-4, IL-13) and Th1 (IFN-γ) cytokines (27, 28), as well as histamine, leukocyte-derived enzymes, and cationic proteins, can decrease epithelial barrier function (29, 30). When considered together, findings from this study and that of Lopez-Sousa and colleagues (16) suggest that airway epithelium that is disrupted by air pollutants, infectious agents, or other noxious agents could allow virus to penetrate past the infection-resistant apical cells and reach basal cells that are more susceptible to RV infection and replication.
RVs enter target cells via ICAM-1 (major group) or LDLR (minor group) receptors (31) and can replicate in tracheobronchial epithelium in vitro (11) and in vivo (32). It was recently reported that differentiated epithelial cells are relatively resistant to RV infection in comparison to undifferentiated cell monolayers (16). Furthermore, the degree of RV infection was inversely correlated to TER. The higher rate of infectibility in cultures with disrupted intercellular junctions may be the consequence of specific pattern of expression of RV-related receptors in mature, pseudostratified epithelium (7, 33, 34). ICAM-1 is almost exclusively restricted to the basal layer of bronchial epithelium (33), although it is substantially induced in inflamed tissues and after cytokine (IFN-γ, TNF-α) treatment of epithelial cells in vitro (35, 36). Our data confirm that ICAM-1 expression in in vitro differentiated tracheobronchial epithelium is largely restricted to basal cells. This observation may in part explain our results in major group RV infection because restriction of ICAM-1 to basal cells makes them more susceptible to RV entry. The distribution and regulation of expression of minor group RV receptor LDLR has not been extensively studied in differentiated bronchial epithelium; however, basolateral sorting of LDL receptors seems to be a characteristic feature of all polarized epithelial cells (37). In addition, RVs infect epithelial cells via ceramide-enriched membrane rafts (38). Differentiation of apical polarized cells during epithelial growth may be associated with changed cellular membrane properties that may interfere with particle trafficking and raft formation. It is also possible that differentiating apical cells acquire more efficient innate antiviral responses.
Epithelium in asthmatic airways can exhibit signs of cellular damage and activation (7, 8, 39). This has led to speculation that the bronchial epithelium is continuously undergoing cycles of injury followed by induction of progenitor cells and subsequent regeneration (40). Bossios and colleagues (15) reported that subconfluent primary bronchial epithelial cells had greater RV-induced cytotoxicity compared with confluent monolayers, suggesting that rapidly dividing cells were more susceptible to infection. There is evidence that CK14-expressing basal cells represent a progenitor cell population capable of regenerating all other epithelial cell fractions (41–43). We found that the basal layer of epithelium differentiated in vitro is composed of CK14+ and CK14− cells, and this pattern has been described in previous studies (44, 45). Here we show that RVs can penetrate to basal cells during epithelial injury and can infect and replicate in CK14+ and CK14− cells. Collectively, these results suggest that RV infection of basal cells in damaged epithelium could inhibit the process of epithelial repair (15).
These experiments were conducted using a model of differentiated epithelium that has some inherent limitations. For example, cells from other lineages (e.g., dendritic cells, lymphocytes, and neuroendocrine cells) are lacking. Although this could be regarded as a limitation of this model, it allows for the study of pure epithelial cell fractions. It will be desirable to repeat these experiments in native bronchial epithelium that is excised from mucosal surfaces, although methods for the separation of specific epithelial cells from native epithelium need to be developed.
In conclusion, we found that basal cells from airway epithelium differentiated in vitro are more susceptible to RV infection. In addition, models that expose basal cells to viruses exhibit higher RV replication due to infection of most sensitive cells. If this is also true in vivo, these findings imply that environmental exposures or diseases that damage the epithelium could promote more severe RV infections. These findings suggest that this mechanism could contribute to increased lower respiratory tract susceptibility to RV infections in asthma, a disease of chronic airway inflammation.
Acknowledgments
The authors thank Lance Rodenkirch for assistance in confocal imaging and Rose Vrtis for assistance in real-time PCR analysis.
This work was supported by the National Heart, Lung and Blood Institute exchange program fellowship (B.J.) and by NIH grant no. P01 HL70831–01.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0050OC on December 6, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 2004;23:327–333. [DOI] [PubMed] [Google Scholar]
- 2.Takizawa H. Bronchial epithelial cells in allergic reactions. Curr Drug Targets Inflamm Allergy 2005;4:305–311. [DOI] [PubMed] [Google Scholar]
- 3.Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc 2004;1:222–230. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 2004;1:93–98. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron C, Boulet LP. Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest 2006;129:1068–1087. [DOI] [PubMed] [Google Scholar]
- 6.Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, Howarth P. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl 2003;44:24s–29s. [DOI] [PubMed] [Google Scholar]
- 7.Trautmann A, Kruger K, Akdis M, Muller-Wening D, Akkaya A, Brocker EB, Blaser K, Akdis CA. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol 2005;138:142–150. [DOI] [PubMed] [Google Scholar]
- 8.Shahana S, Bjornsson E, Ludviksdottir D, Janson C, Nettelbladt O, Venge P, Roomans GM. BHR-group. Ultrastructure of bronchial biopsies from patients with allergic and non-allergic asthma. Respir Med 2005;99:429–443. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol 2005;116:267–273. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc 2005;2:150–156. [DOI] [PubMed] [Google Scholar]
- 11.Mosser AG, Brockman-Schneider R, Amineva S, Burchell L, Sedgwick JB, Busse WW, Gern JE. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis 2002;185:734–743. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000;181:1875–1884. [DOI] [PubMed] [Google Scholar]
- 13.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 2006;12:1023–1026. [DOI] [PubMed] [Google Scholar]
- 15.Bossios A, Psarras S, Gourgiotis D, Skevaki CL, Constantopoulos AG, Saxoni-Papageorgiou P, Papadopoulos NG. Rhinovirus infection induces cytotoxicity and delays wound healing in bronchialepithelial cells. Respir Res 2005;6:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol 2004;286:L373–L381. [DOI] [PubMed] [Google Scholar]
- 17.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, Gern JE. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol 1999;20:1220–1228. [DOI] [PubMed] [Google Scholar]
- 18.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 1996;14:104–112. [DOI] [PubMed] [Google Scholar]
- 19.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med 2005;171:645–651. [DOI] [PubMed] [Google Scholar]
- 20.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med 1998;157:2000–2006. [DOI] [PubMed] [Google Scholar]
- 21.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 1997;10:2398–2403. [DOI] [PubMed] [Google Scholar]
- 22.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol Anim 2003;39:56–62. [DOI] [PubMed] [Google Scholar]
- 23.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003;8:432–446. [DOI] [PubMed] [Google Scholar]
- 24.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol 2004;114:997–1008. [DOI] [PubMed] [Google Scholar]
- 25.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, et al. p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 1999;104:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen HD. Pen ch 13 allergen induces secretion of mediators and degradation of occluding protein of human lung epithelial cells. Allergy 2006;61:382–388. [DOI] [PubMed] [Google Scholar]
- 27.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol 2001;281:C2029–C2038. [DOI] [PubMed] [Google Scholar]
- 28.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003;171:6164–6172. [DOI] [PubMed] [Google Scholar]
- 29.Zabner J, Winter M, Excoffon KJ, Stoltz D, Ries D, Shasby S, Shasby M. Histamine alters E-cadherin cell adhesion to increase human airway epithelial permeability. J Appl Physiol 2003;95:394–401. [DOI] [PubMed] [Google Scholar]
- 30.Uchida DA, Irvin CG, Ballowe C, Larsen G, Cott GR. Cationic proteins increase the permeability of cultured rabbit tracheal epithelial cells: modification by heparin and extracellular calcium. Exp Lung Res 1996;22:85–99. [DOI] [PubMed] [Google Scholar]
- 31.Savolainen C, Blomqvist S, Hovi T. Human rhinoviruses. Paediatr Respir Rev 2003;4:91–98. [DOI] [PubMed] [Google Scholar]
- 32.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med 1997;155:1159–1161. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Rogers AV, Burke-Gaffney A, Hellewell PG, Jeffery PK. Cytokine-induced airway epithelial ICAM-1 upregulation: quantification by high-resolution scanning and transmission electron microscopy. Eur Respir J 1999;13:1318–1328. [DOI] [PubMed] [Google Scholar]
- 34.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 2002;13:3218–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-α on expression of ICAM-1 in human airway epithelial cells in vitro: signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol 2000;22:685–692. [DOI] [PubMed] [Google Scholar]
- 36.Konno S, Grindle KA, Lee WM, Schroth MK, Mosser AG, Brockman-Schneider RA, Busse WW, Gern JE. Interferon-gamma enhances rhinovirus-induced RANTES secretion by airway epithelial cells. Am J Respir Cell Mol Biol 2002;26:594–601. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 2005;6:233–247. [DOI] [PubMed] [Google Scholar]
- 38.Grassme H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem 2005;280:26256–26262. [DOI] [PubMed] [Google Scholar]
- 39.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003;167:1360–1368. [DOI] [PubMed] [Google Scholar]
- 40.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:726–733. [DOI] [PubMed] [Google Scholar]
- 41.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670. [DOI] [PubMed] [Google Scholar]
- 42.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawlins EL, Hogan BLM. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465. [DOI] [PubMed] [Google Scholar]
- 44.Zabner J, Zeiher BG, Friedman E, Welsh MJ. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol 1996;70:6994–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima M, Kawanami O, Jin E, Ghazizadeh M, Honda M, Asano G, Horiba K, Ferrans VJ. Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int 1998;48:944–953. [DOI] [PubMed] [Google Scholar]