Abstract
The water-soluble, hydroxylated fullerene [fullerol, nano-C60(OH)22–26] has several clinical applications including use as a drug carrier to bypass the blood ocular barriers. We have assessed fullerol’s potential ocular toxicity by measuring its cytotoxicity and phototoxicity induced by UVA and visible light in vitro with human lens epithelial cells (HLE B-3). Accumulation of nano-C60(OH)22–26 in the cells was confirmed spectrophotometrically at 405 nm and cell viability estimated using MTS and LDH assays. Fullerol was cytotoxic to HLE B-3 cells maintained in the dark at concentrations higher than 20 µM. Exposure to either UVA or visible light in the presence of >5 µM fullerol induced phototoxic damage. When cells were pretreated with non-toxic antioxidants: 20 µM lutein, 1 mM N-acetyl cysteine, or 1 mM L-ascorbic acid prior to irradiation, only the singlet oxygen quencher lutein significantly protected against fullerol photodamage. Apoptosis was observed in lens cells treated with fullerol whether or not the cells were irradiated, in the order UVA > visible light > dark. Dynamic light scattering (DLS) showed that in the presence of the endogenous lens protein α-crystallin, large aggregates of fullerol were reduced. In conclusion, fullerol is both cytotoxic and phototoxic to human lens epithelial cells. Although the acute toxicity of water soluble nano-C60(OH)22–26 is low, these compounds are retained in the body for long periods, raising concern for their chronic toxic effect. Before fullerols are used to deliver drugs to the eye, they should be tested for photo- and cytotoxicity in vivo.
Keywords: fullerenes, fullerol, ocular toxicology, phototoxicity, human lens epithelial cells, dynamic light scattering
Introduction
The human eye is exposed to ambient radiation that serves the fundamental biological functions of directing vision and circadian rhythm (Roberts, 2005). Light damage to the human lens is prevented by protective chromophores (i.e. 3-OH kynurenine glucoside) (Balasubramanian, 2000; Dillon, 1991) that absorb light but do no harm, and by an efficient antioxidant system (Andley, 2001). The cornea cuts off all light below 295 nm. Long UVB† (295–315 nm) and UVA (315–400 nm) are absorbed by the human lens (Barker et al., 1991). Any substance that absorbs light above 295 nm and produces reactive oxygen species has the potential to damage the human lens. Such damage is seen in patients taking phototoxic prescription drugs, diagnostic dyes or over-the counter herbal medications (Roberts, 2002). This can lead to permanent or transient loss of vision due to early cataract formation.
Water-soluble fullerene derivatives have shown promise as drug carriers to bypass the brain and ocular barriers (Calvo et al., 1996; Da Ros and Prato, 1999; Dugan et al., 1997). They have also exhibited antitumor and antiviral activity, including inhibition of HIV protease (Bogdanovic et al., 2004; Friedman et al., 1993; Nakamura and Isobe, 2003; Schinazi et al., 1993). Although the water-soluble fullerenes are not genotoxic (Zakharenko et al., 1997) they are retained in the body for long periods, raising concerns about chronic toxic effects (Yamago et al., 1995). Fullerol and other water soluble hydroxylated fullerenes have been found to be cytotoxic to human dermal fibroblasts, human liver carcinoma cells (HepG2) and neuronal human astrocytes (Sayes et al., 2004; Sayes et al., 2005).
Photoexcitation of fullerene derivatives efficiently produces an excited triplet state (Arbogast et al., 1991; Guldi and Prato, 2000) and through energy and electron transfer to molecular oxygen produce both singlet molecular oxygen (Prat et al., 1999) and superoxide (Yamakoshi et al., 2003). Although the carboxylated water-soluble fullerene derivatives were not found to be phototoxic to B lymphocytes (Irie et al., 1996), the malonic acid derivatives of fullerenes were found to be cytotoxic and phototoxic to both HeLa (Yang et al., 2002) and Jurkat cells (Rancan et al., 2002).
In the studies presented here, we assessed the ocular toxicity of hydroxylated fullerene [fullerol, C60(OH)22−26] (Fig. 1). We have determined that fullerols are both cytotoxic and phototoxic to human lens epithelial cell model system in the presence of either UVA or visible light. We have also demonstrated that the phototoxicity could be modified by the singlet oxygen quencher lutein that is known to be present in the human lens (Bernstein et al., 2001).
Fig. 1.
The structure of fullerol [C60(OH)22−26].
Materials and Methods
Reagents
L-ascorbic acid 6-palmitate and dehydroascorbic acid were purchased from Aldrich (Milwaukee, WI, USA); fetal bovine serum (FBS) was from Biofluids (Rockville, MD, USA); N-acetyl-L-cysteine was from Fluka (Milwaukee, WI, USA); gentamicin and trypsin-EDTA were from GIBCO Invitrogen Corporation (Carlsbad, CA, USA); Nembutal was from Ovation Pharmaceuticals (Deerfield, IL, USA); and L-(+)-ascorbic acid, Eagle’s Minimum Essential Medium (MEM), L-glutamine, sterile dimethyl sulfoxide (DMSO), lutein, and α-crystallin were from Sigma (St. Louis, MO, USA). All reagents used for experiments were at least analytical grade. Hydroxy fullerene [C60(OH)22−26 >99.5%, M = 1128.8 g/mol] was procured from the MER Corporation (Tuscon, AZ, USA) through a contract with Battelle Memorial Institute (Columbus, OH, USA).
Cell culture
The extended lifespan human lens epithelial cell line (HLE B-3) used in these studies was developed by Andley (Andley et al., 1994), who first prepared them by isolating epithelium fragments from infant human lenses and from patients who underwent treatment for retinopathy of prematurity. These cells were allowed to grow from explants and infected with an adenovirus 12-SV40 hybrid virus (Ad12-SV40) to increase their ability to propagate in culture (Andley et al., 1994).
Cells were grown in Eagle’s MEM (Sigma) containing 2 mM L-glutamine, 50 µg/ml gentamicin, and 20% FBS in an atmosphere of 5% CO2 / 95% air at 37°C. The pH of the medium was adjusted to 7.4 before sterile filtration and addition of serum. Cells were fed 3 times a week and after attaining confluence were passaged using trypsin (0.125%) – EDTA (0.5 mM).
In vitro uptake studies of fullerol in HLE B3 cells
For light microscope analysis lens cells were incubated in: 35 mm glass bottom microwell dishes (MatTek Corp., Ashland, MA, USA) in 1.25 ml of 0–50 µM of fullerol solution in MEM/DMSO (99:1). After 16-hour incubation in the dark and in an atmosphere of 5% CO2 / 95% air at 37°C the cells were washed with Hank’s balanced salt solution (HBSS). Then they were overlaid with 1.25 ml of the cell culture medium and returned to the incubator. Directly before microscopic observation the cells were washed with HBSS and overlaid with 1.25 ml of MEM without phenol red. The cells were observed using phase contrast illumination with an Olympus IX70 microscope equipped with a Zeiss 10X UPlanFl objective (N.A. 0.3 and phase 1). A Zeiss AxioCam XEL color camera was used to acquire the images using and exposure time of 6ms with the AxioVision 4.4 software.
For spectrophotometric determination of fullerol uptake the lens cells were incubated in 96-well plates in 0 or 50 µM of the fullerene derivative in HBSS/DMSO (99:1) or MEM/DMSO (99:1) for up to 25 hours. After a specific time of incubation under the same condition as previously described, the supernatant was transferred quantitatively to an empty plate and left for measurement. The cells in each well were washed twice with HBSS and overlaid with 50 µl of the buffer. The uptake of fullerol by lens cells was confirmed by fullerol absorbance at 405 nm (Fig. 2d) using a GENios plate reader (Tecan US SPECTRAFluor Plus, Research Triangle Park, NC).
Fig. 2.
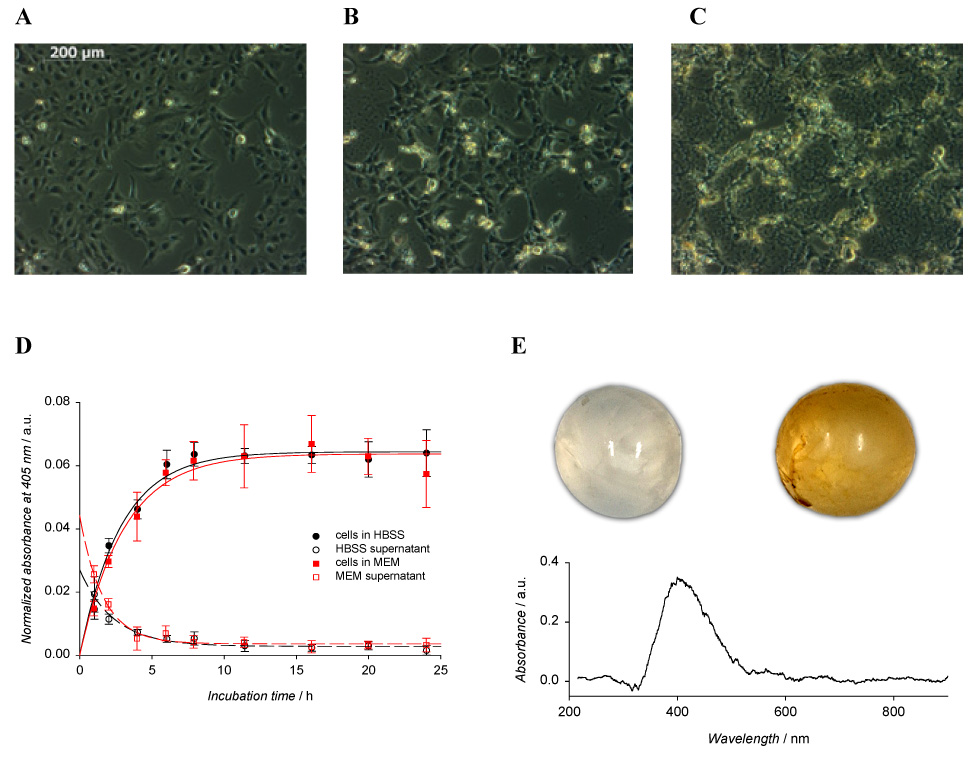
Accumulation of fullerol in HLE B3 cells (A–D) and in the rat’s lens (E). The cells were grown in: A–C) 3.5 cm glass-bottom plates and D) 96-well plates and incubated in: A–C) 1.25 ml of 0, 10, and 50 µM fullerol in MEM/DMSO (99:1), respectively for 16 h and D) 50 µl of 50 µM fullerol in HBSS/DMSO (99:1) or MEM/DMSO (99:1). The lenses in fig. E) were incubated for 16 h in 4 ml of HBSS/DMSO (99:1) (upper left) and in 50 µM fullerol in HBSS/DMSO (99:1) (upper right). Values in figure D) are the means ± SE (n=6). The lower graph (E) shows the absorption spectrum of fullerol accumulated in the rat’s lens.
Ex vivo uptake studies of fullerol
Sprague-Dawley rats were purchased from Charles River Labs and were housed in the NIEHS Animal Facility according to the NIEHS/AAALAC guidelines for animal care. A Sprague-Dawley rat (10 weeks old) was surgically anesthetized by an i.p. injection of Nembutal (1 ml/kg), then sacrificed by terminal bleed, and the eyes were removed. The lenses from the rat were extracted. The right lens was placed in 4 ml of 50 µM fullerol solution in HBSS/DMSO (99:1) while the left one in the same volume of 1% DMSO in HBSS (v/v). Both lenses were incubated in the dark for 24 h at 4°C. Then each lens was washed with PBS, placed on a sheet of blotting paper, and a picture was taken with a camera. For spectrophotometric measurement each lens was placed in appropriately designed holder, and the transmitted absorption spectrum was recorded using a diode array spectrophotometer HP8435 (Hewlett Packard Co., Palo Alto, CA, USA). The spectrum of uptaken fullerol was obtained by subtraction of the spectrum of the control lens from the spectrum of the rat lens incubated with fullerol and smoothing the resultant spectrum.
UVA / visible light treatment
Fullerol was dissolved in DMSO to obtain a stock solution of 5 mM and then diluted in MEM not supplemented with serum. Human lens epithelial cells were then incubated at 37°C in the dark with 1–50 µM fullerol for 16 h in MEM/DMSO (99:1) in 96-well plates for cyto- and phototoxicity studies and with 1–10 µM fullerol in HBSS/DMSO (99:1) for 17 h in 5-cm Petri dishes for apoptosis and necrosis determination. After incubation, the medium or buffer was removed and cells were washed twice with sterile HBSS. The cells were overlaid with sterile HBSS and exposed to UVA or visible light for 10 min and 1 h, respectively. HBSS was used in place of medium for all irradiation studies to prevent the phototoxicity from phenol red, riboflavin, or other medium components which are themselves photoactive with UVA and visible light. For UVA irradiation 4 fluorescent lamps [Houvalite F20T12/BL/HO (PUVA), National Biological Corp., Twinsburg, OH] were used. To remove a wavelengths below 300 nm the cells were covered with a plastic lid. For visible light treatment, cells were irradiated with cool white visible light (Phillips F40AX50 5000K Advantage X) that was filtered to transmit only wavelengths above 400 nm using a liquid filter (Wielgus et al., 2007). The UVA and the visible light irradiance were measured with a spectroradiometer (LuzChem Research Inc., Ottawa, ON, Canada) to be 61.4 W·m−2 and 23.5 W·m−2, respectively. Irradiation of the cells with UVA for 10 min and with visible light for 1 h gave a final UVA dose of 3.7 J cm−2 and light dose of 8.5 J cm−2.
Neither the UVA radiation nor visible light dosages at this energy level had any effect on the viability of the human lens epithelial cells. Control samples were kept in the dark under the same conditions.
Phototoxicity inhibition studies
In selected studies, the human lens cells were incubated with 20 µM lutein, or 1 mM N-acetyl-L-cysteine, or 1 mM L-(+)-ascorbic acid in MEM/DMSO (99:1) for 2 h, prior to exposure to 15 µM fullerol in MEM/DMSO (99:1). The doses of the antioxidants used in our study were chosen to mimic their endogenous concentrations, which were previously determined in the human lens (lutein) (Hankinson et al., 1992; Yeum et al., 1995) or in the aqueous humor which feeds the lens (ascorbic acid) (Iqbal et al., 1999). In other studies, the cells were incubated with 0.5 mM or 1 mM of either L-(+)-ascorbic acid or dehydroascorbic acid (DHA), or 20 µM or 50 µM L-ascorbic acid 6-palmitate (AA6P) in MEM/DMSO (99:1) for 2 h, prior to exposure to 20 µM fullerol in MEM/DMSO (99:1). Dehydroascorbate (DHA) is better absorbed by cells than ascorbate (AscH−) and then is reduced inside the cells (Corti et al., 2004; Sasaki et al., 1995). Therefore we used the oxidized form at the same concentration as AscH− to detect the ability of ascorbate hydrophilic forms to prevent fullerol cyto- and phototoxic effects in cytoplasm. Ascorbate palmitate at a concentration comparable to that of lutein was used to confirm that the cytoplasm membrane is one of the targets of fullerol toxicity. After incubation with a specific phototoxicity inhibitor the supernatant was removed, the cells were washed with HBSS, and exposed to fullerol solution in MEM/DMSO (99:1) for 16 h as described earlier. Only the hydrophilic antioxidants were still present in solutions at corresponding concentrations during incubation with fullerol and UVA exposure but not in the medium in which the cells were incubated after irradiation. Control cells were incubated in 1% DMSO in MEM during both series of experiments.
MTS and LDH assays
After irradiation, the cells were incubated overnight in the cell culture medium at 37°C in 5% CO2 atmosphere. Metabolic activity and lactate dehydrogenase release of the cells were determined as described previously (Wielgus et al., 2007) using MTS (Cell Titer 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega Corp., Madison, WI, USA) and LDH (CytoTox 96® Non-Radioactive Cytotoxicity Assay, Promega Corp., Madison, WI, USA) assays, respectively.
Measurement of apoptotic and necrotic cells
Apoptotic and necrotic cells were quantitatively evaluated by flow cytometry (Martin et al., 1995; Reno et al., 1998). After irradiation, the cells were harvested by trypsinization and collected by centrifugation at 140 g for 6 min at 22°C. Cells were washed with cold phosphate buffered saline (PBS) and stained with Annexin V-FITC and Propidium Iodide (PI) using TACS™ Apoptosis Detection Kit according to the manufacturer’s instruction (Trevigen, Gaithersburg, MD, USA). Cells positive for PI, for Annexin V-FITC, or for both were quantified by flow cytometry using a Becton Dickinson FACSort (Becton Dickinson, Mountain View, CA). In the fluorescence dot plot histogram of Annexin V/PI stained cells, the lower left quadrant shows normal viable cells which are negative for both Annexin V and PI; the lower right quadrant shows early-apoptotic cells which are positive for Annexin V; the upper left quadrant shows necrotic cells which are positive for PI; while the upper right quadrant shows late-apoptotic cells which are positive for both Annexin V and PI (Martin et al., 1995; Reno et al., 1998).
Caspase-3 acitivity
Caspase-3 activity was determined using ApoAlert Caspase Fluorescent Assay Kit (Clontech Laboratories, Palo Alto, CA, USA). After irradiation, the cells were incubated in the cell culture medium at 37°C in 5% CO2 atmosphere for 6.5 h, then removed with a cell lifter, washed with PBS by centrifugation at 400g for 10 min at 4°C, and lysed. The cell lysates were centrifuged at 12000×g for 10 min at 4°C to precipitate cellular debris. Supernatants were used for determination of caspase-3 activity by incubation at 37°C for 1 h with a fluorescent substrate, DEVD-AFC. Fluorescence of cleaved AFC was measured in a plate reader at excitation and emission wavelengths of 405 and 535 nm, respectively. A stock solution of free AFC was used for preparation of a calibration curve. The results were expressed as a ratio of AFC released per hour and normalized to the amount of protein in the cell lysate supernatant.
Particle size measurements
Dynamic light scattering (DLS) was used to determine the particle size of fullerol in the presence and absence of the most abundant lens protein, α-crystalline (Horwitz et al., 1999). HBSS (pH 7.4) was purged with argon before preparation of fullerol and α-crystallin solutions. Fullerol was mixed on a plate stirrer in 2 separate glass vials containing HBSS. After an hour, a specific amount α-crystallin was added to one vial of a dispersion of the fullerol particles (70 µM) and to another one containing only HBSS. Solutions of 1 mg/ml α-crystallin, 70 µM fullerol, and the mixture of fullerol with the protein were formed with gentle stirring for 16 hours. All solutions were prepared in dim light at 37°C. Measurements of particle size were carried out using a light scattering Zetasizer Nano-S light scattering instrument (Malvern Instruments, Southboro, MA, USA).
Statistical analysis
Data in graphs in figure 2–figure 5 and figure 7 are presented as mean ± SE of four to six experiments and in figure 6 as mean ± range of two experiments. All p values were calculated using the ANOVA test.
Fig. 5.
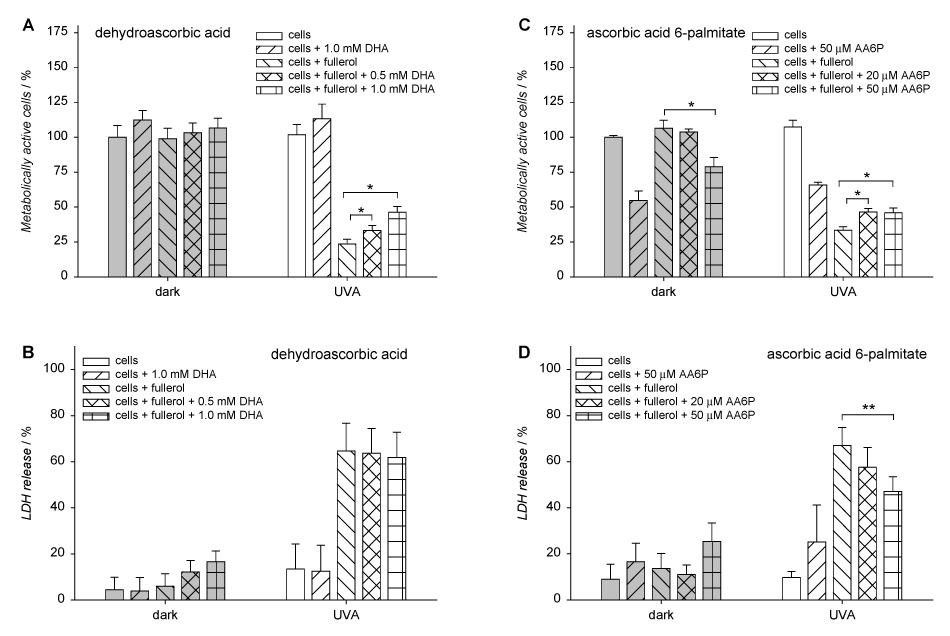
Protective effect of dehydroascorbic acid (DHA) and L-ascorbic acid 6-palmitate (AA6P) from fullerol phototoxicity in human lens epithelial cells. The cells were incubated with an ascorbate derivative in Minimum Essential Medium (MEM)/DMSO (99:1) for 2 h and with 20 µM fullerol in MEM/DMSO (99:1) for the next 16 h. Then the cells were washed and overlaid with HBSS and exposed to UVA for 10 min. After irradiation, the cells were incubated overnight in MEM/FBS (8:2). Metabolic activity (A, C) and lactate dehydrogenase release (B, D) from the cells were determined with MTS and LDH assays, respectively. Results are expressed as mean ± SE (n=4). * p<0.01, and ** p<0.02 comparing metabolic activity or LDH release in cells incubated with antioxidant and fullerol vs. cells incubated only with fullerol both in the “dark” and “UVA exposed” group.
Fig. 7.
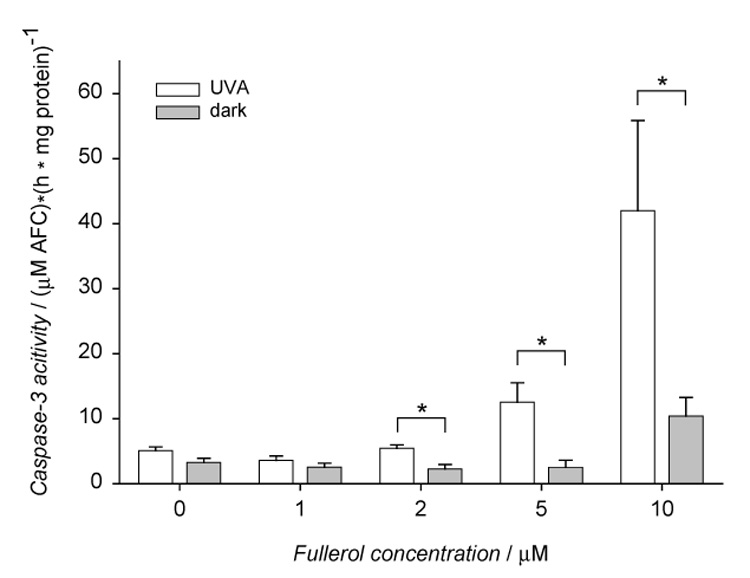
Fullerol effect on caspase-3 activation in HLE cells stored in the dark or exposed to UVA. The cells were incubated with 0–10 µM fullerol in Minimum Essential Medium (MEM)/DMSO (99:1) for 17 h and then exposed to UVA for 10 min. After irradiation the cells were incubated in MEM/FBS (8:2) for 6.5 h. Caspase-3 activity was determined in cytosolic extracts with ApoAlert Caspase Fluorescent Assay Kit, where DEVD-AFC fluorescent substrate was used. Values are expressed as mean ± SE (n=6). * p<0.01 comparing results obtained in cells incubated with fullerol and irradiated with UVA vs. the fullerol-treated cells incubated in the dark. The differences in caspase-3 activity between fullerol-treated cells vs control cells were statistically significant at p<0.01 for 5 and 10 µM fullerol within the group of cells exposed to UVA and 10 µM within the group of cells kept in the dark.
Fig. 6.
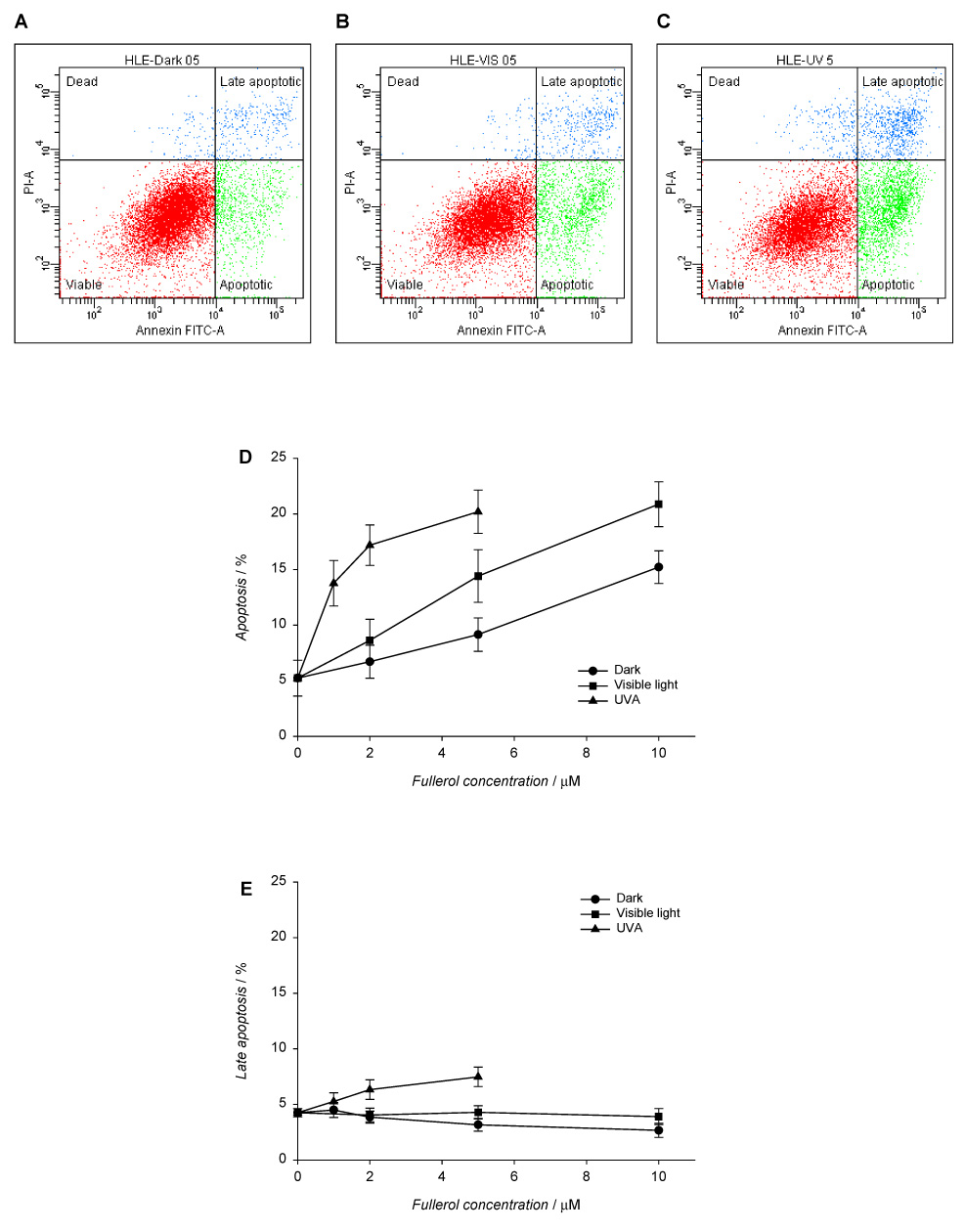
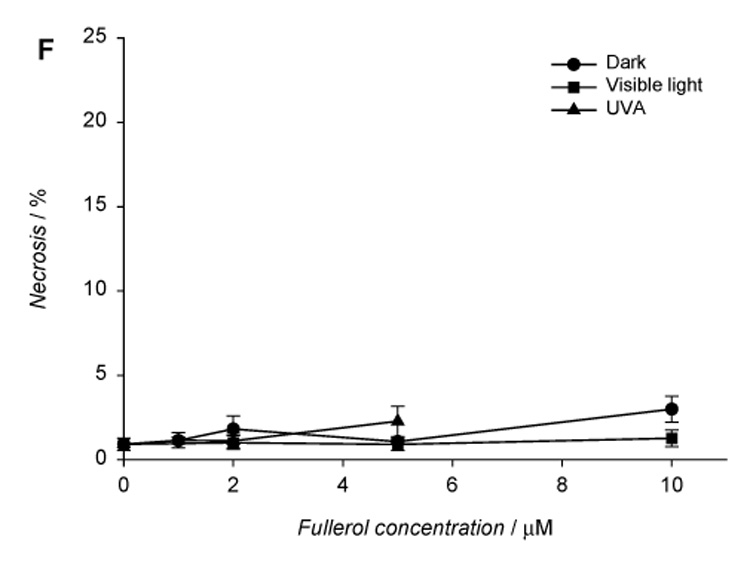
Fullerol-induced apoptotic and necrotic death in human lens epithelial cells. The cells were incubated with fullerol in HBSS/DMSO (99:1) in the dark for 17 h and exposed to visible light (1 h) or UVA (10 min). After irradiation, the cells were incubated overnight in MEM/FBS (8:2) and then stained with Annexin V-FITC and propidium iodide. Apoptotic and necrotic cell death were determined with flow cytometry. Histograms A–C show populations of HLE cells preincubated with 5 µM fullerol in the dark (A) and after exposure to visible light (B) or UVA (C). The graphs illustrate the number cells in apoptotic (D), late apoptotic (E), and necrotic state normalized to the total number of counted cells (F). Values in D–F graphs are expressed as mean ± range (n=2).
Results
In vitro and ex vivo uptake of fullerol into lens tissues
In vitro uptake of fullerol into human lens epithelial cells (HLE B-3) was confirmed by light microscopy (Fig. 2A–C). The cells were incubated in the presence of fullerol in 1% DMSO solution in cell culture medium without serum to avoid binding of the fullerene derivative to the serum proteins (Deguchi et al., 2007). Although most of the cells that were incubated for 16 h in 1% DMSO solution in cell culture medium without serum remained alive, a few bright spots in the microscope image (Fig. 2A) represent dead cells. Uptake of 10 µM fullerol is indicated by a distinct yellow color appearing in the microscopic image (Fig. 2B). At this concentration the cells became somewhat rounded. There is an increase in intensity of this yellow color (Fig. 2C) with 50 µM of fullerol uptake, but there is also increased cytotoxic damage to the lens cells.
Fullerol uptake by lens cells was confirmed by absorbance at 405 nm (Fig. 2D). When the cells were incubated in 50 µM solution of fullerol in HBSS, the amount of accumulated compound increased with time and reached a plateau after ~15 hours. At the same time fullerol concentration proportionally decreased in the cell supernatant.
Further studies with the whole rat lens showed the ex vivo uptake of fullerol (Fig. 2E). The lens incubated over night with fullerol became yellow. Spectrophotometric measurement confirmed accumulation of the fullerene derivative in the lens. The differential absorption spectrum showed a band in the 330–530 nm range with a peak at 405 nm.
Photo- and cytotoxicity of fullerol to lens cells
We measured the metabolic activity (MTS) and lactate dehydrogenase release (LDH) of HLE B3 cells in the presence of 0–50 µM fullerol, either maintained in the dark or in the presence of 3.7 J·cm−2 UVA or 8.5 J·cm−2 visible light. Fullerol was found to decrease metabolic activity and increased the release of lactate dehydrogenase in HLE B-3 cells maintained in the dark at concentrations higher than 20 µM (Fig. 3). Neither exposure to visible light nor UVA alone reduced cell viability, but exposure to either UVA or visible light in the presence of >5 µM fullerol induced phototoxic damage.
Fig. 3.
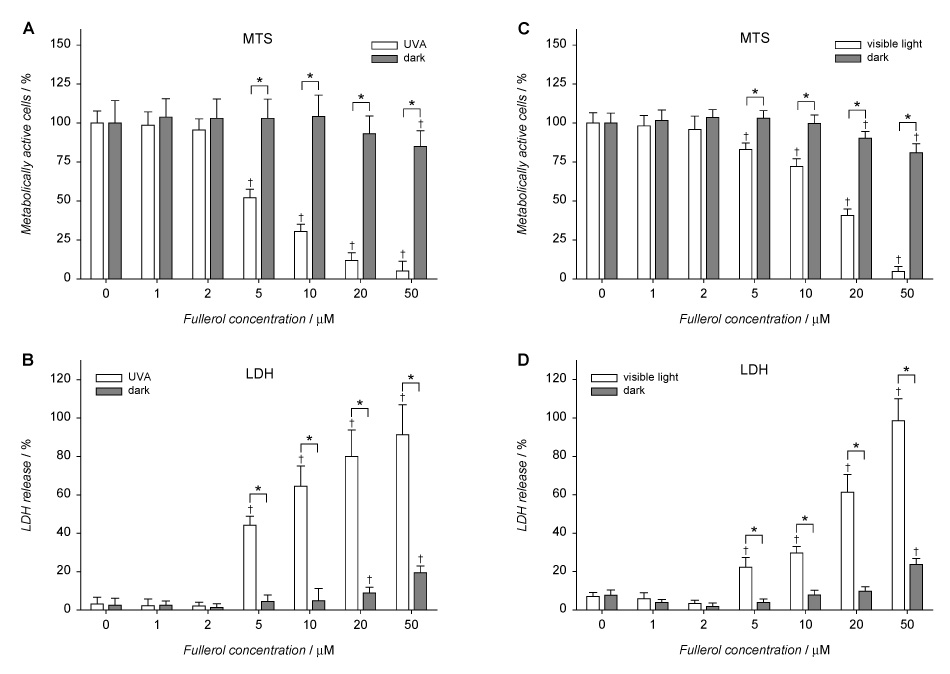
Phototoxicity and cytotoxicity of fullerol in HLE B3 cells. The cells were incubated with 0–50 µM fullerol in Minimum Essential Medium (MEM)/DMSO (99:1) for 16 h. Then they were washed and overlaid with HBSS and exposed to UVA for 10 min (A, B) or visible light for 1 h (C, D). After irradiation, the cells were incubated overnight in MEM/FBS (8:2). Metabolic activity and lactate dehydrogenase release of the cells were determined with MTS and LDH assays, respectively. Values are expressed as mean ± SE (n=6). * p<0.01 comparing corresponding values in each pair of samples (irradiated vs. dark). † p<0.01 comparing results obtained for cells treated with fullerol vs. controls (0 µM fullerol) within each group of cells.
Inhibition of fullerol phototoxicity to lens cells
To determine the mechanism of fullerol phototoxicity and the possible involvement of reactive oxygen intermediates, we examined the effect of specific quenchers (lutein, N-acetyl-Lcysteine – the glutathione mimic, and L-ascorbic acid) that are endogenous to the human lens (Busch et al., 1999; Hammond et al., 1997; Iqbal et al., 1999) and the aqueous humor that feeds the lens. We chose concentrations of fullerol (15–20 µM) that almost completely inhibited metabolic activity and dramatically enhanced lactate dehydrogenase release from the lens cells. In the presence of 20 μM lutein, added 2 h prior to irradiation with UVA, phototoxic damage decreased by half (Fig. 4). On the other hand, no protection against fullerol phototoxicity was offered when cells were preincubated with 1 mM N-acetyl-L-cysteine or 1 mM L-ascorbic acid at 37°C. These antioxidants were present for 2 h prior to and during both incubation with fullerol and UVA exposure (data not shown).
Fig. 4.

Lutein protects human lens epithelial cells from phototoxicity of fullerol. The cells were incubated with 20 µM lutein in Minimum Essential Medium (MEM)/DMSO (99:1) for 2 h and with 15µM fullerol in MEM/DMSO (99:1) for the next 16 h. Then the cells were washed and overlaid with HBSS and exposed to UVA for 10 min. After irradiation, the cells were incubated overnight in MEM/FBS (8:2). Metabolic activity (A) and lactate dehydrogenase release (B) from the cells were determined with MTS and LDH assays, respectively. Values are expressed as mean ± SE (n=4). * p<0.01, comparing metabolic activity or LDH release in cells incubated with lutein and fullerol vs. cells incubated only with fullerol both in the “dark” and “UVA exposed” group.
The quenching properties of other forms of ascorbic acid were also examined. Dehydroascorbic acid as an oxidized form of vitamin C showed a slight protective effect on metabolic activity of the cells (Fig. 5A) but no effect on LDH release (Fig. 5B). The hydrophobic L-ascorbic acid 6-palmitate reduced the release of LDH by about 20% (Fig. 5D), but had almost no protective effect against fullerol-induced inhibition of metabolic activity (Fig. 5C).
Analysis of fullerol-induced apoptosis and necrosis
Apoptosis was quantified using flow cytometry. Early apoptosis is characterized by plasma membrane reorganization (Martin et al., 1995; Reno et al., 1998; Singh, 2000) and is detected by positive staining for Annexin V-FITC while later stage apoptosis indicating DNA damage shows positive staining for both Annexin V and PI. We measured necrosis by determining the percentage of cells which were positive for only PI. As seen in Fig. 6, at concentrations of 1–10 µM the primary damage to the lens cells by fullerol was through early apoptosis (up to 20%).
Incubation with 10 µM fullerol caused activation of caspase-3 in HLE cells (Fig. 7). The protease activity was enhanced by UVA exposure. A significant increase in the enzyme activity was observed in irradiated cells preincubated with fullerol at concentrations higher than 2 µM.
Interaction of fullerol with α-crystallin
α-Crystallin is a major lens protein that is present in human lens epithelial cells and also comprises of up to 40% of nuclear lens proteins (Horwitz et al., 1999). We analyzed the interaction between fullerol and α-crystallin by determination of their particle size (Fig. 8). The average diameter of fullerol particles in 70 µM solution in HBSS buffer at pH 7.4 was 695 ± 80 nm. However, α-crystallin at 1 mg/ml in the same buffer was characterized by 3 types of aggregates with sizes of 21 ± 5 nm, 374 ± 160 nm, and 5053 ± 580 nm, respectively. When fullerol and α-crystallin were incubated together under the same conditions, there was a loss of the largest aggregates of crystallin and of fullerol and only particles of sizes: 23 ± 2 nm and 356 ± 35 nm were detected. This suggests that there is non-specific binding of fullerol to α-crystallin similar to what has been seen with C60 nanoparticles and human serum albumin (HSA) (Deguchi et al., 2007).
Fig. 8.
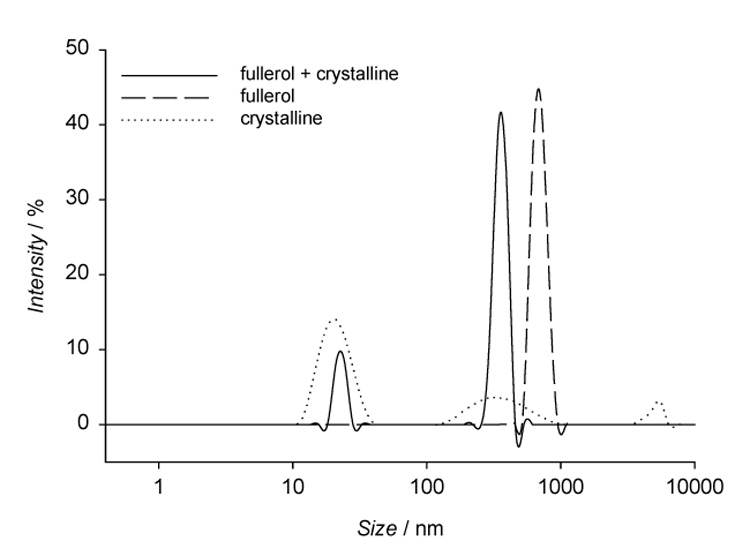
DLS determination of particle size of 70 µM fullerol and 1 mg/ml α-crystallin in HBSS. Measurements of particle size distributions of fullerol and lens protein (solid line), fullerol (dashed line), and lens protein (dotted line).
Discussion
Water-soluble fullerene derivatives show antiviral activity and are of particular interest in drug delivery to the eye and the brain. Concentrations in the range of 5–25 µM (Jensen et al. 1996) have been found to reduce HIV-1 virus infectivity by more than 95%. Intravenous injection of a polar C60 derivative to rats led to accumulation of the compound in many animal organs including the brain and the eye (Jensen et al., 1996). The use of nanosized (colloid) carriers for drug delivery is described and particles have been found months after dosing in the eye (Amrite and Kompella, 2005; Bourges et al., 2003; Jani et al., 1989).
We have determined that fullerol accumulates in the human lens cells. The fullerene derivative has very low fluorescence intensity and so confocal microscopy could not be used to measure its uptake by the lens cells. Instead, uptake was confirmed visually and by spectrophotometric measurements (Fig. 2 A–E). Fullerol exhibits both dark cytotoxicity and phototoxic effects on human lens epithelial cells. The levels of illumination that were chosen for the phototoxicity studies represent the amount of ambient UVA and visible light exposure one would expect of a bright, sunny day (Diffey, 1977; Sliney, 2002). UVA exposure included a cutoff filter to remove all radiation below 295 nm to mimic human corneal transmission (Barker et al., 1991). Light exposure in the absence of fullerol induced no detectable damage in the human lens epithelial cells. Exposure to visible light or UVA in the presence of fullerol, however, induced dose-related apoptosis in lens epithelial cells.
The present results demonstrate that fullerol, which is being considered for future medical use, can damage human lens epithelial cells. Fullerol decreased metabolic activity and increased lactate dehydrogenase release in the HLE B3 cells in the presence of concentrations higher than 20 µM fullerol. When these lens cells were irradiated with either 3.7 J·cm−2 UVA or 8.5 J·cm−2 visible light, metabolic activity was decreased and lactate dehydrogenase release was apparent with less than 5 µM fullerol.
The endogenous antioxidant, lutein, (Hankinson et al., 1992; Yeum et al., 1995) offered some protection against the phototoxic damage induced by fullerol, but neither the glutathione mimic N-acetyl-L-cysteine (Busch et al., 1999) nor L-ascorbic acid appear to provide any protection for the human lens epithelial cells.
Ascorbic acid (Vitamin C) in the reduced form is present in high concentrations (1 mM) in the aqueous humor which feeds the human lens. It primarily protects the lens from damage occurring on the lens surface (Iqbal et al., 1999). When the oxidized form of L-ascorbic acid, dehydroascorbic acid, was tested for its protective effect against phototoxic damage induced by fullerol, there was some protection against metabolic damage. Dehydroascorbic acid is more readily absorbed by the lens than the reduced form (Corti et al., 2004). Additionally, the internalization of DHA is enhanced by glucose transporter proteins (GLUT1 and GLUT3), which are expressed in the lens (Corti et al., 2004). In the cytoplasm, oxidized ascorbic acid is efficiently reduced by a glutathione (GSH) and NADPH dependent mechanism to produce L-ascorbic acid (Corti et al., 2004; Sasaki et al., 1995). We found that the only water-soluble quencher to offer any protection against phototoxic damage inside the cells was the oxidized form of ascorbic acid. During mild oxidative stress, ascorbic acid contained in aqueous humor may provide tissues of the anterior eye with a source of oxidized ascorbic acid which is readily taken up and converted to intracellular reduced ascorbic acid, while intracellular ascorbic acid is maintained in the reduced status by intracellular antioxidant systems. This process could be impaired in the presence of fullerol.
When we exposed the cells to fullerol and either lutein or the ascorbate 6-palmitate, which are lipid soluble quenchers, there was a much greater protective effect, especially in the form of a reduction in the release of LDH from the cells. This would strongly suggest that the phototoxic damage of fullerol to lens cells is to the membranes of the cells. When light excites a phototoxic agent it can lead to the generation of reactive oxygen species including singlet oxygen and superoxide anion (Roberts, 2002). As lutein is a singlet oxygen quencher, the phototoxic mechanism of damage is very likely to involve singlet oxygen as one of the main reactive oxygen species. Enhanced production of reactive oxygen species within the lens of the eye and consequent oxidative damage to the lens proteins is thought to be a major mechanism leading to the onset of cataracts. Additionally, the lack of effect of ascorbic acid and N-acetyl-L-cysteine could be due to compartmentalization of these water-soluble molecules within the cell in hydrophilic regions of the cytoplasm, rendering them ineffective against phototoxic reactions in the membrane.
Fullerol, both with and without irradiation from UVA or visible light, induced early apoptosis, again indicating membrane damage. These experiments also suggest that disruption of the plasma membrane of the lens cells is an important mechanism in the cyto- and phototoxic damage by fullerol. Apoptosis in human lens cells has also been shown to lead to the development of cataracts (Okamura et al., 2002).
Implications for human health
One of the most frequent causes of blindness in humans is cataract formation. The human lens is made up of two distinct layers: the surface of the lens where the lens epithelial cells (cortex) are located and an inner layer (nucleus) which contains the protein fibers. The orderly arrangement of these protein fibers causes the lens to be highly transparent (Benedek, 1971). Any disruption in the function of the lens epithelial cells or in the structural integrity of crystallin lens proteins in the protein fibers leads to a loss of the transparency of the lens known as a cataract. If the damage is to the epithelial cells a cortical cataract is formed while damage to the interior of the lens induces a nuclear cataract (Roberts, 2001).
Both UVA and UVB exposure are risk factors for the development of cortical cataracts (Roberts, 2001), whereas in the presence of a phototoxic agent, UVA and visible light are associated with both cortical and nuclear cataracts (Roberts, 2002). Although senile cataracts are an age related disorder, these normally occur with patients that are over 70 years old. Phototoxic agents can induce cataracts at a much younger age.
Longer wavelengths of light penetrate deeper into biological tissues. Although UVB is stopped by the lens epithelial layer, UVA and visible light can penetrate into the superficial cortex, be absorbed in the interior, and reach the lens proteins. α-Crystallin is a major lens protein which acts as a heat shock protein to prevent UV induced protein aggregation in lens epithelial cells (Andley et al., 1998). α-Crystallin also comprises up to 40% of nuclear lens proteins, where its structural role is to assist in maintaining the proper refractive index in the lens and to prevent the formation of large light-scattering aggregates (Horwitz et al., 1999). We have shown here using DLS method measurement of nanoparticle size that in the presence of α-crystallin, fullerol is in a less aggregated form in the scale measured. We have found previously that compounds with multiple hydroxyl groups (i.e., hypericin) bind to and phototoxidize α-crystallin (Schey et al., 2000). Others have shown that there is non-specific binding with human serum albumin (HSA) and other C60 nanoparticles (Deguchi et al., 2007). Binding of fullerol to serum proteins does not interfere with its accumulation in many tissues (Ji et al., 2006). Binding of a drug to an ocular tissue would increase its retention time in the eye and therefore the drug would be more likely to induce phototoxic damage. Our previous work with photoreactive compounds has shown that the lens protein most susceptible to phototoxic oxidative damage is α-crystallin, and that damage to this protein alone is sufficient to cause a cataract (Roberts et al., 1991).
Although UVA-blocking sunglasses will protect critical components of the lens against some of the damage by fullerol, the visible light wavelengths that induce a phototoxic effect by fullerol are in the same range as those used for sight and therefore are not blocked by typical sunglasses. Before fullerols are used in the future to deliver drugs to the eye, their potential side effects on the human eye should be further examined.
Conclusion
Our results with in vitro and ex vivo lens models indicate that exposure to fullerols, particularly in the presence of sunlight, may lead to early cataractogenesis. Although the acute toxicity of water soluble nano-C60 is low, these compounds are retained in the body for long periods, raising concern for their chronic toxic effect. Before fullerols are used to deliver drugs to the eye, they should be thoroughly tested for photo- and cytotoxicity.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. We wish to thank Dr. Ann Motten National Institute of Environmental Health Sciences (NIEHS) for help in preparation of this manuscript and Dr. Carl Bortner and Maria Sifre for their assistance with flow cytometry measurements. Images of the cells were taken by C. Jeff Tucker at the NIEHS Fluorescence Microscopy and Imaging Center. The images of the whole lenses were taken by Steve McCaw and the figure of fullerol was designed by David Green both of NIEHS Arts and Photograph Center. We also wish to thank Dr. Nigel Walker of National Toxicology Program, NIEHS for supplying the fullerol used in these experiments and Dr. Laura Degn of US Environmental Protection Agency (USEPA) for assistance with dynamic light scattering measurements. This manuscript has been reviewed by the National Health and Environmental Effects Research Laboratory, USEPA and NIEHS and approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation for use.
Footnotes
Abbreviations: AA6P, L-ascorbic acid 6-palmitate; DHA, dehydroascorbic acid; DLS, dynamic light scattering; DMSO, dimethyl sulfoxide; EDTA, ethylenediamine tetraacetic acid; FBS, fetal bovine serum; GSH, reduced form of glutathione; HBSS, Hank’s balanced salt solution; HLE, human lens epithelial cells; HSA, human serum albumin; LDH, lactate dehydrogenase; MEM, minimum essential medium, MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; NADPH, reduced form of nicotinamide adenine dinucleotide phosphate; PBS, phosphate buffered saline; PI, propidium iodide; UV, ultraviolet radiation; UVA, ultraviolet radiation at 315–400 nm; UVB, ultraviolet radiation at 280–315 nm
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J. Pharm. Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- Andley UP. Ocular lens photobiology. In: Coohill TP, Valenzeno DP, editors. Photobiology for the 21st Century. Overland Park, Kansas: Valdenmar Publishing Company; 2001. [Google Scholar]
- Andley UP, Rhim JS, Chylack LT, Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J. Biol. Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Arbogast JA, Darmanyan AP, Foote CS, Rubin Y, Diederich FN, Alvarez MM, Anz SJ, Whetten RL. The photophysical properties of C60. J. Phys. Chem. 1991;95:11–12. [Google Scholar]
- Balasubramanian D. Ultraviolet radiation and cataract. J. Ocul. Pharmacol. Ther. 2000;16:285–297. doi: 10.1089/jop.2000.16.285. [DOI] [PubMed] [Google Scholar]
- Barker FM, Brainard GC, Dayhaw-Barker P. Transmission of the human lens as a function of age. Invest. Ophthalmol. Vis. Sci. 1991;32S:1083. [Google Scholar]
- Benedek GB. Theory of transparency of eye. Appl. Opt. 1971;10:459. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao D-Y, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp. Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Bogdanovic G, Kojic V, Dordevic A, Canadanovic-Brunet J, Vojinovic-Miloradov M, Baltic VV. Modulating activity of fullerol C60(OH)22 on doxorubicin-induced cytotoxicity. Toxicol. In Vitro. 2004;18:629–637. doi: 10.1016/j.tiv.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest. Ophthalmol. Vis. Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- Busch EM, Gorgels TG, Roberts JE, van Norren D. The effects of two stereoisomers of N-acetylcysteine on photochemical damage by UVA and blue light in rat retina. Photochem. Photobiol. 1999;70:353–358. [PubMed] [Google Scholar]
- Calvo P, Sanchez A, Martinez J, Lopez MI, Calonge M, Pastor JC, Alonso MJ. Polyester nanocapsules as new topical ocular delivery systems for cyclosporin A. Pharm. Res. 1996;13:311–315. doi: 10.1023/a:1016015803611. [DOI] [PubMed] [Google Scholar]
- Corti A, SM F, Lazzarotti A, Del Corso A, Mura U, Casini AF, Paolicchi A. UV light increases vitamin C uptake by bovine lens epithelial cells. Mol. Vis. 2004;10:533–536. [PubMed] [Google Scholar]
- Da Ros T, Prato M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999;8:663–669. [Google Scholar]
- Deguchi S, Yamazaki T, Mukai S, Usami R, Horikoshi K. Stabilization of C(60) nanoparticles by protein adsorption and its implications for toxicity studies. Chem. Res. Toxicol. 2007;20:854–858. doi: 10.1021/tx6003198. [DOI] [PubMed] [Google Scholar]
- Diffey BL. The calculation of the spectral distribution of natural ultraviolet radiation under clear day conditions. Phys. Med. Biol. 1977;22:309–316. doi: 10.1088/0031-9155/22/2/010. [DOI] [PubMed] [Google Scholar]
- Dillon J. The photophysics and photobiology of the eye. J. Photochem. Photobiol. B. 1991;10:23–40. doi: 10.1016/1011-1344(91)80209-z. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, Shen CK, Luh TY, Choi DW, Lin TS. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. U S A. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives: Model building studies and experimental verification. J. Am. Chem. Soc. 1993;115:6506–6509. [Google Scholar]
- Guldi DM, Prato M. Excited-state properties of C(60) fullerene derivatives. Acc. Chem. Res. 2000;33:695–703. doi: 10.1021/ar990144m. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Wooten BR, Snodderly DM. Density of the human crystalline lens is related to the macular pigment carotenoids, lutein and zeaxanthin. Optom. Vis. Sci. 1997;74:499–504. doi: 10.1097/00006324-199707000-00017. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, Willett WC. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992;305:335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Bova MP, Ding LL, Haley DA, Stewart PL. Lens alpha-crystallin: function and structure. Eye. 1999;13:403–408. doi: 10.1038/eye.1999.114. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Midgley JM, Watson DG, Karditsas SD, Dutton GN, Wilson WS. Effect of oral administration of vitamin C on human aqueous humor ascorbate concentration. Zhongguo Yao Li Xue Bao. 1999;20:879–883. [PubMed] [Google Scholar]
- Irie K, Nakamura Y, Ohigashi H, Tokuyama H, Yamago S, Nakamura E. Photocytotoxicity of water-soluble fullerene derivatives. Biosci. Biotechnol. Biochem. 1996;60:1359–1361. doi: 10.1271/bbb.60.1359. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J. Pharm. Pharmacol. 1989;41:809–812. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg. Med. Chemistry. 1996;4:767–779. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
- Ji ZQ, Sun H, Wang H, Xie Q, Liu Y, Wang Z. Biodistribution and tumor uptake of C60(OH)x in mice. J. Nanopart. Res. 2006;8:53–63. [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003;36:807–815. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- Okamura N, Ito Y, Shibata M-A, Ikeda T, Otsuki Y. Fas-mediated apoptosis in human lens epithelial cells of cataracts associated with diabetic retinopathy. Med. Electron Microsc. 2002;35:234–241. doi: 10.1007/s007950200027. [DOI] [PubMed] [Google Scholar]
- Prat F, Stackow R, Bernstein R, Qian W, Rubin Y, Foote CS. Triplet-state properties and singlet oxygen generation in homologous series of functionalized fullerene derivatives. J. Phys. Chem. 1999;103:7230–7235. [Google Scholar]
- Rancan F, Rosan S, Boehm F, Cantrell A, Brellreich M, Schoenberger H, Hirsch A, Moussa F. Cytotoxicity and photocytotoxicity of a dendritic C(60) mono-adduct and a malonic acid C(60) tris-adduct on Jurkat cells. J. Photochem. Photobiol. 2002;B 67:157–162. doi: 10.1016/s1011-1344(02)00320-2. [DOI] [PubMed] [Google Scholar]
- Reno F, Burattini S, Rossi S, Luchetti F, Columbaro M, Santi S, Papa S, Falcieri E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem. Cell Biol. 1998;110:467–476. doi: 10.1007/s004180050308. [DOI] [PubMed] [Google Scholar]
- Roberts JE. Ocular phototoxicity. J. Photochem. Photobiol. B. 2001;64:136–143. doi: 10.1016/s1011-1344(01)00196-8. [DOI] [PubMed] [Google Scholar]
- Roberts JE. Screening for ocular phototoxicity. Int. J. Toxicol. 2002;21:491–500. doi: 10.1080/10915810290169918. [DOI] [PubMed] [Google Scholar]
- Roberts JE. Update on the positive effects of light in humans. Photochem. Photobiol. 2005;81:490–492. doi: 10.1562/2004-12-02-IR-391. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Atherton SJ, Dillon J. Detection of porphyrin excited states in the intact bovine lens. Photochem. Photobiol. 1991;54:855–857. doi: 10.1111/j.1751-1097.1991.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Giblin FJ, Winkler BS, Chakrapani B, Leverenz V, Shu CC. A protective role for glutathione-dependent reduction of dehydroascorbic acid in lens epithelium. Invest. Ophthalmol. Vis. Sci. 1995;36:1804–1817. [PubMed] [Google Scholar]
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004;4:1881–1887. [Google Scholar]
- Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE. Photooxidation of lens alpha-crystallin by hypericin (active ingredient in St. John's Wort) Photochem. Photobiol. 2000;72:200–203. doi: 10.1562/0031-8655(2000)072<0200:polcbh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schinazi RF, Sijbesma R, Srdanov G, Hill CL, Wudl F. Synthesis and virucidal activity of a water-soluble, configurationally stable, derivatized C60 fullerene. Antimicrob. Agents Chemother. 1993;37:1707–1710. doi: 10.1128/aac.37.8.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP. A simple method for accurate estimation of apoptotic cells. Exp. Cell Res. 2000;256:328–337. doi: 10.1006/excr.2000.4810. [DOI] [PubMed] [Google Scholar]
- Sliney DH. How light reaches the eye and its components. Int. J. Toxicol. 2002;21:501–509. doi: 10.1080/10915810290169927. [DOI] [PubMed] [Google Scholar]
- Wielgus AR, Chignell CF, Miller DS, Van Houten B, Meyer J, Hu D-N, Roberts JE. Phototoxicity in human retinal pigment epithelial cells promoted by hypericin, a component of St. John's Wort. Photochem. Photobiol. 2007;83:706–713. doi: 10.1562/2006-08-09-RA-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K, Nakahara H, Enomoto S, Ambe F. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem. Biol. 1995;2:385–389. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J. Am. Chem. Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- Yang XL, Fan CH, Zhu HS. Photo-induced cytotoxicity of malonic acid [C(60)]fullerene derivatives and its mechanism. Toxicol. In Vitro. 2002;16:41–46. doi: 10.1016/s0887-2333(01)00102-3. [DOI] [PubMed] [Google Scholar]
- Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest. Ophthalmol. Vis. Sci. 1995;36:2756–2761. [PubMed] [Google Scholar]
- Zakharenko LP, Zakharov IK, Vasiunina EA, Karamysheva TV, Danilenko AM, Nikiforov AA. Determination of the genotoxicity of fullerene C60 and fullerol using the method of somatic mosaics on cells of Drosophila melanogaster wing and SOS-chromotest. Genetika. 1997;33:405–409. [PubMed] [Google Scholar]


