Introduction
Although an underlying vulnerability to acute sleep disturbance and the subsequent development of chronic insomnia has been proposed by several investigators [1–3], relatively little data regarding specific factors that might predispose individuals to insomnia have been identified. This, despite the fact that one of the most commonly held models of insomnia proposes a predisposition (i.e., an individual difference in vulnerability) to insomnia as a central tenant of the model [4]. The identification of specific characteristics that increase the risk for insomnia is an important scientific endeavor for a variety of reasons. First, understanding these characteristics could help identify at-risk individuals early in the evolution of insomnia and may lead to improvements in treatment and even prevention of disease progression. Second, identification of at-risk individuals will allow the study of physiological, behavioral, and cognitive processes involved in the development of insomnia, even prior to its full development. This would be an important advance as early underlying components of the disorder could be identified and studied separately from morbidity components. For example, it would allow for determination of whether hyperarousal contributes to the evolution of insomnia or is a consequence of insomnia. Finally, the identification of predisposed individuals would facilitate the investigation of phenotypic traits which may interact with environmental stress and could be linked to the development of chronic insomnia, and the identification of such traits could help parse out environmental effects from predisposing factors, which could facilitate future genetic studies of insomnia and its predisposition in vulnerable individuals.
Several lines of research have begun to study systematic differences in vulnerability to sleep disturbance using a variety of methods. Recently, Bonnet and Arand [1] showed that individuals with low sleep efficiency on a first night in the laboratory had consistent sleep disturbance in response to circadian as well as caffeine challenges. Their data support the notion of a stable trait characteristic associated with sleep disturbance in response to common sleep challenges. In a study of norepinephrine knockout mice, disturbed sleep (i.e., increased latency to sleep) in response to handling stress was entirely dependent upon an intact norepinephrine system [5–7]. Data from our laboratory provide further evidence for a trait vulnerability to sleep disturbance. Specifically, subjects’ self-evaluation of sleep-related vulnerability to common stressors predicts the degree of sleep disturbance (measured polysomnographically) to both a first-night effect [2] and caffeine administration [3]. At present, the relevance of these data for the development of chronic insomnia is not known. However, the finding that norepinephrine knockout mice have normal sleep prior to being exposed to stress, in addition to the fact that in the data from both Drake and Bonnet there was increased risk of disturbed sleep in only a subset of normal sleeping volunteers, supports the possibility of a biological substrate representing a sleep-response system, which may identify the population at risk for developing insomnia.
It must be emphasized that at present there is very little data directly linking vulnerability to acute sleep disturbance to the eventual development of chronic insomnia. Even if there is a subset of individuals who are particularly sensitive to acute sleep disturbance, this characteristic may be completely unrelated to chronic insomnia. For instance, the subset of individuals with transient symptoms may simply reflect those individuals with high exposure to significant sleep disruptive stressors. Clearly much work remains to be done investigating the links between transient sleep disturbance and chronic insomnia, but one recent study has provided some evidence for this relationship [8]. In that study, a previous history of transient insomnia was an independent and prospective predictor of the development of chronic insomnia following hospitalization for various disorders. Interestingly, length of hospital stay, hospital sleep quality, and cardiac surgery were not predictive of post-hospital insomnia, suggesting that a history of short-term sleep disturbance may be an even more important variable than more proximal factors.
The data reviewed above suggest that a trait of sleep-related responsivity to stress may exist. If such a trait does exist, one would hypothesize that this predisposition is genetically transmitted and thus insomnia would also have a genetic component. Indeed, a recent twin study has shown that more than 50% of the variance in the risk for insomnia can be attributed to genetic components [9]. It then follows that if increased sleep responsivity is a significant risk factor for insomnia, it too should show a significant familial aggregation.
As the study of potential predisposing factors in insomnia has increased in recent years, several measures have been developed to aid in identifying individuals predisposed to insomnia prior to the onset of the condition [2,10]. Of these measures, the Ford Insomnia Response to Stress Test (FIRST) has been validated by a priori identifying individuals at risk for disturbed sleep using polysomnography in response to a challenge [3]. Furthermore, preliminary data suggests that this measure has some predictive validity in determining the development of insomnia over a 13-month follow-up period [11].
Twin studies of insomnia suggest the possibility that other related constructs such as vulnerability to acute/transient sleep disturbance in response to stress may also have a familial component. Thus, if the FIRST is a reliable and valid measure of the vulnerability to acute sleep disturbance, there may be a relationship between siblings in FIRST scores reflecting the familial aggregation of this construct. The present study tested this hypothesis using the FIRST as a measure of individual vulnerability to stress-related sleep disturbance in non-insomniac siblings. Data on stress-related sleep disturbance were also collected for individuals who met criteria for insomnia in order to further characterize the discriminative validity of the FIRST in the sleep problem population.
Methods
Participants
Individuals ages 18 to 70 years were recruited from a sample of individuals who had previously (1–3 years prior) participated in an epidemiological random-digit-dial phone survey in conjunction with the Henry Ford Hospital Sleep Center. Details regarding the survey methodology for that study have been reported elsewhere [12]. For the present study, individuals were randomly selected, contact was made by phone and informed consent was obtained verbally for participation. Following consent, individuals were administered a brief interview by phone. Individuals who accepted participation were asked to provide contact information on a non-twin full biological sibling. If there was more than one sibling, the sibling closest in age to the initial participant was contacted to reduce potential age-related differences in sleep. Sibling pair interviews were conducted an average of 17 days apart. The participants were instructed to refrain from discussing study-specific information with their siblings. Four siblings refused participation and 15 siblings could not be contacted. Thus, data for a total of 62 individuals (31 sibling pairs) was collected. A total of 6 brother-brother pairs, 15 sister-sister pairs, and 10 brother-sister pairs were completed. All subjects were paid for participation.
The questionnaire consisted of a series of questions regarding demographic, health, and sleep-related information. The average duration of the interview was 15–20 minutes. Specific demographic information included age, gender, height, and weight. Health information included current medical conditions, any history of depression, anxiety or other psychiatric disorder, and average daily alcohol intake. Sleep-related information included the following: current shift work (evening, night, rotating shift), weekday/weekend bed time, wake time, and total sleep time.
The FIRST questionnaire was administered as a measure of stress-related vulnerability to sleep disturbance. The FIRST is a standardized questionnaire with high test-retest reliability (0.92) and has been validated as a sensitive measure of vulnerability to sleep disturbance in normal non-insomniac individuals using polysomnographic assessment [2]. The measure includes nine items and requires the individual to rate the likelihood of having sleep disruption in association with specific and common stressful events and more broadly described periods of stress occurring during the day or evening. The actual FIRST questionnaire has been published previously along with its psychometric validation [2]. High scores on the FIRST are indicative of greater vulnerability to sleep disruption. Additional studies have demonstrated that this measure is able to predict individual responses to pharmacological sleep-disruptive challenges and that FIRST scores are significantly higher in individuals with insomnia in comparison to controls [13]. While previous measures of vulnerability to sleep disturbance have been developed [10], the accuracy of these measures in predicting polysomnographically measured sleep disturbance remains unclear.
Assessment to rule out insomnia was determined with the following questions and criteria based on those of the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) for insomnia, using a modified version of the Global Sleep Assessment Questionnaire items for insomnia [14]. Specifically, individuals were asked: “In the past 4 weeks, how often…1) Have you had difficulty falling asleep?”; 2) “Had difficulty staying asleep?”; 3) Had a problem feeling poorly rested despite an adequate amount of sleep?” Individuals were also asked: 4) “Did sleep difficulties or daytime sleepiness interfere with your daily activities?” Response choices for each question were “never,” “sometimes,” “usually,” or “always.” Individuals were identified as insomniacs and excluded from the present study if they endorsed “usually” or “always” in response to any of the first three questions as well as responding “usually” or “always” to question 4 regarding daytime interference. For the entire sample of 62 individuals (22 males; 31 pairs), 8 met criteria for insomnia (8/62; 12.9%). After exclusion of insomniacs and their respective siblings (n=16) the total non-insomniac sample included 46 individuals (23 sibling pairs).
Statistical Analyses
The values and interpretation of sibling correlations can be affected by several conditions. Importantly, the relationship to be measured should be linear. Although the degree to which this assumption is met is difficult to determine for the construct measured here, we believe this is a reasonable assumption as the construct of individual vulnerability to sleep disturbance is likely to be on a continuum from relatively low to very high vulnerability [15]. This seems to hold true using the FIRST as a measure of this construct, as previous studies have shown a normal distribution across a population-based sample [2]. The second assumption is that there should be no outliers in the sample. Data for the current study meet this assumption according to the following criteria. FIRST scores were converted to z-scores and outliers were considered to be present if a score deviated by ±2. As documented in previous sibling studies [16], the most appropriate method for assessing the relationship between sibling pairs on a given variable when there is no reason for assigning one member of a pair to a particular group (X’s) and the second to another (Y’s) is the intraclass correlation coefficient (ICC). Thus, the ICC was utilized in the present study to determine the correlation between sibling pairs on the FIRST scale. Partial correlations were also computed using covariates of age, gender, shift work (night or rotating), history of depression/anxiety, and alcohol use.
Results
Demographic data for all participants following exclusion of individuals meeting criteria for insomnia are shown in Table 1. Only one score deviated by two or more standard deviations (z = 2.3) from the mean. In order to examine the effect of this outlier, data were analyzed with and without this individual and their sibling. Finally, restriction of range can lead to underestimations of true correlation values. However, data from the present sample were widely distributed across the entire range of possible scores (9–36) reducing the likelihood of underestimation in the present study.
Table 1.
Mean and standard deviations of demographic variables for the study sample (n=46)
| Demographic Variables | |
|---|---|
| Age (years) | 51.04 ± 12.22 |
| Gender %F | 60.9 |
| Body Mass Index | 27.51 ± 5.28 |
| TST weekday (hrs) | 6.77 ± 1.52 |
| TST weekend (hrs) | 7.26 ± 1.67 |
| Overall TST (hrs) | 6.91± 1.42 |
| TIB weekday (hrs) | 7.52 ± 1.02 |
| TIB weekend (hrs) | 8.20 ± 1.22 |
| SE weekday % | 90.03 ± 15.51 |
| SE weekend % | 88.53 ± 15.98 |
| ESS | 6.15 ± 3.63 |
| FIRST | 18.30 ± 7.01 |
TST= total sleep time; TIB= time in bed; SE= Sleep Efficiency; ESS= Epworth Sleepiness Scale; FIRST= Ford Insomnia Response to Stress Test.
The mean score on the nine-item FIRST questionnaire for the combined sample (n = 62) was 19.48 ± 7.25. The mean FIRST score for the eight individuals who were excluded due to insomnia was 25.89 ± 5.87. After exclusion of these individuals (and their respective siblings) the mean FIRST score for the non-insomniac sample was 18.30 ± 7.01 with a range from 9 to 36. This is consistent with population-based studies using this measure where a mean of 19.9 ± 5.7 was obtained [2]. As can be seen from Table 1, individuals in the final sample had an average reported total sleep time, time in bed, and sleep efficiency consistent with normal sleep values of a non-insomniac sample.
Using the ICC, the value of the correlation for the FIRST between sibling pairs was r = 0.61, df = 23, p = 0.001. This indicated that 37.2% of the variance in FIRST scores can be accounted for by familial aggregation. A scatterplot showing this relationship is presented in Figure 1. When the single potential outlier and their sibling were removed from the data, the results were essentially unchanged (ICC-r = 0.64, df = 22, p < 0.001). The relationship between FIRST scores in the insomniacs and their non-insomniac siblings was r = 0.45, p = 0.12, but this did not reach statistical significance due to the small sample size (8 pairs). When data from all participants were included, the overall ICC for the entire sample (insomniacs and non-insomniacs) was r = 0.58, p < 0.001. As a number of important demographic and health-related variables could potentially affect the vulnerability to sleep disturbance relationship between siblings, an additional analysis was performed to determine the strength of the sibling relationship for FIRST scores while controlling for age, gender, shift work (night or rotating), history of depression/anxiety, and alcohol use. Despite the high number of covariates included in this analysis relative to the number of participants, a strong sibling correlation remained rp = 0.716, df = 13, p < 0.01. Thus, the present results appear to be stable and provide evidence for a moderate to high familial aggregation for FIRST scores.
Figure 1.
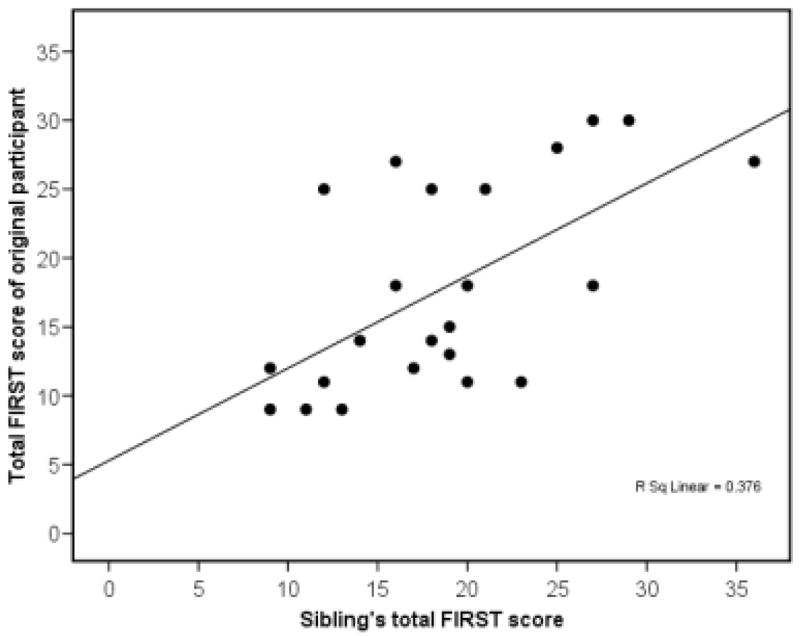
Scatterplot of the correlation (r = .61) between siblings on the Ford Insomnia Response to Stress Test (FIRST).
DISCUSSION
The aim of the present study was to determine the degree of familial aggregation of vulnerability to stress-related sleep disturbance in the absence of insomnia. The results showed a moderate to high degree of association between sibling pairs on a valid measure of vulnerability to sleep disturbance. To our knowledge, this is the first study to have assessed a familial relationship in the degree to which individuals are vulnerable to stress-related sleep disturbance. The robust positive correlation found for vulnerability between siblings in the present study is consistent with previous studies that have found a high genetic component to insomnia [9,17]. Taken together these results provide support for the idea that a trait vulnerability to stress-induced sleep disturbance exists and that it has a familial component. Future studies using samples of monozygotic and dizygotic twins will be needed to confirm the present findings and put the results in the context of a potential genetic predisposition.
The present results are suggestive of a potential genetic component to a predisposition towards insomnia. One prospective study has shown that a history of transient sleep disturbance predicts the future development of chronic insomnia [8]. Thus, a direct link between trait vulnerability to transient sleep disturbance and insomnia remains tenuous, and definitive conclusions will require large scale long-term prospective studies examining predictors of the incidence of insomnia. Nonetheless, previous studies provide evidence for the existence of a stable trait that predisposes individuals to acute sleep disturbance [1–3]. There is also preliminary evidence that at least one component of the predisposition to insomnia may include a pre-morbid vulnerability to stress-related sleep disruption [11,15]. The data presented here suggest that this latter individual characteristic has a familial component. This finding is consistent with twin studies in insomnia [9,17,18] and further suggests that individual vulnerability to sleep disturbance may be easily measured both in individuals with and without current insomnia.
The present results extend previous twin studies of insomnia and sleep disturbance and also provide further validation of the FIRST scale as a measure of vulnerability to stress-related sleep disturbance. The demonstration of a familial component to this vulnerability suggests that a subset of individuals may have an inherent sensitivity to stressful challenges of the sleep system. The fact that a number of individuals had FIRST scores above the mean of the insomnia group (13/54 > 25; 24.1%), but did not have insomnia, suggests that stress-related vulnerability to sleep disturbance is not an uncommon trait in the population. This raises the question of why such vulnerable individuals have not developed insomnia. One possibility is that stress-related vulnerability to sleep disturbance is completely independent of the disorder of insomnia, but the high FIRST scores of the insomnia patients in the present study and that of a previous sample argue against this possibility [13]. Previous studies have provided evidence for stressful events as triggers for insomnia [19–21]. It may be that these individuals have not been exposed to sufficient amounts of stress to produce insomnia. However, this presumes an interaction between an environmental trigger (e.g., a major psychosocial stressor) and a predisposition to chronic insomnia. While the notion of an interaction between these components may appear self-evident, it is an important question for which there is a paucity of data available.
It is important to highlight the significance of the current findings for future studies aimed at determining the underlying biological components of vulnerability to sleep disturbance and its potential association with chronic insomnia. Although there have been some discrepant results [22], numerous studies in insomnia have demonstrated elevated arousal measures in patients compared with controls using sympathetic nervous system activity [23], metabolic rate [24], brain metabolism [25], brain electrical activity [26], cognitive activity [27], and elevations in cortisol [28]. What is important but unknown at present is whether basal elevations (or other abnormalities) in these broad arousal systems are present prior to the development of insomnia or conversely are a consequence of the condition itself. The ability to identify individuals who are vulnerable to insomnia prior to developing the disorder is an important and necessary step in answering this question. The present study suggests the presence of a familial component in the predisposition to vulnerability to acute stress-related sleep disturbance and provides some initial evidence that individuals with the phenotypic trait can be identified a priori. It should be emphasized that, while the present data support a familial aggregation for the trait vulnerability to insomnia, this familial contribution could be genetic or environmental. Future long-term prospective studies will be needed to determine the potential interaction of the diathesis towards sleep disturbance with the appearance of environmental triggers thought to be common precipitants of chronic insomnia.
Acknowledgments
The authors would like to thank the Henry Ford Hospital Sleep Center Staff for their assistance with the completion of this project. We would also like to that the National Institutes of Health for providing the funding for this research study.
Disclosure statement: This was not an industry supported study. This study was supported by NIMH Grant 068372. No significant financial interest/other relationship to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26(8):1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 2.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Drake CL, Jefferson C, Roehrs T, Roth T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Med. 2006;7(7):567–72. doi: 10.1016/j.sleep.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–53. [PubMed] [Google Scholar]
- 5.Hunsley MS, Curtis WR, Palmiter RD. Behavioral and sleep/wake characteristics of mice lacking norepinephrine and hypocretin. Genes Brain Behav. 2006;5(6):451–7. doi: 10.1111/j.1601-183X.2005.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26(5):521–6. [PubMed] [Google Scholar]
- 7.Hunsley MS, Palmiter RD. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol Biochem Behav. 2004;78(4):765–73. doi: 10.1016/j.pbb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths MF, Peerson A. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49(3):245–53. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 9.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29(5):645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 10.Coren S. Prediction of insomnia from arousability predisposition scores: scale development and cross-validation. Behav Res Ther. 1988;26(5):415–20. doi: 10.1016/0005-7967(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 11.Drake C, Jefferson C, Roehrs T, Richardson G, Roth T. Vulnerability to chronic insomnia: a longitudinal population-based prospective study. Sleep. 2004;27(Abstract Supplement):A270–A1. [Google Scholar]
- 12.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson C, Roth T, Roehrs T, Drake CL. Sleep reactivity to stress in insomniacs. In: Penzel T, Fietze I, Chokroverty S, editors. Proceedings of the World Association of Sleep Medicine; 2005; Berlin, Germany: Medimond; 2005. pp. 79–82. [Google Scholar]
- 14.Roth T, Zammit G, Kushida C, Doghramji K, Mathias SD, Wong JM, et al. A new questionnaire to detect sleep disorders. Sleep Med. 2002;3(2):99–108. doi: 10.1016/s1389-9457(01)00131-9. [DOI] [PubMed] [Google Scholar]
- 15.Drake CL, Roth T. Predisposition in the evolution of insomnia: evidence, potential mechanisms, and future directions. In: Roth T, Lee-Chiong TJ, editors. Sleep Medicine Clinics. Philadelphia: Elsevier; 2006. pp. 333–49. [Google Scholar]
- 16.Paul SM. Sibling resemblance in mental ability: a review. Behav Genet. 1980;10(3):277–90. doi: 10.1007/BF01067773. [DOI] [PubMed] [Google Scholar]
- 17.McCarren M, Goldberg J, Ramakrishnan V, Fabsitz R. Insomnia in Vietnam era veteran twins: influence of genes and combat experience. Sleep. 1994;17(5):456–61. doi: 10.1093/sleep/17.5.456. [DOI] [PubMed] [Google Scholar]
- 18.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13(4):318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 19.Hall M, Buysse D, Reynolds C, Kupfer D, Baum A. Stress related intrusive thoughts disrupt sleep onset and continuity. Sleep Research. 1996;25:163. [Google Scholar]
- 20.Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, 3rd, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62(2):227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosomatic medicine. 1981;43(5):439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Varkevisser M, Van Dongen HP, Kerkhof GA. Physiologic indexes in chronic insomnia during a constant routine: evidence for general hyperarousal? Sleep. 2005;28(12):1588–96. [PubMed] [Google Scholar]
- 23.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 25.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 26.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 27.Harvey AG. Pre-sleep cognitive activity: a comparison of sleep-onset insomniacs and good sleepers. Br J Clin Psychol. 2000;39(Pt 3):275–86. doi: 10.1348/014466500163284. [DOI] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]


