Abstract
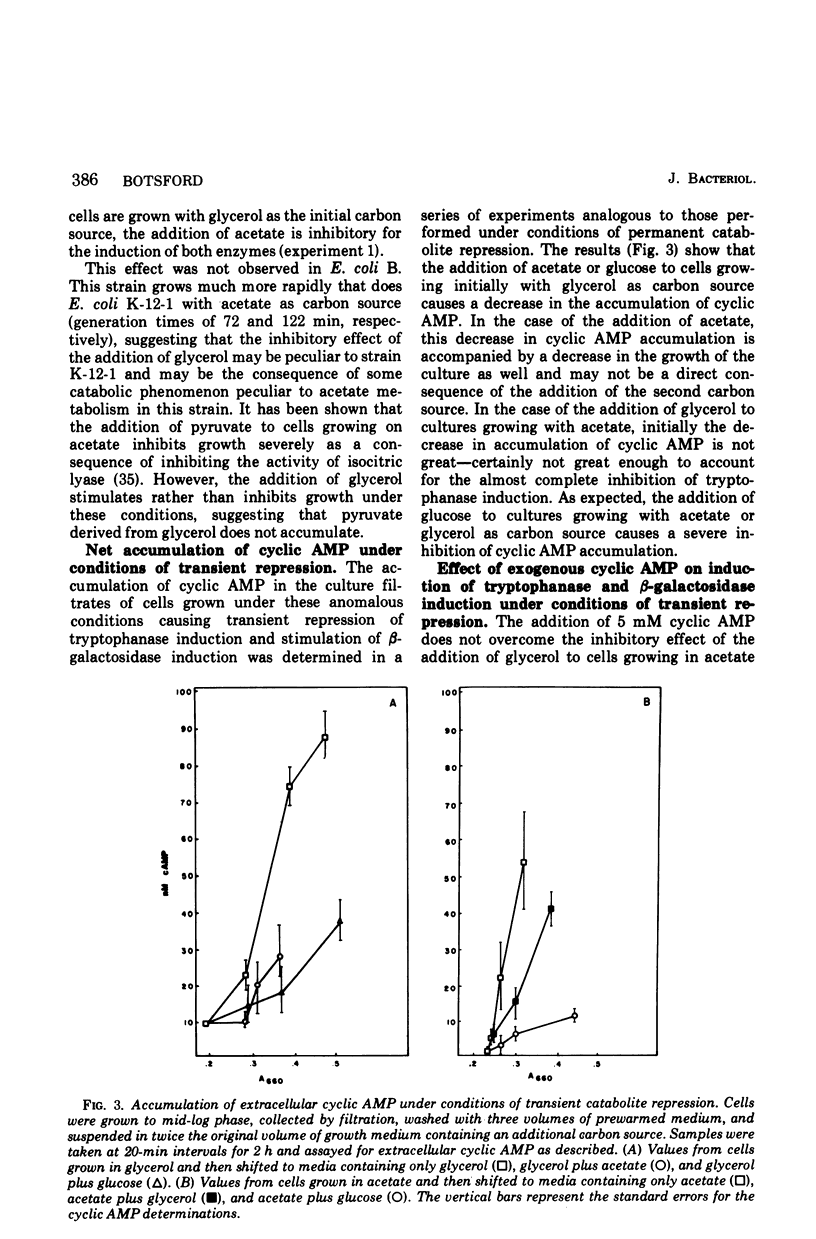
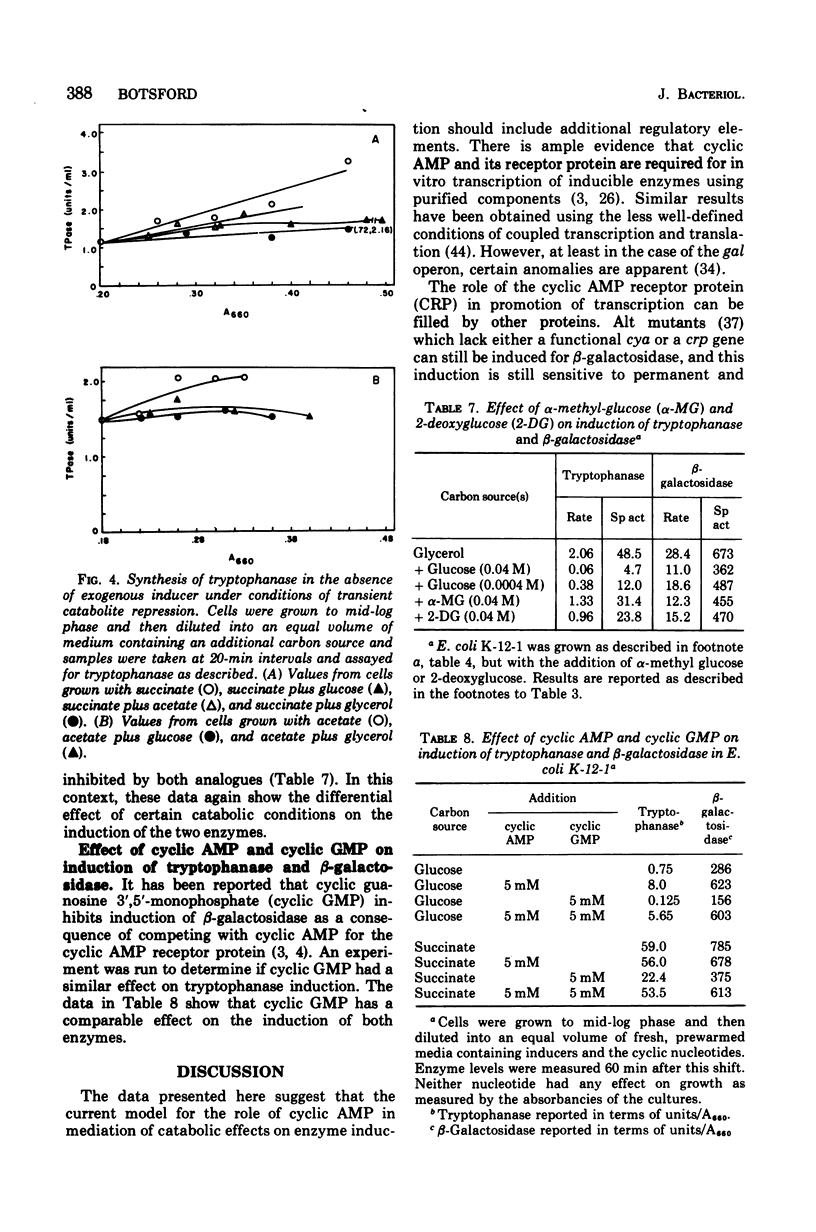
The relationship between cyclic adenosine 3',5'-monophosphate (cyclic AMP) metabolism and the induction of tryptophanase and beta-galactosidase was studied in several strains of Escherichia coli grown with succinate, acetate, glycerol, or glucose as the carbon source. No consistent relationship between the intracellular concentration of cyclic AMP in the several strains cultured and the various carbon sources was discerned. In E. coli K-12-1 the induction of tryptophanase was found to vary in the order: succinate greater than acetate greater than glycerol greater than glucose, and that of beta-galactosidase was found in the order: glycerol greater than acetate greater than succinate greater than glucose. Rate of accumulation of cyclic AMP in the culture filtrate was in the order: succinate greater than acetate greater than glycerol greater than glucose. The addition of glycerol to E. coli K-12-1 grown in acetate caused inhibition of tryptophanase and slight inhibition of accumulation of extracellular cyclic AMP. These same conditions caused beta-galactosidase induction to be stimulated. The addition of exogenous cyclic AMP to cultures grown with four different carbon sources had an effect characteristic for each of the two enzymes studied as well as each individual carbon source. The results suggest that there are control elements distinct from cyclic AMP and its receptor protein which respond to the catabolic situation of the cell.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman R. S., Cozzarelli N. R., Epstein W. Accumulation of toxic concentrations of methylglyoxal by wild-type Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):357–362. doi: 10.1128/jb.119.2.357-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Pastan I. The cyclic AMP receptor of Escherichia coli: immunological studies in extracts of Escherichia coli and other organisms. Biochim Biophys Acta. 1973 Oct 5;320(3):577–587. doi: 10.1016/0304-4165(73)90137-2. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Perlman R. L., Pastan I. Effect of adenosine 3',5'-monophosphate analogues on the activity of the cyclic adenosine 3',5'-monophosphate receptor in Escherichia coli. J Biol Chem. 1972 May 10;247(9):2717–2722. [PubMed] [Google Scholar]
- Artman M., Werthamer S. Effect of cyclic guanosine 3,5-monophosphate on the synthesis of enzymes sensitive to caatabolite repression in intact cells of Escherichia coli. J Bacteriol. 1974 Nov;120(2):980–983. doi: 10.1128/jb.120.2.980-983.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Botsford J. L., DeMoss R. D. Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol. 1971 Jan;105(1):303–312. doi: 10.1128/jb.105.1.303-312.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Demoss R. D. Escherichia coli tryptophanase in the enteric environment. J Bacteriol. 1972 Jan;109(1):74–80. doi: 10.1128/jb.109.1.74-80.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Kon C. An improved protein binding assay for cyclic AMP. Anal Biochem. 1974 Apr;58(2):459–468. doi: 10.1016/0003-2697(74)90214-0. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3'.5'-nucleotide phosphodiesterase. Effect of binding protein on the hydrolysis of cyclic AMP. Biochem Biophys Res Commun. 1972 Jan 14;46(1):99–105. doi: 10.1016/0006-291x(72)90635-3. [DOI] [PubMed] [Google Scholar]
- Dahl R., Wang R. J., Morse M. L. Effect of pleiotropic carbohydrate mutations (ctr) on tryptophan catabolism. J Bacteriol. 1971 Aug;107(2):513–518. doi: 10.1128/jb.107.2.513-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss R. D., Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969 Apr;98(1):167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., LICHSTEIN H. C. Inhibitory effect of glucose on tryptophanase. J Bacteriol. 1960 Nov;80:633–638. doi: 10.1128/jb.80.5.633-638.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- McFall E. Role of adenosine 3',5'-cyclic monophosphate and its specific binding protein in the regulation of D-serine deaminase synthesis. J Bacteriol. 1973 Feb;113(2):781–785. doi: 10.1128/jb.113.2.781-785.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses V., Sharp P. B. Intermediary metabolite levels in Escherichia coli. J Gen Microbiol. 1972 Jun;71(1):181–190. doi: 10.1099/00221287-71-1-181. [DOI] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Stimulation of tryptophanase synthesis in Escherichia coli by cyclic 3',5'-adenosine monophosphate. J Biol Chem. 1969 Apr 25;244(8):2226–2232. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Hesse J. E., Epstein W. Role of cyclic adenosine 3',5'-monophosphate in the in vivo expression of the galactose operon of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1040–1044. doi: 10.1128/jb.114.3.1040-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam K. T., Valentine R. C. Molecular biology of nitrogen fixation. Science. 1975 Mar 14;187(4180):919–924. doi: 10.1126/science.238283. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Goman M., Scaife J. G. ALT: a new factor involved in the synthesis of RNA by Escherichia coli. Mol Gen Genet. 1972;118(3):223–234. doi: 10.1007/BF00333459. [DOI] [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J Bacteriol. 1970 May;102(2):411–422. doi: 10.1128/jb.102.2.411-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. K., Rosen O. M. Cyclic 3':5'-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1436–1440. doi: 10.1073/pnas.71.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Wu F. Y. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A temperature-jump study. Biochemistry. 1974 Jun 4;13(12):2573–2578. doi: 10.1021/bi00709a016. [DOI] [PubMed] [Google Scholar]
- Wu F. Y., Nath K., Wu C. W. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A fluorescent probe study. Biochemistry. 1974 Jun 4;13(12):2567–2572. doi: 10.1021/bi00709a015. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]



