Abstract
Background
Methamphetamine (MA) use among pregnant women is an increasing problem in the United States. How prenatal MA exposure affects neonatal neurobehavior is unknown.
Objective
To examine the neurobehavioral effects of prenatal MA exposure.
Design
The Infant Development, Environment and Lifestyle (IDEAL) study screened 13,808 subjects and 1632 were eligible and consented. 166 (n=74 exposed) were enrolled in a longitudinal follow up. Exposure was determined by meconium assay and self-report with alcohol, marijuana, and tobacco present in both groups. The NICU Network Neurobehavioral Scale (NNNS) was administered within the first 5 days of life. Analyses conducted on NNNS summary scores included exposure group effects, heavy MA use effects, association with frequency of use by trimester, and dose-response relationships with amphetamine metabolites.
Results
After adjusting for covariates, exposure to MA was associated with increased physiological stress. Heavy MA use was related to lower arousal, more lethargy, and increased physiological stress. First trimester MA use was related to elevated physiological stress. Third trimester use was related to poorer quality of movement. Higher level of amphetamine metabolites in meconium was associated with increased CNS stress.
Conclusions
Prenatal MA exposure was associated with neurobehavioral patterns of decreased arousal, increased stress, and poor quality of movement. The dose response relationships may represent neurotoxic effects from MA.
Keywords: prenatal exposure, neurodevelopment, drug, meconium
1. INTRODUCTION
Methamphetamine (MA) is the dominant drug problem in the western and midwestern portions of the United States and is the most widely abused drug worldwide [36;47]. The number of adults age 12 and over who have tried MA once in their lifetime has increased to 5.3% in 2002 from 4.3% in 1999 and 2.5% in 1997. This has led to the concern that MA is the growing drug of choice for adults in the United States [2;43;44]. Data from treatment centers in 2003 showed that 45% of patients treated for MA abuse were women[45]. In addition, substance use by pregnant women continues to be a serious problem[39]. The Substance Abuse and Mental Health Services Administration (SAMHSA) reported that among pregnant women age 15–44 years, 4.6% used illicit drugs in the previous month [42]. Consistent with the SAMHSA report, we found approximately 6% of women reported drug use during pregnancy in the multisite Infant Development, Environment and Lifestyle (IDEAL) study[2].
The effects of prenatal MA exposure on the developing fetus have not been well characterized. Isolated cases of cardiac defects, cleft lip and biliary atresia have been reported in infants exposed to MA in utero[34]. Similar to findings reported in neonates exposed to cocaine, increased rates of premature birth, fetal distress and growth restriction in the offspring of women using MA during pregnancy have been reported[15]. MA is frequently compared to cocaine as both are sympathomimetic agents. However, the neurotoxic effects of MA may be greater than cocaine due to its longer half life and more sympathomimetic mechanisms. Hansen and colleagues reported poorer visual recognition memory, a measure correlated with subsequent IQ in MA and cocaine exposed newborns[20]. The most extensive follow-up data regarding amphetamine exposed children are from a series of reports from Sweden by Billing and colleagues who have followed a group of amphetamine exposed children from birth to age 14. In the first few months of life, increased drowsiness was noted [5]. Among children exposed to amphetamine continuously throughout pregnancy, emotional characteristics of autism, speech problems and signs of wariness of strangers were noted by age one[5]. By age 4, IQ was lower than a normative group of Swedish children [6] and at age 8 prenatal exposure predicted aggressive behavior and problems with peers[4]. At age 14, the children showed problems with advancement in school due to delays in math and language and had difficulties with physical fitness activities[10]. The limitations of these reports of children exposed to MA include the lack of a control group, small sample size and confounding with other prenatal drug use.
Although the available data suggest MA-exposed children are at risk for poor developmental outcome, the cocaine epidemic highlights the danger of over-interpretation based on limited findings[28;31;49]. The multisite IDEAL study is a prospective, longitudinal study of children with prenatal MA exposure and neurobehavioral outcome from birth to 36 months. This study reports preliminary findings in neonates utilizing the Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS). The NNNS is a standardized neurobehavioral exam for the healthy and at-risk neonate that has been used in studies of intrauterine exposure to cocaine[30;33], opiates[12;25], and nicotine[27]. In addition, preliminary findings have demonstrated that neurobehavioral measures assessed by the NNNS strongly correlate with brain volumes in the newborn period and scores on the 24 month Bayley exam in neonates born <30 weeks’ gestation[8]. This is the first report that we are aware of from a prospectively controlled investigation of neurobehavioral outcome in neonates exposed to MA in utero.
2. METHODS
Because the primary goal of the research was to investigate the outcomes associated with prenatal MA exposure, clinical sites in specific geographic areas known to have MA problems were chosen to participate in the IDEAL study. The cities chosen were Los Angeles, CA; Des Moines, IA; Tulsa, OK; and Honolulu, HI. The study was approved by the Institutional Review Boards at all participating sites. Prior to initiation of recruitment, personnel from all sites met for a week-long training session so that procedures could be standardized[2]. Recruitment is planned for two years; we present neonatal neurodevelopmental findings in the cohort recruited by year one.
The exclusion criteria for the mother were: age <18; opiate, lysergic acid diethylamide (LSD), phencyclidine (PCP), hallucinogens, and cocaine use during pregnancy; institutionalized for retardation or emotional disorders; overtly psychotic or a documented history of psychosis; unable to speak English. Exclusion criteria for children were: critically ill and unlikely to survive; multiple birth; major life threatening congenital anomaly; documented chromosomal abnormality associated with mental or neurologic deficiency; overt TORCH infection; sibling previously enrolled in the IDEAL study. A National Institute on Drug Abuse Certificate of Confidentiality was obtained for the project that assured confidentiality of information regarding the subjects’ drug use, superseding mandatory reporting of illegal substance use. The certificate was explained to the mother during the recruitment and informed consent process, including the condition that the certificate did not exclude reporting of evidence of child abuse or neglect.
After obtaining informed consent, drug use during pregnancy was ascertained through maternal interview using the Substance Use Inventory and sociodemographic information was determined using the Lifestyle Interview. All questions for both the interviews were read to the mothers to ensure standardization. The Lifestyle Interview includes information about the course of the pregnancy, household composition, demographics [socioeconomic status (SES), education, age, race, and marital status], type of home and neighborhood, and services received during pregnancy. Education and occupation information was collected to calculate the 4-factor Hollingshead index of SES which has been adapted to single parent and non-nuclear families[21;26]. Meconium was collected on all infants. Meconium samples were collected in the nursery and began immediately after obtaining consent in order to attempt to collect the first and/or earliest discharge of meconium. In some cases, more than one collection of meconium from an infant was used to ensure an adequate amount that could be tested. The samples were shipped to a central laboratory (United States Drug Testing Laboratory in Des Plaines, IL) for analysis of the amphetamine class, cocaine metabolites, cannabinoids, opiates and cotinine. The specimen was initially screened with a sensitive enzyme multiplied immunoassay test (EMIT II; Dade-Behring, Cupertino, CA). If positive results were obtained, the specific drug analyte or metabolite was confirmed by gas chromatography-mass spectrometry (GC/MS).
Exposed subjects were identified by maternal report of MA use during the pregnancy based on the hospital interview or a negative self-report and positive GC/MS confirmation of amphetamine and/or metabolites in infant meconium. Although amphetamines and ecstasy using mothers were included in the MA group, a small percentage (<1%) reported using during pregnancy. There were also low levels of benzodiazepines and barbiturates in the sample (<1%). Comparison subjects were defined as denial of MA use during this pregnancy and a negative meconium screen.
All exposed infants and their mothers were enrolled in a longitudinal follow up from birth to 36 months postmenstrual age. Comparison neonates within each center were matched based on race, birth weight category (<1500 grams, 1500–2500 grams, >2500 grams), type of insurance (private versus public), and education (high school education completed versus not completed). The Substance Use Inventory, administered to the enrolled mothers, is a detailed questionnaire of frequency and quantity of drugs of abuse used during the three months before and each trimester of pregnancy. A calendar covering the previous year, with holidays and personal events added, serves as the anchor for memory. A history of maternal alcohol, marijuana, and tobacco use during the pregnancy was considered as background variables in both the exposed and comparison groups. Both the exposed and comparison groups excluded use of opiates, PCP, LSD, and cocaine. Targeted medical data were obtained from the mothers’ and infants’ charts after obtaining HIPAA authorization.
Participants
All births in 7 hospitals were screened from September 2002 to August 2003. Of the 13,808 mother-infant dyads screened, 10,510 mothers were available to approach, 7119 (76.1%) met the eligibility criteria, and 1632 (23.0%) of eligible mothers consented to participate in the study. The study team was forbidden to ask questions of a personal nature such as drug use history to individuals not consenting to participate in the study. Therefore, drug use information is limited to the 1632 consented subjects. Though potential enrollees cited numerous reasons for refusal, the most common reason for study refusal among eligible mothers was the difficulty to commit to the three year follow-up protocol
Among the eligible and consented subjects, 166 (10.8%) were enrolled in the longitudinal follow up (74 exposed and 92 comparison). Among the 74 exposed subjects, 72 (97.3%) were self-reporters and 21 (28.4%) meconium samples were confirmed positive for MA. Two exposed subjects were identified by confirmation of MA in meconium only. Exposed subjects were enrolled immediately after knowledge of their MA status. For the sample included in this study, 60 (36%) subjects were from California, 53 (31.9%) from Hawaii, 28 (16.9%) from Oklahoma, and 25 (15.1%) from Iowa. Since all subjects were matched within each site we did not expect site to affect our findings regarding exposure effects. However, site was included in all preliminary analyses.
Comparison subjects were group matched to exposed subjects on race, type of insurance, maternal education, and birth weight soon after the enrollment of the exposed subject. Typically the first consented subject to match an exposed subject was enrolled with a one to one ratio. In cases where the pattern of characteristics for the exposed subject was difficult to match, a few comparison subjects were enrolled in advance of identification of exposed subjects, leading to uneven group sizes. The method of enrollment ensured a community rather than a convenience or clinical sample of MA using women with comparison subjects selected from the same hospital population. Matching criteria reduced disparities between groups related to race, social class and poverty, and prematurity. The exposed and unexposed groups enrolled in follow up differed in demographic characteristics from the larger sample[2].
NNNS
The NNNS exam was administered on all subjects born at term within the first 5 days of life. Premature newborns were examined at 36–40 weeks’ gestation. There were 8 (n=3 [3.2%] comparison, n=5 [6.8%] exposed) assessments conducted outside these parameters (range: 6 to 12 days of age). The exam was administered by examiners who were certified on the examination and masked to exposure status. Gold standard reviewers both trained and determined certification for specified measures and procedures. To maintain reliability, examiners were periodically rechecked during the two-year period of recruitment. The NNNS provides an assessment of neurologic, behavioral, and stress/abstinence neurobehavioral function[29]. The neurologic component includes active and passive tone, primitive reflexes, and items that reflect the integrity of the central nervous system and maturity of the infant. The behavior component is based on items from the Neonatal Behavioral Assessment Scale (NBAS)[7] modified to be sensitive to putative drug effects. The stress/abstinence component is a checklist of “yes” or “no” items organized by organ system based primarily on the work of Finnegan[17]. The NNNS follows a relatively invariant sequence of administration that starts with a pre-examination observation, followed by the neurologic and behavioral components. The Stress/Abstinence scale is based on signs observed throughout the examination. The NNNS items are summarized into the following scales: Habituation, Attention, Arousal, Regulation, Handling, Quality of Movement, Excitability, Lethargy, Nonoptimal Reflexes, Asymmetric Reflexes, Hypertonicity, Hypotonicity, and Stress/Abstinence. The actual sequence of administration and the means used by the examiner to maintain an infant’s participation in the examination are recorded. The examination is administered in a quiet room, midway between feedings with the infant initially asleep and covered if possible.
Statistical Analysis
Five sets of analyses were conducted. First, one-way analysis of variance (ANOVA) and Chi-square statistics were used to compare the MA and comparison groups on medical, demographic characteristics, as well as on the twelve NNNS summary scores for MA exposure effects. Mann-Whitney median tests were used to test the average quantities of alcohol, tobacco, and marijuana use across pregnancy. In the second set of analyses, MA exposure effects on the summary scores were tested by General Linear Model (GLM) with Type III sum of squares after adjustment for covariates. This method calculates the sums of squares of an effect in the design as the sums of squares adjusted for any other effects in the model. That is, the Type III Sum of squares for a factor is corrected for as many other factors in the model as possible. The Type III sums of squares are invariant with respect to the cell frequencies as long as the general form of estimability remains constant which is useful for an unbalanced model with no missing cells. Habituation was not analyzed, as too few infants were sleeping at the start of the exam. In the third set of analyses, the effects of heavy MA use, defined as average use of MA >=3 days per week across pregnancy, were examined before and after adjusting for the same covariates. In the fourth set of analyses, the association between the NNNS summary scores and the frequency of MA use (days per week) during each trimester was tested by step-forward regression analyses with the following steps of forced entry of predictors: 1). First trimester MA use frequency; 2) Second trimester MA use frequency; 3). Third trimester MA use frequency; 4) birth weight in 100g, average quantities of alcohol use, tobacco use, marijuana use across pregnancy, SES, assessment >5 days postpartum, first born, and site. Finally, correlation analyses tested dose-response relations of GC/MS amphetamine metabolites and NNNS summary scores. Metabolite values were log transformed to normalize the distribution. All parametric values are reported as unadjusted means (SD) in the tables. All nonparametric data are reported as absolute number and percentages. Significance was accepted at P<.05.
Standard Covariate Set
Variables in Tables 1 and 2 were examined for possible inclusion as covariates. Covariates were selected based on conceptual reasons, published literature, and characteristics from Tables 1 and 2 that differed between groups if not highly correlated with other covariates. The effect of prenatal exposures to alcohol, tobacco, and marijuana on NNNS measures have been previously reported[27], as have birth weight effects[30].
Table 1.
Maternal Characteristics of Methamphetamine Exposed and Comparison Groups (N = 166)
| Mean (SD)/Number (Percent)
|
||
|---|---|---|
| Exposed (N = 74) | Comparison (N = 92) | |
| Race | ||
| White | 33 (45%) | 38 (41%) |
| Hispanic | 14 (19%) | 19 (21%) |
| Pacific Islander | 10 (14%) | 11 (12%) |
| Asian | 12 (16%) | 13 (14%) |
| Black | 4 (5%) | 8 (9%) |
| Other | 1 (1%) | 3 (3%) |
| Low SES (Hollingshead V) * | 29 (39.2%) | 11 (12.0%) |
| Household Income < $10,000 | 21 (32%) | 18 (21%) |
| No partner* | 43 (58.1%) | 33 (35.9%) |
| Public Insurance | 62 (83.8%) | 77 (83.7%) |
| Education <12 years | 38 (51.4%) | 38 (41.3%) |
| Age, yr | 25.1 (5.4) | 23.9 (5.8) |
| Gest Age 1st Prenatal Visit, wk * | 15 (7.7) | 9 (5.1) |
| Out of Home Placement* | 20 (28.2%) | 2 (2.2%) |
| Prenatal Heavy METH Use | 12 (16.7%) | |
| Prenatal Tobacco Use* | 58 (78.4%) | 25 (27.2%) |
| Heavy Tobacco Use* | 22 (29.7%) | 8 (8.7%) |
| #of Cigarettes Per Day (Median & Range)* | 3.83 (0–20) | 0 (0–25) |
| Prenatal Alcohol Use* | 28 (38%) | 15 (16%) |
| Heavy Alcohol Use* | 3 (4.1%) | 0 |
| #of Absolute Alcohol Per Day (Median & Range)* | 0 (0–1.36) | 0 (0– .14) |
| Prenatal Marijuana Use* | 24 (32%) | 7 (7.6%) |
| Heavy Marijuana Use* | 15 (20.3%) | 6 (6.5%) |
| #of Joints Per Day (Median & Range)* | 0 (0–4) | 0 (0–1.36) |
P<.05
Table 2.
Newborn Characteristics of Methamphetamine and Comparison Groups (N = 166)
| Mean (SD) Number (Percent) | ||
|---|---|---|
| Exposed (N = 74) | Comparison (N = 92) | |
| Gestational age, wk | 38.3 (2.3) | 38.9 (2.2) |
| ≤ 35 wk, Gestational Age,% | 8 (10.8%) | 8 (8.7%) |
| Birth weight, g | 3160 (640) | 3267 (627) |
| Length, cm * | 49.4 (3.9) | 50.9 (3.3) |
| Head Circumference, cm | 33.7 (1.8) | 34.0 (2.0) |
| Apgar 1 minute, < 5, % | 6 (8.1%) | 3 (3.3%) |
| Apgar 5 minute, < 5, % | 0 | 1 (1.4%) |
| Male, % | 42 (57%) | 47 (52%) |
| First Born, % * | 20 (27%) | 46 (50%) |
P<.05
The covariates included in analyses 2 and 3 were birth weight, SES, and 3-level alcohol, tobacco, marijuana use (Heavy/Some/No Use), assessment > 5 days postpartum, first born, and site. Cutoffs for levels of drug use were based on thresholds for detecting effects that have been reported by others[13;18;22–24;38]. For alcohol, heavy use was >=.5 oz of absolute alcohol per day (1 standard drink). For tobacco, heavy use was defined as >=10 cigarettes per day. For marijuana, heavy use was defined as >=.5 joints per day. All other uses were defined as some use. Assessment> 5 days postpartum and first born were dichotomous [yes/no] variables. SES and birth weight were continuous variables. Summary statistics of these covariates by MA exposure are shown in Tables 1 and 2.
In analysis 4, the main effects of MA use frequency was examined. Frequency of MA use in each trimester was defined as number of days using MA per week. The covariates included in analysis 4 were birth weight, SES, and quantities of alcohol, tobacco, and marijuana use, assessment > 5 days postpartum, first born, and site. Alcohol quantity was defined as number of absolute alcohol per day. Tobacco quantity was defined as number of cigarettes per day. Marijuana quantity was defined as number of joints per day.
Site was a 4-group categorical variable in the GLM analyses and was recoded into 3 dummy coded variables (Site 1 versus the other sites; Site 2 versus the other sites; Site 3 versus the other sites) in the regression analyses. Since each MA exposed subject was matched with a comparison within each site, site was kept in the final model as a covariate only if it made significant contribution to the model (P < .05) in preliminary analyses. Otherwise, it was removed from the final models. Household income less than $10,000, no partner, and gestational age at first prenatal visit (Table 1) were highly correlated with SES and were not included in the final analysis. The variable, “1 minute Apgar < 5” (Table 2), had too few subjects per group to include as a covariate. We ran a second analyses after dropping the few subjects with very low 1-minute Apgar and no change in our findings were noted regarding the main effects of drug exposure.
3. RESULTS
3.1 Maternal and Newborn Characteristics
Relative to the comparison group, the MA exposed women were more likely to have a lower socioeconomic status and to be without a partner (Table 1). In addition, the mothers in the exposed group attended their first prenatal visit later in gestation and their newborns were more likely to be placed out of the home. No difference in age was observed between the groups. As expected, no differences in racial distribution, insurance, or education were observed because these characteristics were matched between groups. Comparison newborns were more likely to be first born and have a longer birth length. There were no differences in gestational age, incidence of prematurity, birth weight, 1 and 5-minute Apgar scores or gender distribution between the groups (Table 2).
3.2 Maternal Drug Use
More mothers in the MA exposed group used tobacco, alcohol, and marijuana during pregnancy than in the comparison group. Further, MA using mothers were greater than 4 times more likely to be heavy users of these substances than mothers in the comparison group (Table 1). These findings are similar to those in previous studies addressing maternal substance use[30]. Although amphetamines and ecstasy using mothers were included in the MA group, a small percentage (<1%) reported using during pregnancy. There were also low levels of benzodiazepines and barbiturates in the sample (<1%).
Table 3 shows the patterns of MA use by the mothers who admitted MA use in the exposed group (n=72) by trimester. There was a decline in use during the three trimesters. Of the 72 admitted users, 15.3% admitted to daily MA use during the first trimester, yet only 2.8% were daily users in the third trimester. The percentage of women who abstained from MA use during pregnancy increased from 16.7% in the first trimester to 58.3% in the third trimester. The quantity of MA use in the exposed study population was deemed unreliable in the study set because many did not know the grams conversion of the street term amount they were using. Although there was assistance from drug enforcement, there was no consensus across the study locations.
Table 3.
Patterns of MA Use for Admitted Users (n=72)
| MA Use | Trimester
|
|||||
|---|---|---|---|---|---|---|
| First | Second | Third | ||||
| N | (%) | N | (%) | N | (%) | |
| Daily | 11 | (15.3%) | 4 | (5.6%) | 2 | (2.8%) |
| 3–6 d/wk | 19 | (26.4%) | 10 | (13.9%) | 7 | (9.7%) |
| 1–2 d/wk | 9 | (12.5%) | 7 | (9.7%) | 5 | (6.9%) |
| 1–3 d/mo | 8 | (11.1%) | 6 | (8.3%) | 3 | (4.2%) |
| 1–2 d/3mo | 13 | (18.1%) | 14 | (19.4%) | 13 | (18.1%) |
| Not at all | 12 | (16.7%) | 31 | (43.1%) | 42 | (58.3%) |
Smoking was the most common route of administration (81%) for the mothers who admitted to using MA. The less common routes were snorting/sniffing (28%), injection (14%) and ingestion (7%). About 18% of admitted users reported using 2 or more routes of administration. In order to address the effects of route of administration, the data were recoded into two groups: smoking vs. other routes. No differences were found between the two groups except in Skin Stress and State Stress. The smoking group had less skin stress and state stress than the other route group.
3.3 Neurodevelopmental Outcome on the NNNS
Results of analyses 1 and 2 are presented in Table 4. The unadjusted means and standard deviations are shown in the table. In the univariate analysis without adjusting for covariates (Analysis 1), exposure to MA was associated with lower arousal scores (unadjusted, P=.009), suggesting hypoarousal of these infants. The effect size was .42. However, when covariates were added to the model in Analysis 2, the MA effect on arousal was not significant. Instead, MA exposed infants showed greater physiological stress (P=.007) as observed by difficulty maintaining normal, regular respirations. The effect size was .20. There were no significant differences in the remaining summary scores between the groups.
Table 4.
NNNS Scales by Methamphetamine Exposure Status (N = 166)
| Meth exposed | Comparison | Unadjusted P | Adjusted P | |
|---|---|---|---|---|
| N = 74 | N = 92 | |||
| Means (SD) | Means (SD) | |||
| Attention | 4.89 (1.21) | 4.94 (1.06) | .801 | .418 |
| Arousal | 3.90 (0.69) | 4.18 (0.63) | .009 | .129 |
| Regulation | 5.35 (0.66) | 5.26 (0.72) | .379 | .928 |
| Handling | 0.30 (0.26) | 0.32 (0.27) | .674 | .542 |
| Quality of Movement | 4.30 (0.64) | 4.43 (0.61) | .196 | .292 |
| Excitability | 3.15 (2.24) | 3.70 (1.98) | .097 | .796 |
| Lethargy | 5.05 (2.71) | 4.45 (2.78) | .158 | .077 |
| Non-Optimal Reflexes | 3.46 (2.16) | 3.32 (2.01) | .657 | .479 |
| Asymmetrical Reflexes | 0.38 (0.57) | 0.30 (0.64) | .438 | .557 |
| Hypertonicity | 0.05 (0.23) | 0.01 (0.10) | .107 | .736 |
| Hypotonicity | 0.22 (0.50) | 0.16 (0.40) | .450 | .454 |
| Total Stress/Abstinence | 0.12 (0.06) | 0.11 (0.06) | .262 | .138 |
| Physiological | 0.03 (0.13) | 0.01 (0.05) | .052 | .012 |
| Autonomic | 0.11 (0.14) | 0.10 (0.12) | .656 | .519 |
| CNS | 0.16 (0.08) | 0.15 (0.07) | .398 | .276 |
| Skin | 0.10 (0.15) | 0.10 (0.15) | .895 | .417 |
| Visual | 0.13 (0.10) | 0.12 (0.10) | .443 | .344 |
| GI | 0.07 (0.19) | 0.07 (0.16) | .989 | .904 |
| State | 0.13 (0.16) | 0.11 (0.15) | .520 | .065 |
We tested the correlations between Apgar scores and NNNS outcomes in univariate analyses. Although 5-minute Apgar was negatively correlated with non-optimal reflexes (r= −.158, p = .043) and skin stress (r=−.181, p = .02), including 5-minute Apgar in the model did not result in any changes of the main effects.
Table 5 shows the results of heavy MA use effects in Analysis 4. The unadjusted means and standard deviations by heavy use, some use, and no use groups are shown in the table. In the univariate analysis (unadjusted by covariates), heavy MA use was associated with lower arousal (P = .005) than some use and no use. The effect size was .53 for heavy use versus some use and .85 for heavy use versus no use. No unadjusted heavy MA use effects were found on other NNNS summary variables. When covariates were included, the effects of heavy MA use on arousal remained. In addition, heavy MA use was also associated with more lethargy (P = .05) and more physiological stress (P = .043). The effect size for lethargy was .39 for heavy use versus some use and .52 for heavy use versus no use. The effect size for physiological stress was .07 for heavy use versus some use and .29 for heavy use versus no use.
Table 5.
NNNS Scales by Level of Methamphetamine Use Status (N=166)
| Exposed | Comparison | Unadjusted P | Adjusted P | |||||
|---|---|---|---|---|---|---|---|---|
| Heavy use* | Some use | |||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| Attention | 7 | 4.67 (1.34) | 46 | 4.88 (1.20) | 76 | 4.94 (1.06) | .819 | .560 |
| Arousal | 12 | 3.56 (0.81) | 60 | 3.97 (0.66) | 92 | 4.18 (0.63) | .005 | .033 |
| Regulation | 11 | 5.26 (0.55) | 59 | 5.35 (0.68) | 90 | 5.26 (0.72) | .693 | .906 |
| Handling | 7 | 0.32 (0.35) | 48 | 0.30 (0.26) | 78 | 0.32 (0.27) | .897 | .767 |
| Quality of Movement | 12 | 4.05 (0.49) | 60 | 4.35 (0.67) | 92 | 4.43 (0.61) | .138 | .160 |
| Excitability | 12 | 2.42 (1.78) | 60 | 3.33 (2.32) | 92 | 3.70 (1.98) | .117 | .355 |
| Lethargy | 12 | 6.08 (3.45) | 60 | 4.90 (2.55) | 92 | 4.45 (2.78) | .131 | .050 |
| Non-Optimal Reflexes | 12 | 3.50 (1.88) | 60 | 3.47 (2.25) | 92 | 3.32 (2.01) | .891 | .800 |
| Asymmetrical Reflexes | 12 | 0.33 (0.49) | 60 | 0.40 (0.59) | 92 | 0.30 (0.64) | .643 | .741 |
| Hypertonicity | 12 | 0.00 (0.00) | 60 | 0.07 (0.25) | 92 | 0.01 (0.10) | .122 | .297 |
| Hypotonicity | 12 | 0.17 (0.39) | 60 | 0.23 (0.53) | 92 | 0.16 (0.40) | .636 | .692 |
| Stress Abstinence | 12 | 0.13 (0.05) | 60 | 0.12 (0.06) | 92 | 0.11 (0.06) | .402 | .271 |
| Stress Physiological | 12 | 0.04 (0.14) | 60 | 0.03 (0.13) | 92 | 0.01 (0.05) | .136 | .043 |
| Stress Autonomic | 12 | 0.11 (0.11) | 60 | 0.11 (0.14) | 92 | 0.10 (0.12) | .892 | .709 |
| Stress CNS | 12 | 0.19 (0.06) | 60 | 0.15 (0.08) | 92 | 0.15 (0.07) | .130 | .116 |
| Stress Skin | 12 | 0.15 (0.19) | 60 | 0.09 (0.14) | 92 | 0.10 (0.15) | .424 | .254 |
| Stress Visual | 12 | 0.12 (0.10) | 60 | 0.13 (0.10) | 92 | 0.12 (0.10) | .691 | .609 |
| Stress GI | 12 | 0.03 (0.10) | 60 | 0.07 (0.19) | 92 | 0.07 (0.16) | .688 | .648 |
| Stress State | 12 | 0.13 (0.18) | 60 | 0.13 (0.16) | 92 | 0.11 (0.15) | .737 | .167 |
Heavy use is defined as using any methamphetamine 3 or more days per week.
Table 6 presents the results of regression analysis on the frequency of MA use by trimester after adjusting for covariates. First trimester MA use was related to greater stress abstinence (β =.22, P = .025). Third trimester use was related to poorer quality of movement (β = −.21, P = .035). Poor quality of movement indicates the neonate is jittery with little or no smooth movement of the arms and legs, startles easily and has high overall activity. Use of MA during the 3 trimesters was not so highly correlated as to cause the multicollinearity. The correlations are .602 between 1st and 2nd trimesters, .326 between 1st and 3rd trimesters, and .655 between 2nd and 3rd trimesters.
Table 6.
Regression Models Predicting Quality of Movement and Stress Abstinence (N=166)
| Quality of Movement | Stress Abstinence | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficient (SE) | βa | P | R2 | Unstandardized Coefficient (SE) | β | P | R2 |
| Meth Use Frequency (# of days per week) | 0.254 | 0.244 | ||||||
| 1st Trimester: | 0.01 (0.01) | 0.04 | .716 | 0.01 (0.00) | 0.22* | .025 | ||
| 2nd Trimester: | −0.01 (0.05) | −0.02 | .833 | −0.00 (0.01) | −0.06 | .591 | ||
| 3rd Trimester: | −0.12 (0.06) | −0.21* | .035 | 0.00 (0.01) | 0.07 | .498 | ||
| Birth weight in 100g | 0.03 (0.01) | 0.25* | .002 | 0.00 (0.00) | 0.00 | .982 | ||
| Marijuana Use: # of joints per day | −0.03 (0.04) | −0.05 | .475 | −0.00 (0.00) | −0.02 | .791 | ||
| Alcohol Use: # of absolute alcohol per day | −0.23 (0.30) | −0.06 | .438 | −0.04 (0.03) | −0.09 | .226 | ||
| Tobacco Use: # of cigarettes per day | 0.00 (0.01) | 0.04 | .655 | 0.00 (0.00) | 0.03 | .679 | ||
| SES (Hollingshead Index) | 0.00 (0.01) | 0.03 | .682 | 0.00 (0.00) | 0.04 | .618 | ||
| >5 days Postpartum | 0.20 (0.23) | 0.07 | .369 | −0.02 (0.02) | −0.05 | .500 | ||
| First born | −0.22 (0.07) | −0.17* | .023 | 0.00 (0.01) | 0.01 | .849 | ||
| Site Iowa vs others | 0.06 (0.14) | 0.04 | .670 | −0.02 (0.01) | −0.10 | .256 | ||
| Site Oklahoma vs others | 0.19 (0.07) | 0.23* | .008 | −0.02 (0.01) | −0.19 | .028 | ||
| Site Hawaii vs others | −0.06 (0.11) | −0.05 | .587 | −0.06 (0.01) | −0.49** | .000 | ||
Standardized Regression Coefficient
P<0.05
P< .01
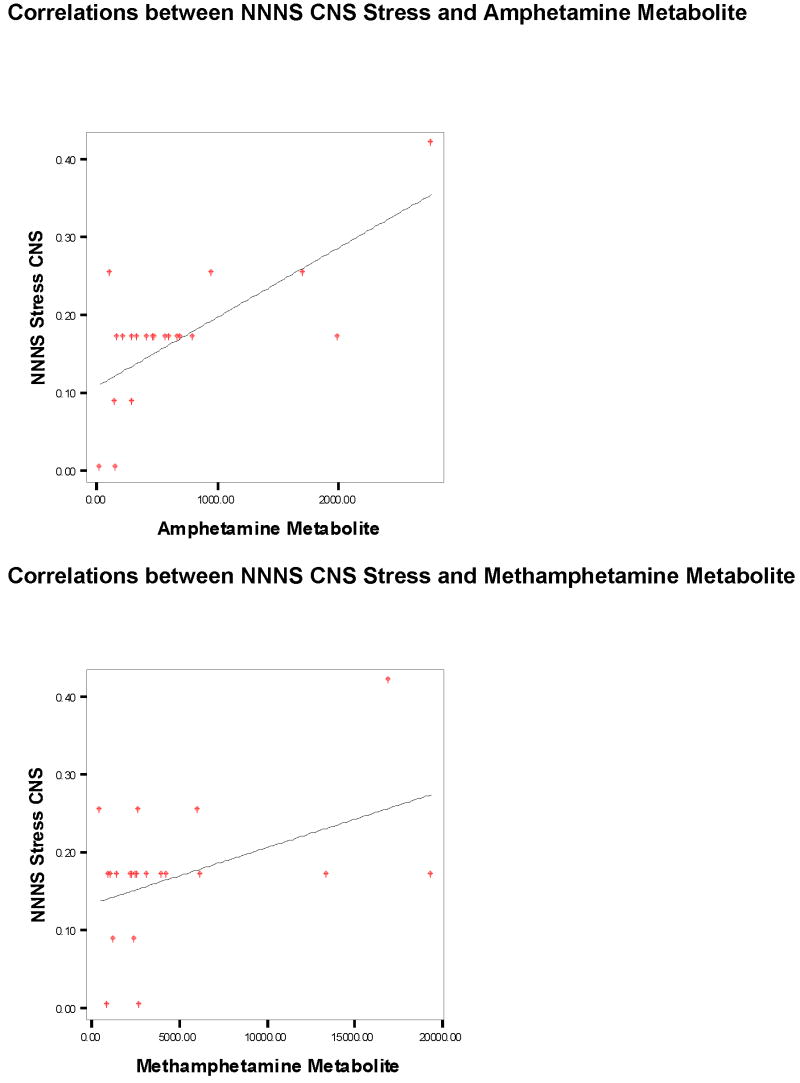
The results from the correlation analysis testing dose-response relationships of the GC/MS amphetamine and methamphetamine metabolites and the NNNS CNS Stress are shown in Figure 1. CNS Stress was associated with increased levels of both metabolites. There were no univariate outliers for either the metabolites or the NNNS variables. No multivariate outliers were found either. Although a few data points appeared to be outliers in the graphs, removing them did not result in changes of the correlations.
Figure 1.
4. DISCUSSION
This is the first prospective investigation reporting the effects of prenatal MA exposure on neurobehavioral outcome at birth. We found MA exposure in utero was associated with neurobehavioral patterns of increased physiological stress and higher levels of amphetamine metabolites were associated with increased CNS stress. In addition, heavy MA exposure was associated with decreased arousal, increased lethargy and physiological stress. MA use frequency in the first trimester was positively associated with stress abstinence and third trimester MA use frequency was negatively associated with the quality of movement. All of these MA effects were adjusted for covariates including prenatal exposure to other drugs, SES, birth weight, first born, out-of-window status, and site.
These subtle neurobehavioral findings are consistent with previous findings in cocaine[30]- and nicotine[27]-exposed children and suggest potential MA-induced neurotoxic effects. Also consistent with previous findings in cocaine exposed children, there are no identifiable patterns of neurobehavior that are consistent with a methamphetamine exposure “syndrome”. The limitations of the previous findings in cocaine[30] - and nicotine[27]-exposed children are that the population may not be representative of all drug-exposed infants. In addition, the use of saliva rather than meconium collection in the nicotine study only allowed measurement of recent nicotine use and not use throughout pregnancy.
The neurotoxic effects of prenatal MA exposure have not been well characterized, but many studies suggest that MA is neurotoxic to the developing central nervous system. Studies in the ovine model have found MA increases fetal blood pressure and decreases fetal oxyhemoglobin saturation and arterial pH[9;41]. In addition, studies in rodents have shown that MA is toxic to dopaminergic and serotonergic neurons[19;35]. Positron emission tomography (PET) studies in abstinent MA users demonstrated decreased dopamine transporters, suggesting long-lasting neurotoxicity due to MA abuse[48]. Cranial ultrasound studies of human newborns exposed to prenatal MA and cocaine demonstrated an increased incidence of intraventricular hemorrhage and white matter densities[14]. In addition, a study of MA-exposed children aged 3–16 years employing magnetic resonance spectroscopy demonstrated increased concentrations of creatine in the striatum suggesting a possible abnormality in energy metabolism in the brains of exposed children[40]. Volumetric assessments of magnetic resonance images in exposed children have demonstrated smaller subcortical volumes including the putamen, globus pallidus and hippocampus[11]. Collectively these data suggest areas of the striatum are vulnerable to prenatal MA exposure. Because executive functioning is thought to be mediated in part by the frontal-striatal pathway, these findings may have important clinical implications. The limitations of the previous findings in MA-exposed children include recording of prenatal MA exposure relied solely on maternal self-reporting, lack of a control group, small sample size and confounding with other prenatal drug use.
In contrast to the marked MA-induced neurotoxicity previously reported in pre-clinical animal models, adult users and children exposed in utero, we report only subtle neurobehavioral findings in exposed newborns. We found MA-exposed newborns were more difficult to arouse, but once aroused, exhibited an increase in physiologic stress. Lower arousal scores and an impaired ability to self-regulate have also been observed in cocaine-exposed children one month after birth[30] and are consistent with findings of drowsiness in amphetamine-exposed infants in the first few months after birth[5]. In addition, we found dose response and trimester related effects in the MA-exposed children. In particular, increased stress signs were seen with higher levels of metabolites and greater frequency of MA use in the first trimester, whereas higher frequency of MA use in the third trimester was related to poorer quality of movement. Although there are limitations to analyses of trimester related effects and correlating meconium metabolite concentrations with drug dosing, our observations are consistent with previous findings in cocaine-exposed newborns. Greater cocaine use in the third trimester is associated with impaired state regulation and ability to orient to the environment in the early neonatal period[16] and greater cocaine metabolite concentrations in meconium correlates to impaired fetal growth [32]. Consistent with our NNNS findings, heavier maternal use of cocaine determined by self report and meconium metabolite values is associated with poor state regulation and greater excitability[46]. Because these dose-related effects of cocaine on neurobehavioral performance were observed at 3 weeks of age[46] and were similar to the findings in methamphetamine-exposed newborns, it is likely that these neurobehavioral differences represent MA-induced neurotoxicities rather than acute “withdrawal-like” effects.
The subtle effects observed in children exposed to prenatal MA by the NNNS exam have short and potentially long term implications. In the immediate neonatal and early infancy period, the neurobehavioral vulnerability manifested in the MA group may be exacerbated by the home environment. Not only could the children be at risk from the chemicals involved in making methamphetamines in the home, but also from potential parental abuse and neglect[1]. These small differences may become amplified and lead to permanent developmental deficits. Therefore, these at-risk newborns can potentially avoid long term insults with the appropriate caregiving environments.
There are limitations to the current study; therefore, these preliminary results should be interpreted with caution. First, despite the significant neurobehavioral differences between the groups, our sample size is small. The subtle differences between the two groups versus the more robust dose response effects observed within the MA group suggest that MA exposed neonates are a heterogeneous group. A larger sample size would allow for more extensive analyses potentially identifying more affected subgroups of infants. Second, the exposed group of subjects is primarily based on self report. However, the reported use of other drugs is consistent with national surveillance data and only two subjects were ascertained by GC/MS without also having self reported. Third, while the sample was recruited randomly at seven separate hospitals, the characteristics of the hospital may limit generalizability to other ethnic and/or income groups. Fourth, since meconium production begins at 14–16 weeks’ gestation, meconium testing primarily reflects maternal drug use during the second and third trimester[3]. Thus, information regarding drug use in the first trimester was ascertained only by self report. Finally, given that we hypothesized negative effects of drug exposure on infant neurobehavior a priori and anticipated that these effects would be subtle, we did not correct for the possibility of a Type I error on primary planned analyses. The systematic pattern of findings across analyses suggests that the results are not due to chance. To correct for multiple tests would invite Type II error in which true effects are missed[37]. Findings of subtle effects not only enhance scientific understanding but can have enormous impact on public policy and treatment.
In summary, this is the first report from the prospective, matched comparison designed IDEAL study of children exposed to MA in utero. Similar to findings in cocaine-exposed infants, we found subtle neurobehavioral effects in newborns exposed to MA. Caution needs to be exercised when interpreting these preliminary results of the IDEAL study. When initial findings regarding the effects of prenatal cocaine were reported in the 1980s, there was a “rush to judgment”[31;49] regarding the long-term outcomes of these children. Similar to cocaine exposed children, long-term follow-up is required to determine if the observed dose response relationships represent permanent neurotoxic effects or acute effects that may not have long term significance.
Acknowledgments
This study was supported by NIDA Grant# 1RO1DA014918 and in part by the National Center on Research Resources, Grant # 3 M01 RR00425 and P20 RR11091.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altshuler SJ. Drug-Endangered children need a collaborative community resonse. Child Welfare. 84(5 AD):171–190. [PubMed] [Google Scholar]
- 2.Arria AM, Derauf C, LaGasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Grotta SD, Liu J, Lester B. Methamphetamine and Other Substance Use During Pregnancy: Preliminary Estimates From the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2006:1–10. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Oz B, Klein J, Karaskov T, Koren G. Comparison of meconium and neonatal hair analysis for detection of gestational exposure to drugs of abuse. Arch Dis Child Fetal Neonatal Ed. 2005;88:F98–F100. doi: 10.1136/fn.88.2.F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billing L, Eriksson M, Jonsson B, Steneroth G, Zatterstrom R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse and Neglect. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 5.Billing L, Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr Scand. 1980;69:675–680. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 6.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse Negl. 1988;12:503–507. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.Brazelton TB. Neonatal Behavioral Assessment Scale. JB Lippinicott; Philadelphia, PA: 1984. [Google Scholar]
- 8.Brown NC, Anderson PJ, Howard K, Bear MJ, Wang H, Hunt RW, Doyle LW, Inder TE. Clinical Validity of Early Neurobehavioral Assessments of Very Preterm Infants. Pediatric Research. 2004;55:582A. [Google Scholar]
- 9.Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991;265:1968–1973. [PubMed] [Google Scholar]
- 10.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Coyle MG, Ferguson A, LaGasse L, Liu J, Lester B. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonatal Ed. 2005;90:F73–F74. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis PJ, Partridge JW, Storrs CN. Alcohol consumption in pregnancy. How much is safe? Arch Dis Child. 1982;57:940–943. doi: 10.1136/adc.57.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- 15.Ericksson M, Larsson C, Windbladh B, Zetterstrom R. The influence of amphetamine addiction on pregnancy and the newborn infant. Acta Paediatrica Scandinavica. 1978;67:95–99. doi: 10.1111/j.1651-2227.1978.tb16283.x. [DOI] [PubMed] [Google Scholar]
- 16.Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: II. Interactive and dose effects on neurobehavioral assessment. Pediatrics. 1998;101:237–241. doi: 10.1542/peds.101.2.237. [DOI] [PubMed] [Google Scholar]
- 17.Finnegan LP. Neonatal abstinence syndrome:assessment and pharmacotherapy. In: Rubatelli FF, Granati B, editors. Neonatal Therapy and Update. Excerpta Medica; New York, NY: 1986. [Google Scholar]
- 18.Fried PA, O’Connell CM. A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis and caffeine on birth size and subsequent growth. Neurotoxicol Teratol. 1987;9:79–85. doi: 10.1016/0892-0362(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 19.Fuller RW, Hemrick-Luecke SK. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 1982;21:433–438. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 20.Hansen RL, Struthers JM, Gospe SM., Jr Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev Med Child Neurol. 1993;35:798–805. doi: 10.1111/j.1469-8749.1993.tb11731.x. [DOI] [PubMed] [Google Scholar]
- 21.Hollingshead A. Four Factor Index of Social Status. Yale University, Dept. of Sociology; New Haven, CT: 1975. [Google Scholar]
- 22.Jacobson JL, Jacobson SW. Methodological issues in human behavioral teratology. In: Rovee-Collier C, Lipsitt L, editors. Advances in Infancy Research. 1990. pp. 111–148. [Google Scholar]
- 23.Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, Wilkins DG, Golden AS, Huggins GR, Lester BM. Buprenorphine treatment of pregnant opioid--dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 26.LaGasse L, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Smeriglio VL. The Maternal Lifestyle Study (MLS): The caretaking environment of infants exposed to cocaine/opiates. Pediatric Research. 1999;45:247A. [Google Scholar]
- 27.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 28.Lester BM, Lagasse L, Freier K, Brunner S. Studies of cocaine-exposed human infants. NIDA Res Monogr. 1996;164:175–210. [PubMed] [Google Scholar]
- 29.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–667. [PubMed] [Google Scholar]
- 30.Lester BM, Tronick EZ, Lagasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, Maza PL. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 31.Mayes LC, Granger RH, Bornstein MH, Zuckerman B. The problem of prenatal cocaine exposure. A rush to judgment. JAMA. 1992;267:406–408. [PubMed] [Google Scholar]
- 32.Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. J Pediatr. 1995;126:636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- 33.Napiorkowski B, Lester BM, Freier MC, Brunner S, Dietz L, Nadra A, Oh W. Effects of in utero substance exposure on infant neurobehavior. Pediatrics. 1996;98:71–75. [PubMed] [Google Scholar]
- 34.Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstetrics and Gynecology Clinics of North America. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- 35.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993;72:325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 36.Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Methamphetamine and cocaine users: differences in characteristics and treatment retention. J Psychoactive Drugs. 2000;32:233–238. doi: 10.1080/02791072.2000.10400234. [DOI] [PubMed] [Google Scholar]
- 37.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 38.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 39.Smeriglio VL, Wilcox HC. Prenatal drug exposure and child outcome. Past, present, future. Clin Perinatol. 1999;26:1–16. [PubMed] [Google Scholar]
- 40.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 41.Stek AM, Baker RS, Fisher BK, Lang U, Clark KE. Fetal responses to maternal and fetal methamphetamine administration in sheep. Am J Obstet Gynecol. 1995;173:1592–1598. doi: 10.1016/0002-9378(95)90654-1. [DOI] [PubMed] [Google Scholar]
- 42.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2004 National Survey on Drug Use and Health: National Findings. 2004. Ref Type: Report. [Google Scholar]
- 43.Substance Abuse and Mental Health Services Administration (SAMHSA) Preliminary Results from the 1997 National Household Survey on Drug Abuse. 1998. Ref Type: Report. [Google Scholar]
- 44.Substance Abuse and Mental Health Services Administration (SAMHSA) National Household Survey on Drug Abuse (NHSDA) National Institute on Drug Abuse. 1999. Ref Type: Generic. [Google Scholar]
- 45.O.o.A.S. Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS). Highlights - 2003. National Admissions to Substance Abuse Treatment Services. Rockville, M.D: 2005. Ref Type: Report. [Google Scholar]
- 46.Tronick EZ, Frank DA, Cabral H, Mirochnick M, Zuckerman B. Late dose-response effects of prenatal cocaine exposure on newborn neurobehavioral performance. Pediatrics. 1996;98:76–83. [PMC free article] [PubMed] [Google Scholar]
- 47.United Nations Office on Drugs and Crime. World Drug Report, vol 1: Analysis. Vienna: 2004. Ref Type: Report. [Google Scholar]
- 48.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 49.Zuckerman B, Frank DA. “Crack kids”: not broken. Pediatrics. 1992;89:337–339. [PubMed] [Google Scholar]