Abstract
Localization of a mutation affecting ribonuclease III activity (an enzyme specific for double-stranded ribonucleic acid) in Escherichia coli was attempted. By a series of matings and transduction experiments, the mutation rnc-105 was mapped near the nadB gene. In strains carrying this mutation, another mutation (ranA2074) was also found. Based on available data, their order on the E. coli chromosome appears to be tyrA, ranA, nadB, rnc, purI. Strains carrying either the ranA2074 or the rnc-105 mutation fail to grow at 45 C in enriched medium, whereas strains carrying only the rnc-105 mutation are defective in ribonuclease III activity. Strains carrying either of these mutations grow more slowly than corresponding wild-type strains in all media tested at all temperatures; the rnc-105 mutation reduces the growth rate more than the ranA2074 mutation. T4 and T7 bacteriophages form plaques with a lower efficiency on strains carrying the rnc-105 mutation than on other strains. Thus we suggest that ribonuclease III is beneficial for normal growth of E. Coli and that at higher temperatures it becomes indispensable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Robertson H. D. RNA precursor molecules and ribonucleases in E. coli. Mol Cell Biochem. 1973 May 11;1(1):83–93. doi: 10.1007/BF01659941. [DOI] [PubMed] [Google Scholar]
- Apirion D. Altered ribosomes in a suppressor strain of Escherichia coli. J Mol Biol. 1966 Apr;16(2):285–301. doi: 10.1016/s0022-2836(66)80173-0. [DOI] [PubMed] [Google Scholar]
- Apirion D. Degradation of RNA in Escherichia coli. A hypothesis. Mol Gen Genet. 1973 May 28;122(4):313–322. doi: 10.1007/BF00269431. [DOI] [PubMed] [Google Scholar]
- Apirion D. The fate of mRNA and rRNA in Escherichia coli. Brookhaven Symp Biol. 1975 Jul;(26):286–306. [PubMed] [Google Scholar]
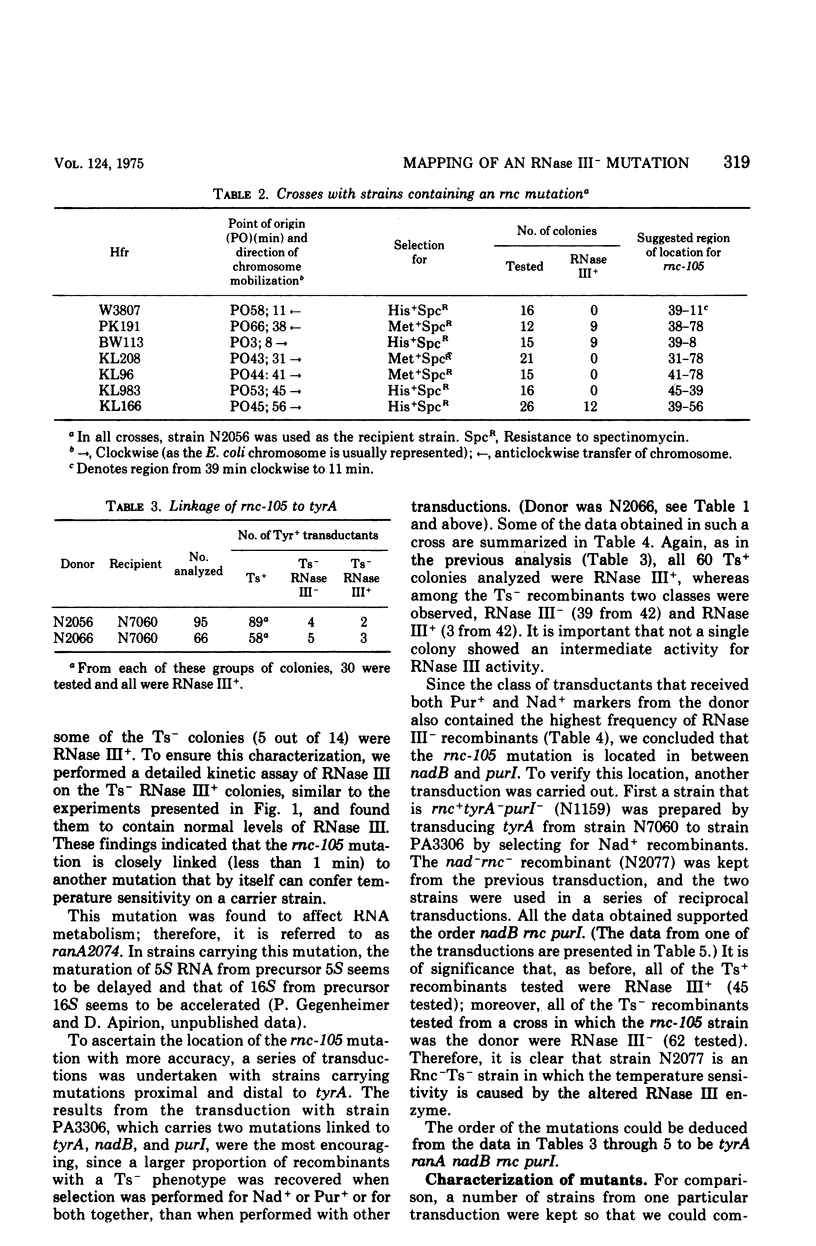
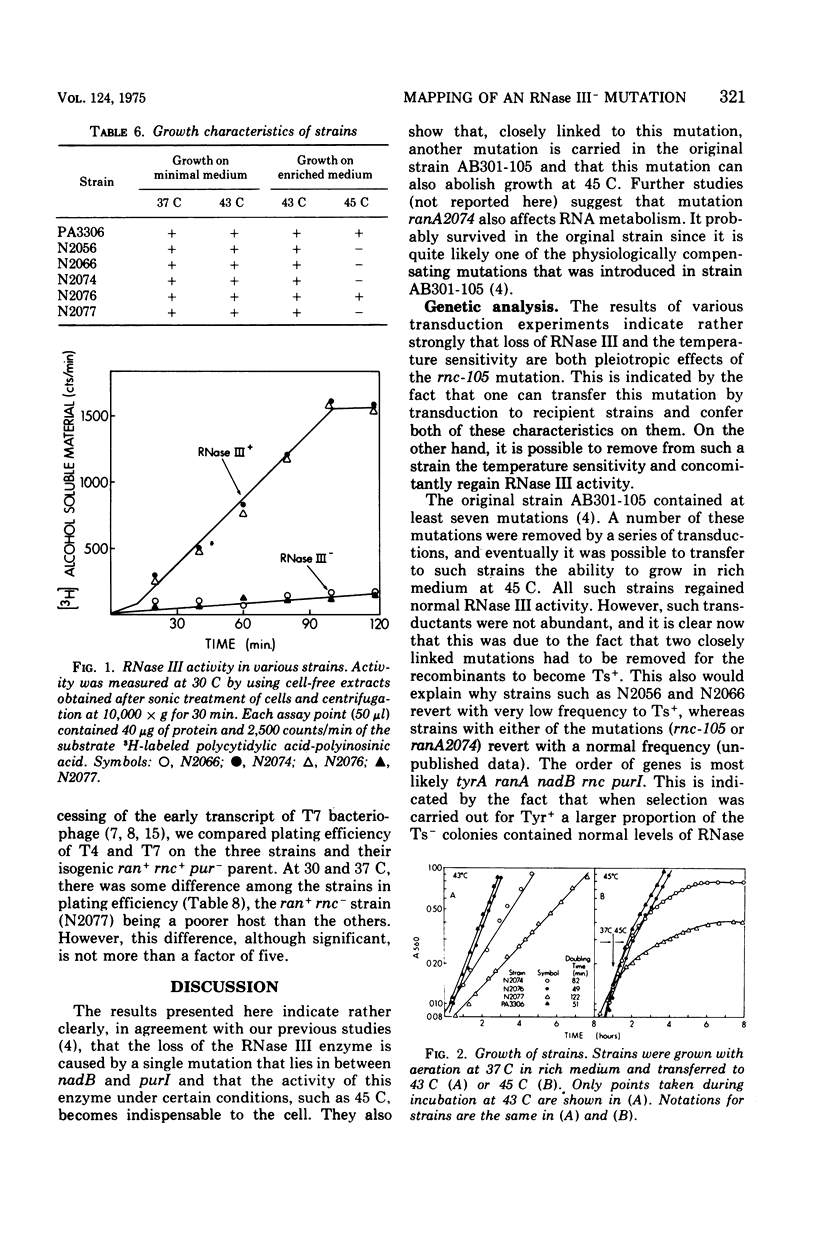
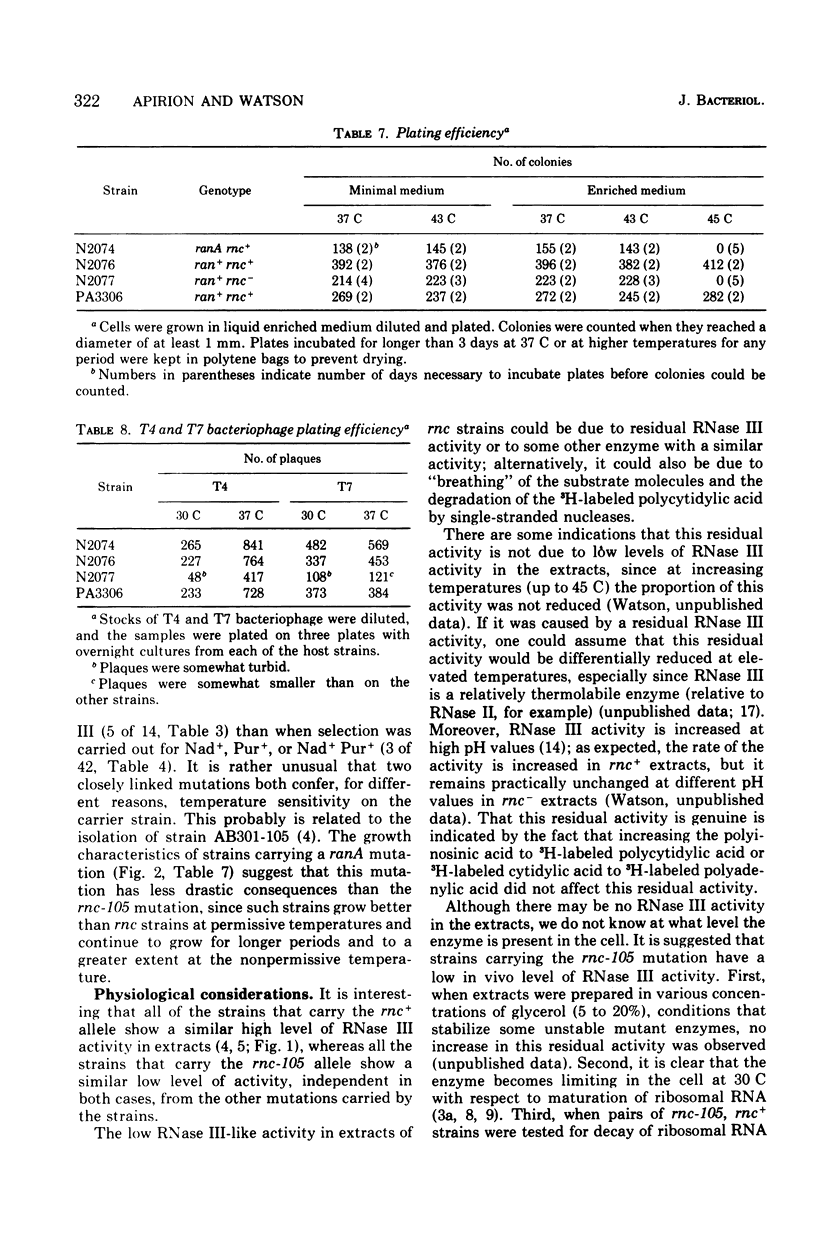
- Apirion D., Watson N. Analysis of an Escherichia coli strain carrying physiologically compensating mutations one of which causes an altered ribonuclease 3. Mol Gen Genet. 1974;132(2):89–104. doi: 10.1007/BF00272175. [DOI] [PubMed] [Google Scholar]
- Apirion D., Watson N. Unaltered stability of newly synthesized RNA in strains of Escherichia coli missing a ribonuclease specific for double-stranded RNA. Mol Gen Genet. 1975;136(4):317–326. doi: 10.1007/BF00341716. [DOI] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Escherichia coli ribosomal ribonucleic acids are not cut from an intact precursor molecule. J Biol Chem. 1975 Mar 25;250(6):2407–2409. [PubMed] [Google Scholar]
- Kindler P., Keil T. U., Hofschneider P. H. Isolation and characterization of a ribonuclease 3 deficient mutant of Escherichia coli. Mol Gen Genet. 1973 Oct 16;126(1):53–59. doi: 10.1007/BF00333481. [DOI] [PubMed] [Google Scholar]
- Lennette E. T., Apirion D. Genetic analysis of an Escherichia coli syndrome. J Bacteriol. 1971 Dec;108(3):1322–1328. doi: 10.1128/jb.108.3.1322-1328.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyhack B., Meyhack I., Apirion D. Colicin E3: a unique endoribonuclease. Proc Natl Acad Sci U S A. 1973 Jan;70(1):156–160. doi: 10.1073/pnas.70.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherford S. C., Rosen L., Gorelic L., Apirion D. Escherichia coli strains with thermolabile ribonuclease II activity. J Biol Chem. 1972 Sep 10;247(17):5404–5408. [PubMed] [Google Scholar]
- Weatherford S. C., Weisberg L. S., Achord D. T., Apirion D. Separation of Escherichia coli ribonucleases on a DNA agarose column and the identification of an RNase H activity. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1307–1315. doi: 10.1016/0006-291x(72)90609-2. [DOI] [PubMed] [Google Scholar]



