Abstract
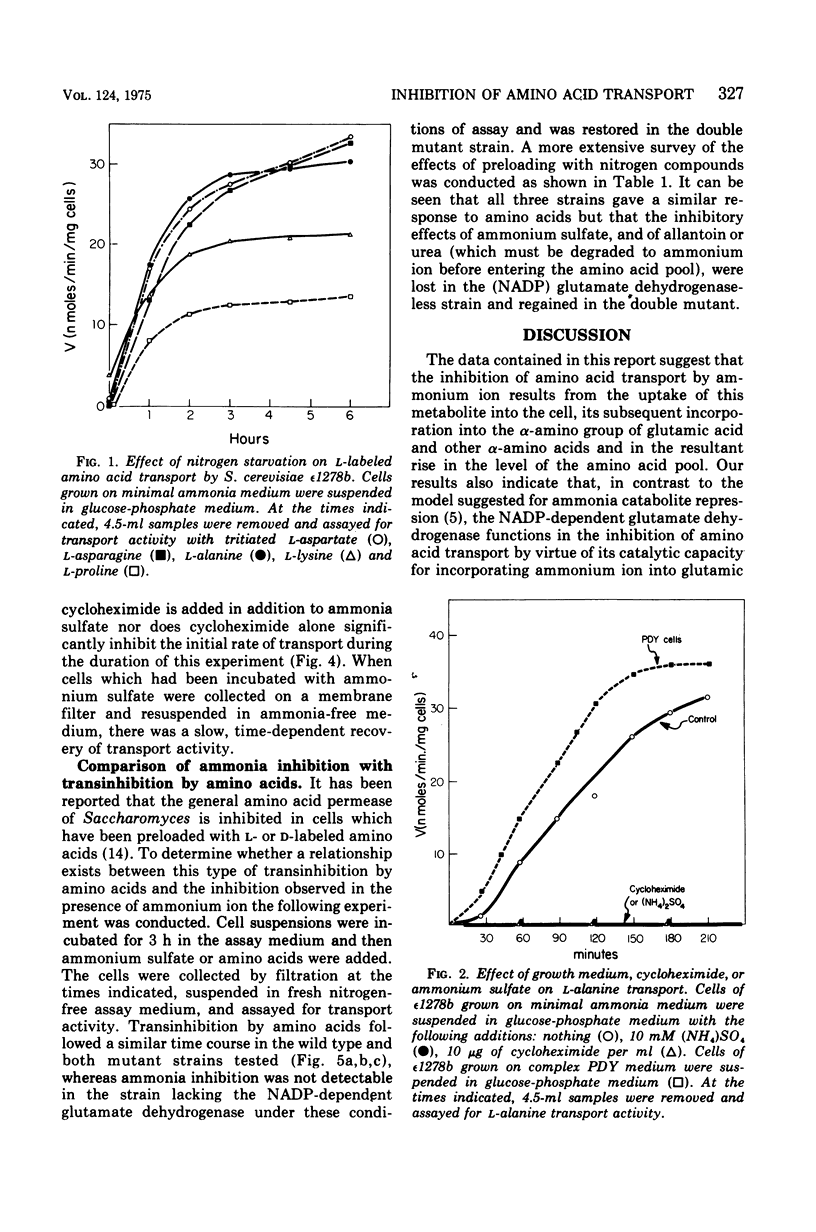
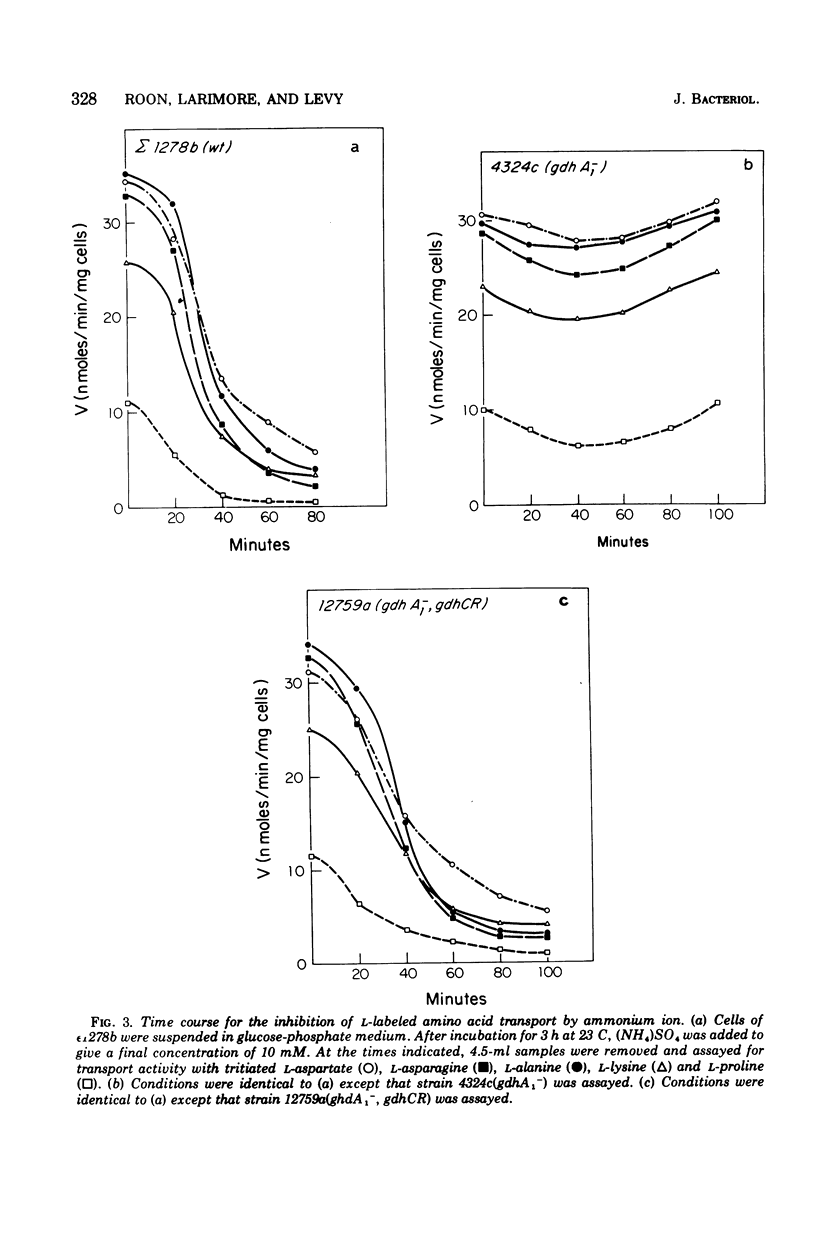
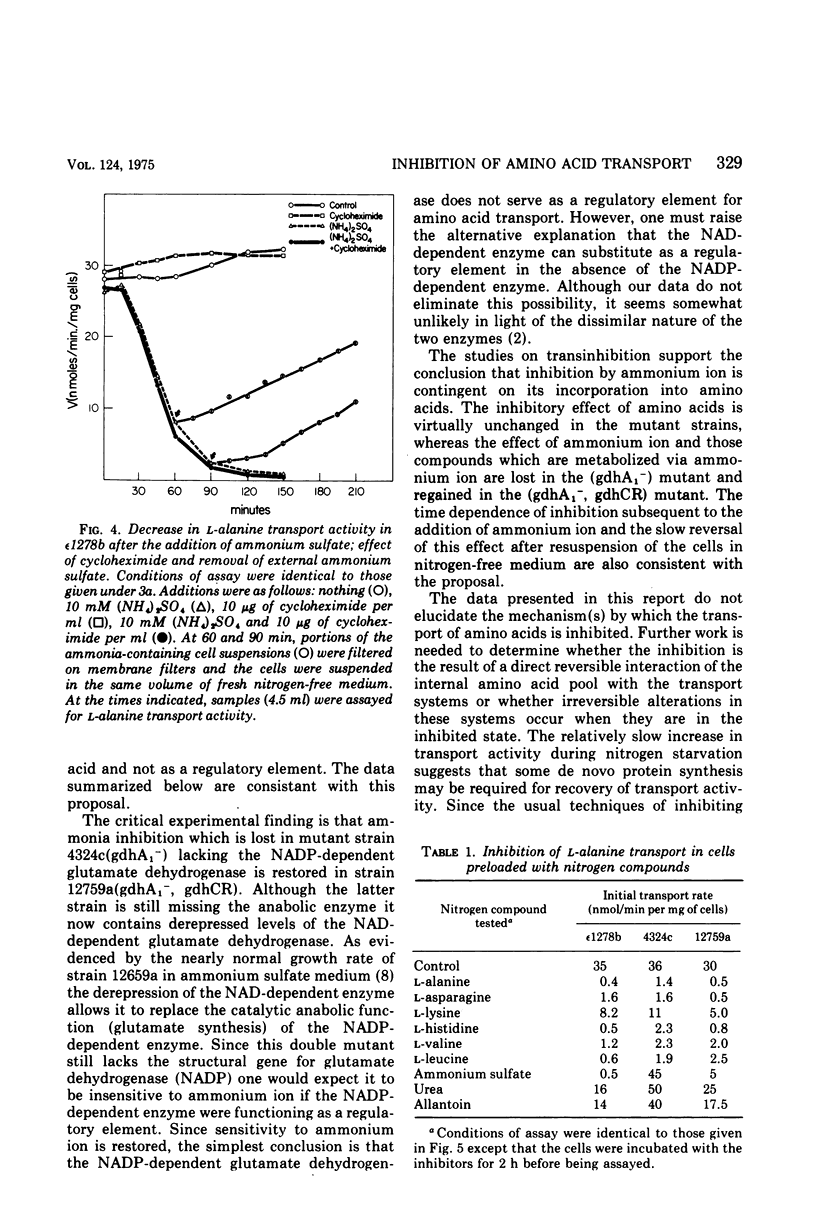
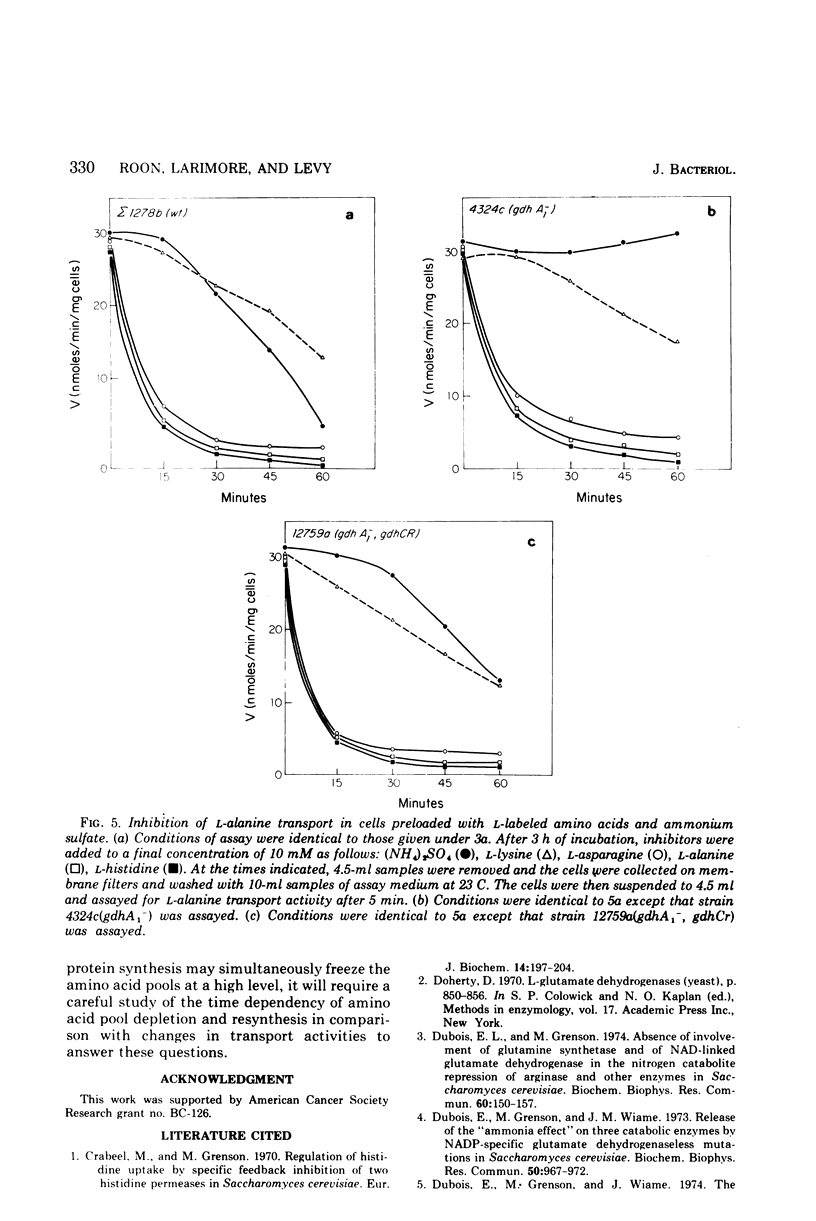
The rate of transport of L-amino acids by Saccharomyces cerevisiae epsilon 1278b increased with time in response to nitrogen starvation. This increase could be prevented by the addition of ammonium sulfate or cycloheximide. A slow time-dependent loss of transport activity was observed when ammonium sulfate (or ammonium sulfate plus cycloheximide) was added to cells after 3 h of nitrogen starvation. This loss of activity was not observed in the presence of cycloheximide alone. In a mutant yeast strain which lacks the nicotinamide adenine dinucleotide phosphate-dependent (anabolic) glutamate dehydrogenase, no significant decrease in amino acid transport was observed when ammonium sulfate was added to nitrogen-starved cells. A double mutant, which lacks the nicotinamide adenine dinucleotide phosphate-dependent enzyme and in addition has a depressed level of the nicotinamide adenine dinucleotide-dependent (catabolic) glutamate dehydrogenase, shows the same sensitivity to ammonium ion as the wild-type strain. These data suggest that the inhibition of amino acid transport by ammonium ion results from the uptake of this metabolite into the cell and its subsequent incorporation into the alpha-amino groups of glutamate and other amino acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Grenson M. Absence of involvement of glutamine synthetase and of NAD-linked glutamate dehydrogenase in the nitrogen catabolite repression of arginase and other enzymes in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Sep 9;60(1):150–157. doi: 10.1016/0006-291x(74)90185-5. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. Release of the "ammonia effect" on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Feb 20;50(4):967–972. doi: 10.1016/0006-291x(73)91500-3. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. The participation of the anabolic glutamate dehydrogenase in the nitrogen catabolite repression of arginase in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 2;48(2):603–616. doi: 10.1111/j.1432-1033.1974.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Dubois E., Piotrowska M., Drillien R., Aigle M. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. Evidence for the gdhA locus being a structural gene for the NADP-dependent glutamate dehydrogenase. Mol Gen Genet. 1974;128(1):73–85. doi: 10.1007/BF00267295. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Aug 21;48(4):749–756. doi: 10.1016/0006-291x(72)90670-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Joiris C. R., Grenson M. Spécificité et régulation d'une perméase des acis aminés dicarboxyliques chez "Saccharomyces crevisiae". Arch Int Physiol Biochim. 1969 Feb;77(1):154–156. [PubMed] [Google Scholar]
- Roon R. J., Even H. L., Dunlop P., Larimore F. L. Methylamine and ammonia transport in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):502–509. doi: 10.1128/jb.122.2.502-509.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytka J. Positive selection of general amino acid permease mutants in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):562–570. doi: 10.1128/jb.121.2.562-570.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N. Derepression of a proline transport system in Saccharomyces chevalieri by nitrogen starvation. Biochim Biophys Acta. 1969 Mar 11;173(2):302–312. doi: 10.1016/0005-2736(69)90113-8. [DOI] [PubMed] [Google Scholar]



