Abstract
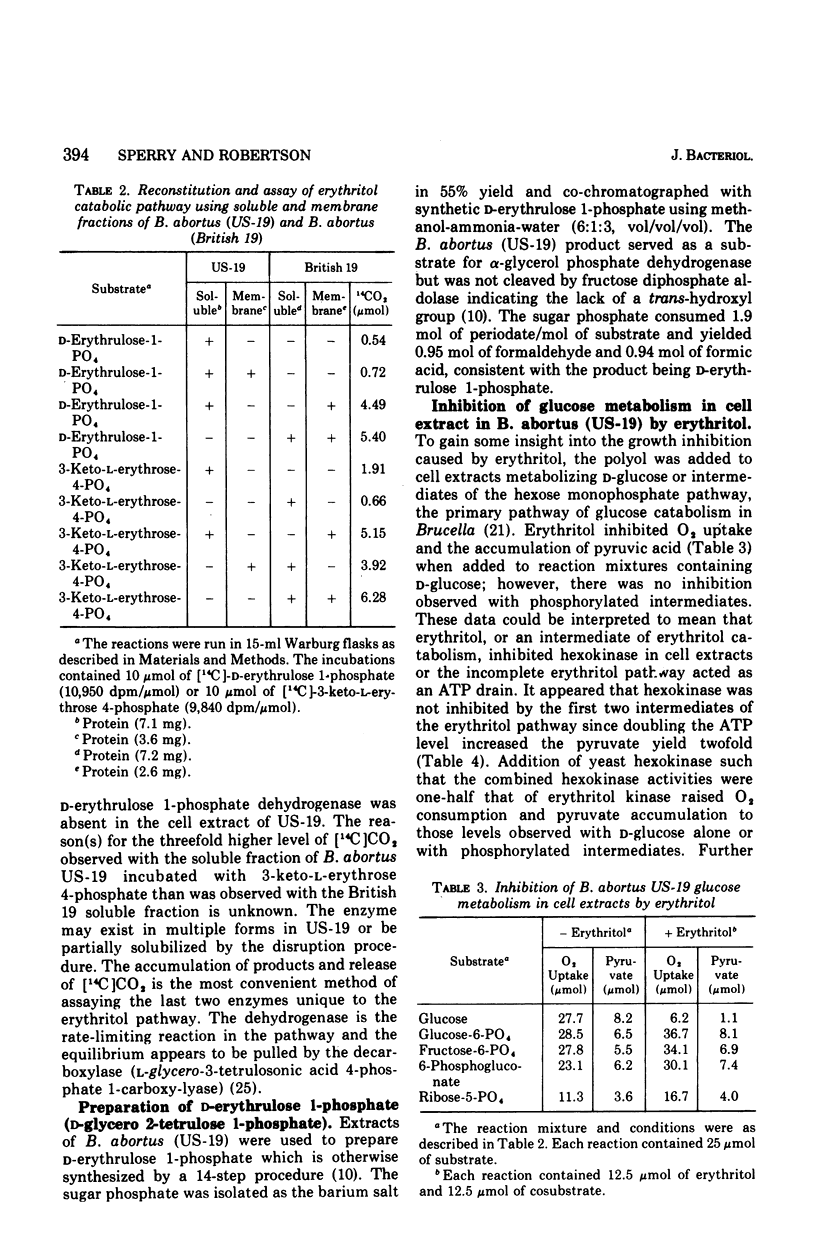
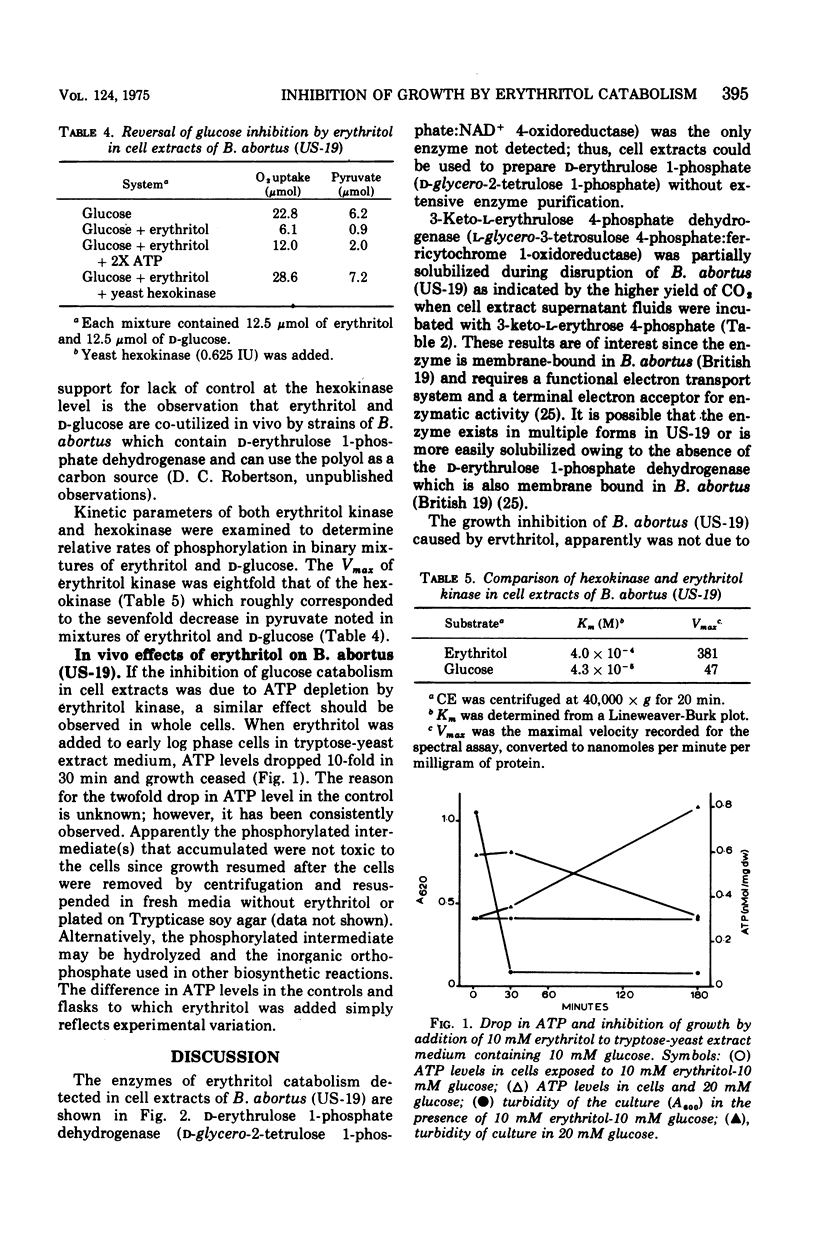
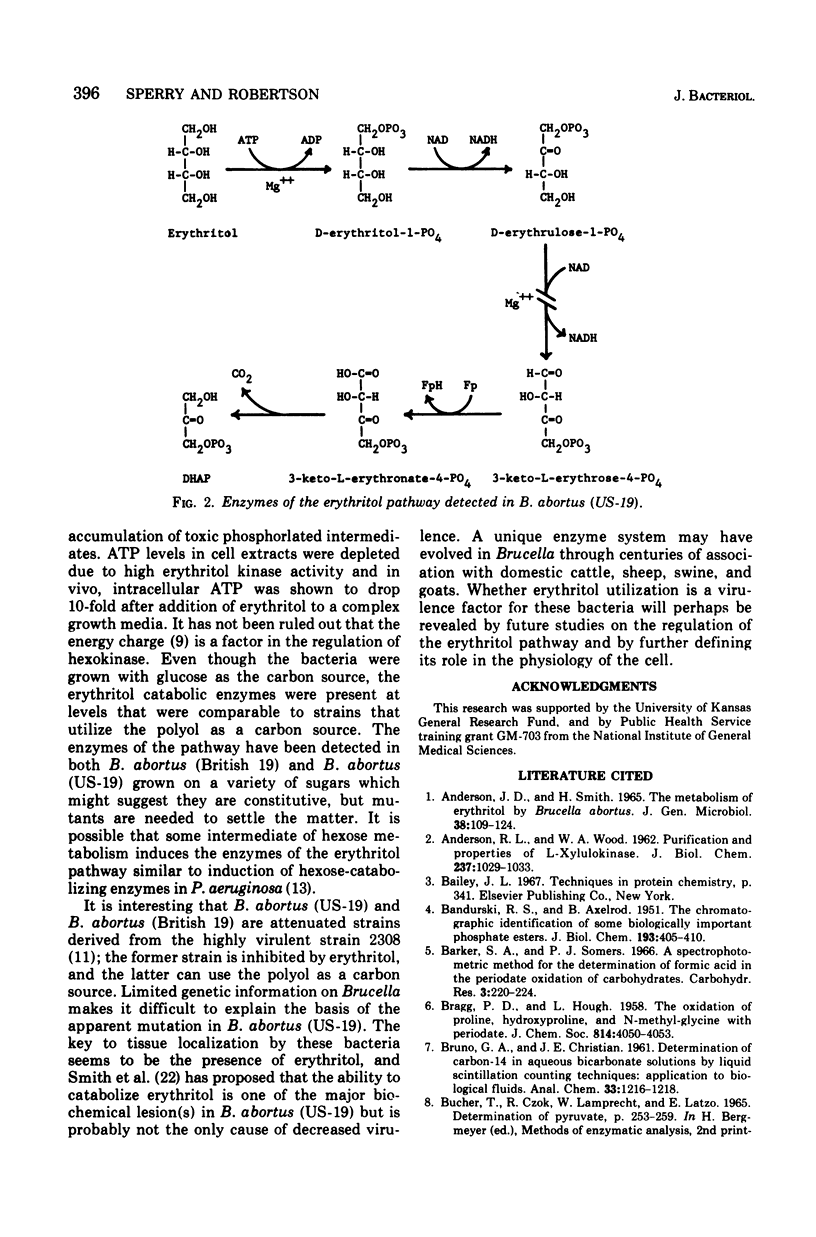
The growth of Brucella abortus (US-19) in a complex tryptose-yeast extract medium containing D-glucose is inhibited by 10 mM erythritol. The enzymes of the erythritol pathway, except for D-erythrulose 1-phosphate dehydrogenase (D-glycero-2-tetrulose 1-phosphate:nicotinamide adenine dinucleotide (NAD+) 4-oxidoreductase) were detected in the soluble and membrane fractions of cell extracts. Glucose catabolism by cell extracts was inhibited by erythritol, whereas, phosphorylated intermediates of the hexose monophosphate pathway were converted to pyruvic acid with oxygen consumption. Erythritol kinase (EC 2.7.1.27; adenosine 5'-triphosphate (ATP): erythritol 1-phosphotransferase) was found to be eightfold higher in activity than the hexokinase in cell extracts. In vivo, ATP is apparently consumed with the accumulation of D-erythrulose 1-phosphate (D-glycero-2-tetrulose 1-phosphate) and no substrate level phosphorylation. ATP levels dropped 10-fold in 30 min after addition of erythritol to log phase cells in tryptose-yeast extract medium with D-glucose as the carbon source. These data suggest bacteriostasis in the presence of erythritol results from the ATP drain caused by erythritol kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. D., SMITH H. THE METABOLISM OF ERYTHRITOL BY BRUCELLA ABORTUS. J Gen Microbiol. 1965 Jan;38:109–124. doi: 10.1099/00221287-38-1-109. [DOI] [PubMed] [Google Scholar]
- ANDERSON R. L., WOOD W. A. Purification and properties of L-xylulokinase. J Biol Chem. 1962 Apr;237:1029–1033. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTEN D., FROMM H. J. Purification and properties of erythritol kinase from Propionibacterium pentosaceum. J Biol Chem. 1961 Oct;236:2581–2584. [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- JONES L. M., MONTGOMERY V., WILSON J. B. CHARACTERISTICS OF CARBON DIOXIDE-INDEPENDENT CULTURES OF BRUCELLA ABORTUS ISOLATED FROM CATTLE VACCINATED WITH STRAIN 19. J Infect Dis. 1965 Jun;115:312–320. doi: 10.1093/infdis/115.3.312. [DOI] [PubMed] [Google Scholar]
- KALNITSKY G., TAPLEY D. F. A sensitive method for estimation of oxaloacetate. Biochem J. 1958 Sep;70(1):28–34. doi: 10.1042/bj0700028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPIE J., WILLIAMS A. E., WITT K., SMITH H. THE ROLE OF ERYTHRITOL IN THE TISSUE LOCALIZATION OF THE BRUCELLAE. Br J Exp Pathol. 1965 Feb;46:104–108. [PMC free article] [PubMed] [Google Scholar]
- Keppie J., Witt K., Smith H. The effect of erythritol on the growth of S19 and other attenuated strains of Brucella abortus. Res Vet Sci. 1967 Jul;8(3):294–296. [PubMed] [Google Scholar]
- Rest R. F., Robertson D. C. Glucose transport in Brucella abortus. J Bacteriol. 1974 Apr;118(1):250–258. doi: 10.1128/jb.118.1.250-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. I. Evaluation of pathways. Arch Biochem Biophys. 1968 Sep 20;127(1):263–273. doi: 10.1016/0003-9861(68)90225-7. [DOI] [PubMed] [Google Scholar]
- SMITH H., WILLIAMS A. E., PEARCE J. H., KEPPIE J., HARRIS-SMITH P. W., FITZ-GEORGE R. B., WITT K. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962 Jan 6;193:47–49. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- Smith H. Biochemical challenge of microbial pathogenicity. Bacteriol Rev. 1968 Sep;32(3):164–184. doi: 10.1128/br.32.3.164-184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. F., Robertson D. C. Erythritol catabolism by Brucella abortus. J Bacteriol. 1975 Feb;121(2):619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



