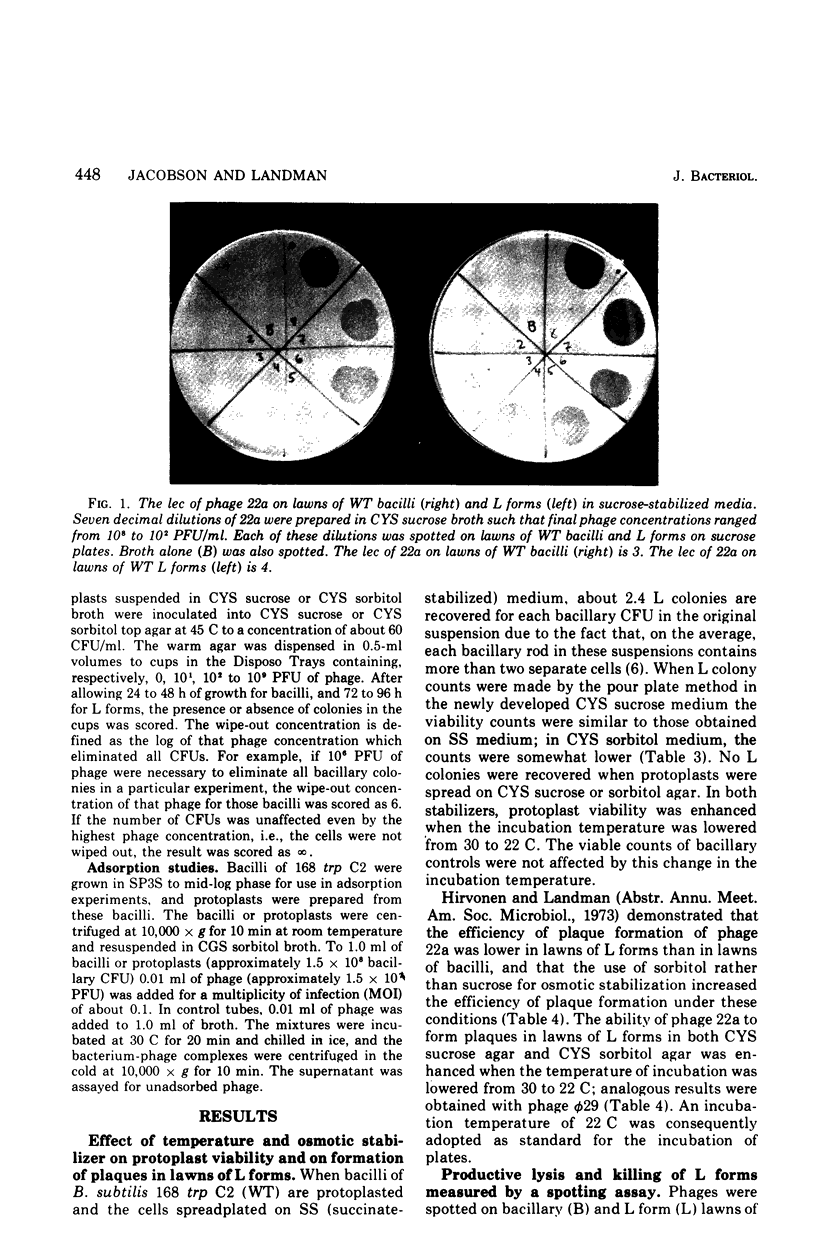
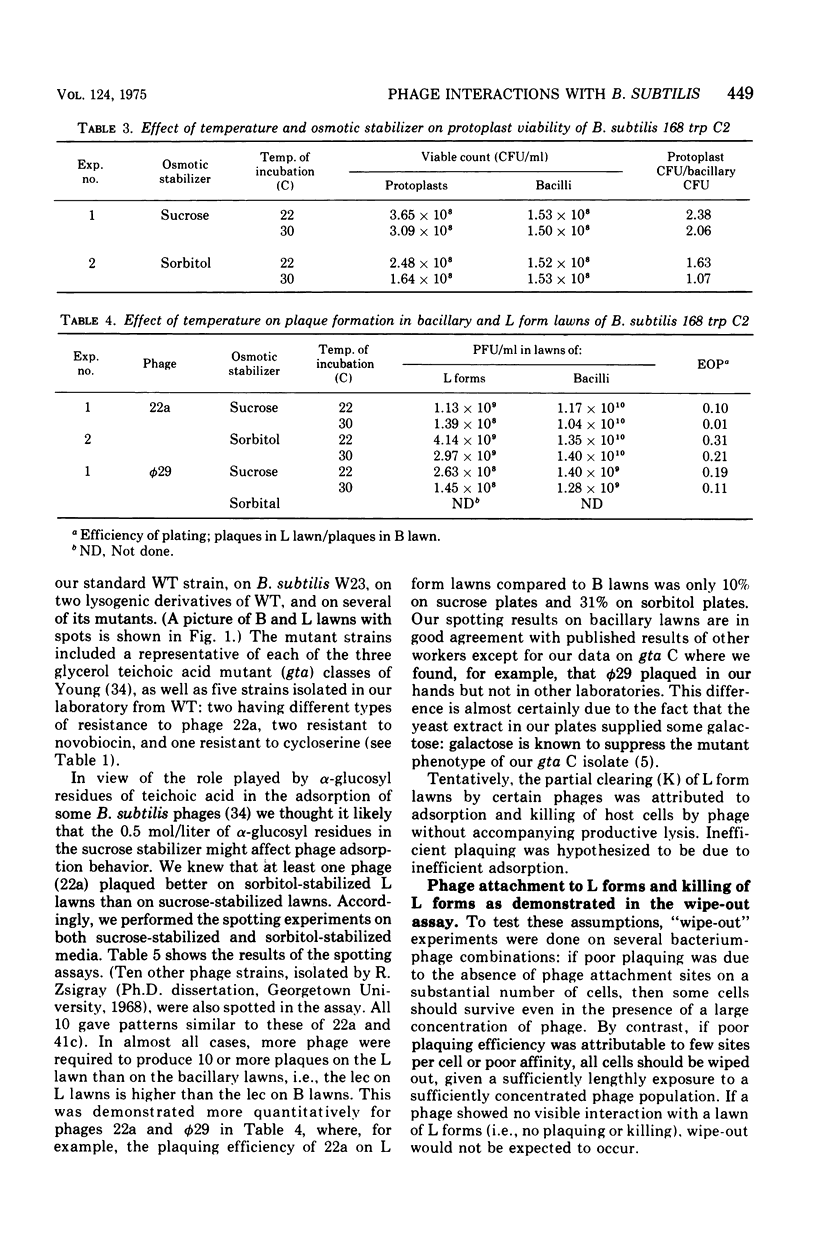
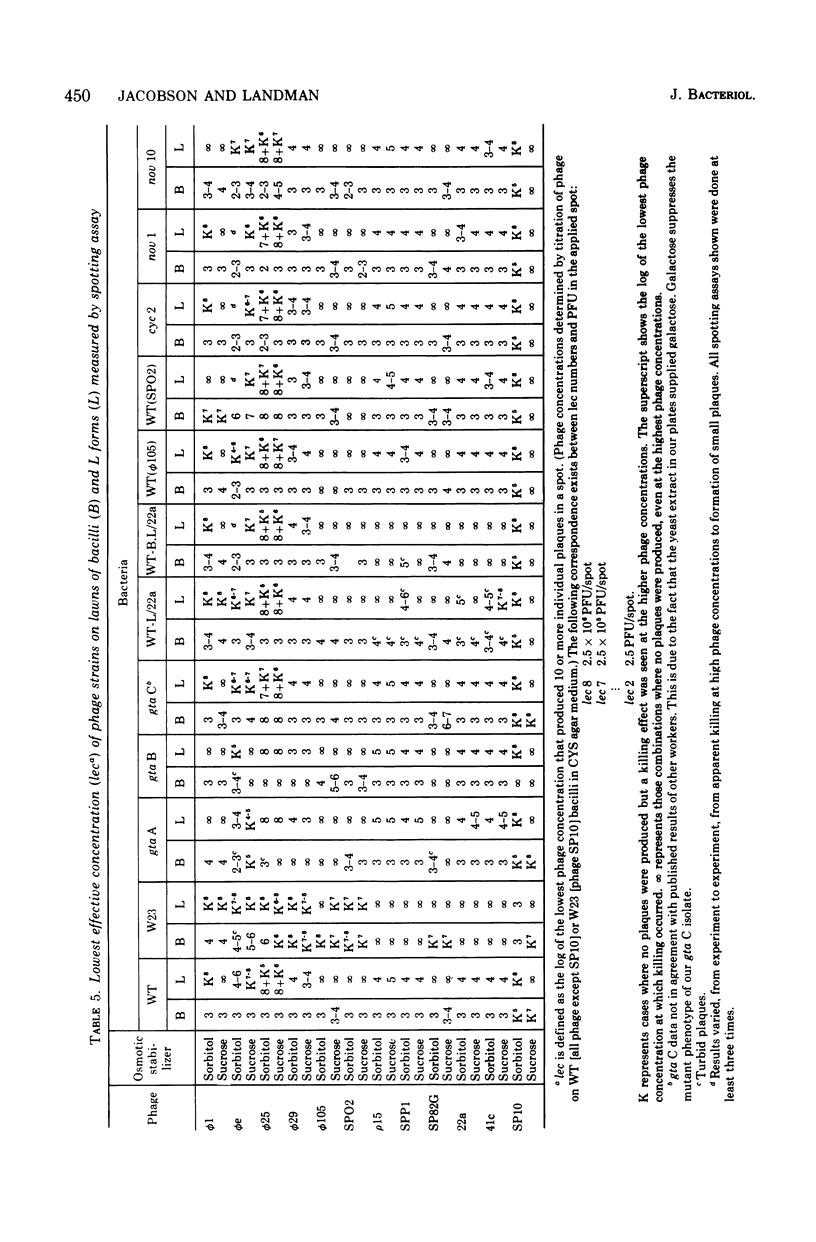
Abstract
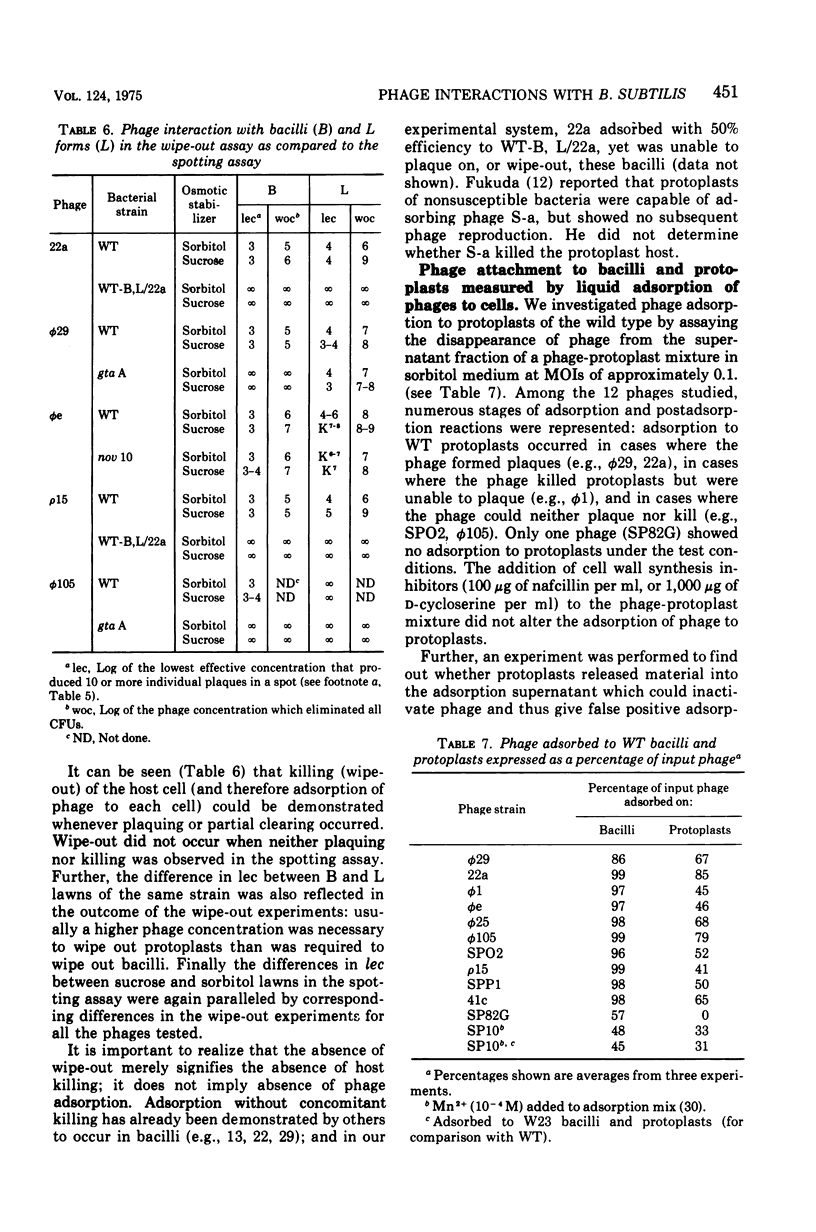
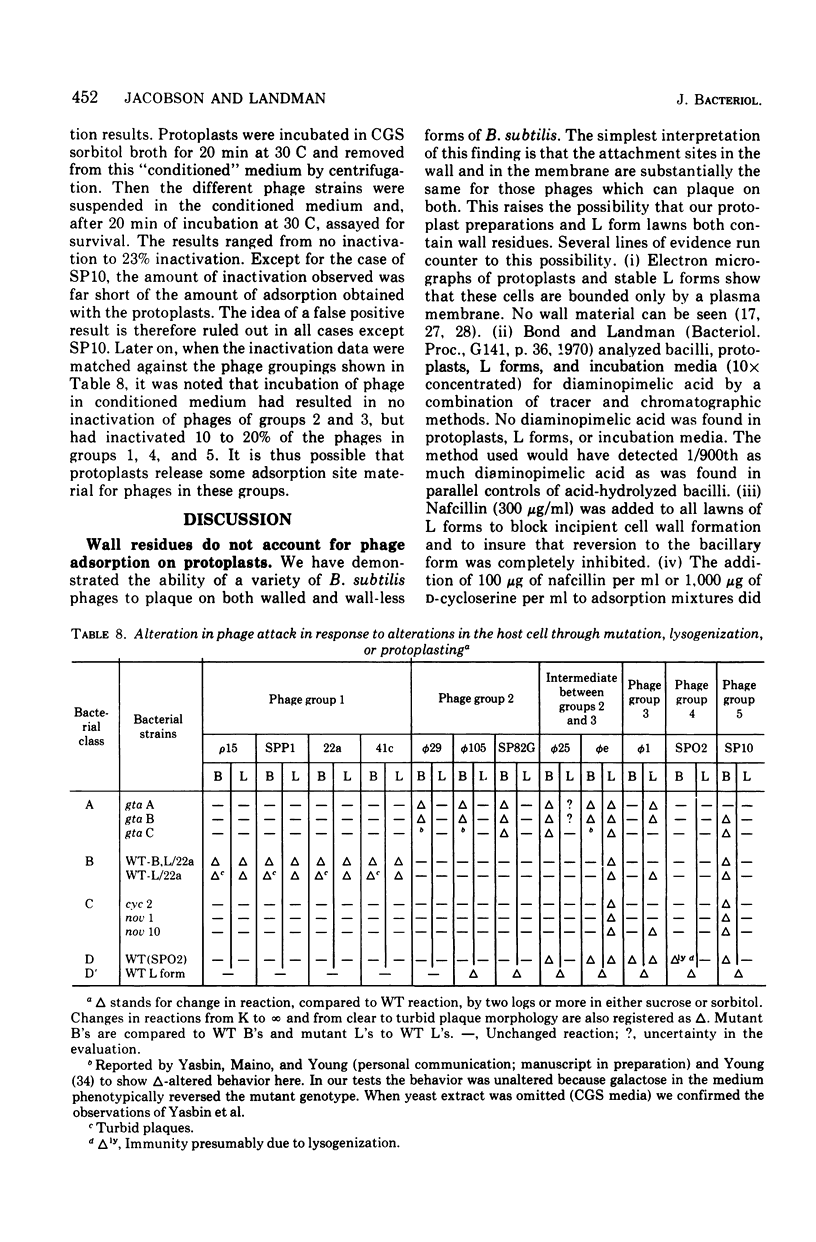
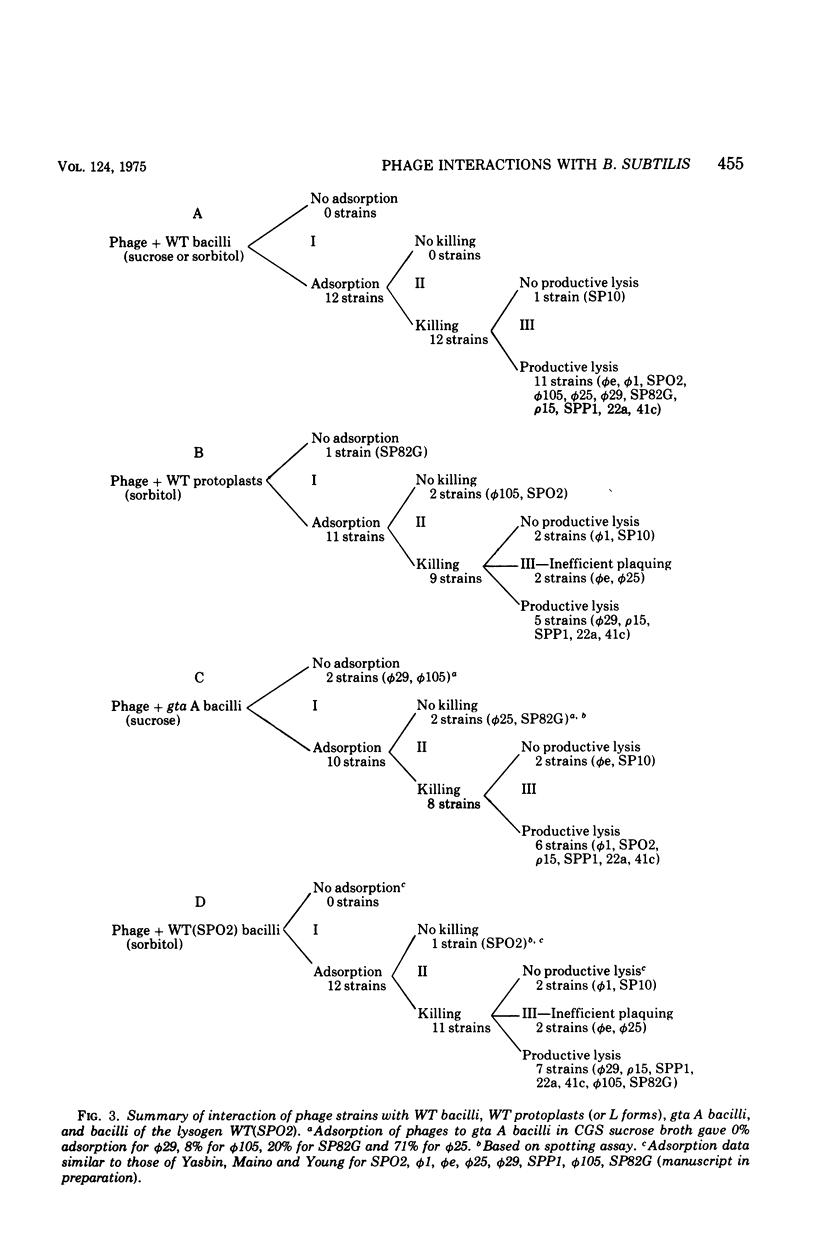
The interaction of 12 phage strains with bacilli, protoplasts, and L forms of Bacillus subtilis 168 and with eight of its mutants and two of its lysogens is described qualitatively and quantitatively. After removal of the cell wall from B. subtilis 168, 11 of the 12 phage strains can still adsorb to the protoplasts, nine kill their wall-less host cells, and five multiply in the naked bacteria, forming plaques on L form lawns. Individual gene mutations can have similarly pleiotropic effects, strongly dependent upon the plating medium. Thus, the gta A mutation, which causes loss of glucosylation of the wall teichoic acid, results in loss of wall adsorption sites for phi (but not membrane sites) and for phi105. Phages phi25, SP82G and phie can still adsorb to gta A bacilli and plaque in unstabilized and sorbitol-stabilized lawns of this mutant, but they can not plaque in sucrose-stabilized lawns. The lysogenized wild type, B. subtilis 168 (SPO2), also exhibits a pleiotropic pattern, showing different levels of resistance to phages SPO2, phi1, phie, and phi25. Its resistance pattern is very similar to that of wild-type protoplasts. On the basis of such patterns, the bacterial mutants and strain B. subtilis 168 (SPO2) could be ordered into four classes and the phage strains classified into four to six groups. Together, they form four to six interaction complexes, based partly on adsorption sites and perhaps partly on metabolic blocks in phage development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku N., Landman O. E. Control of the synthesis of macromolecules during amino acid and thymine starvation in Bacillus subtilis. J Bacteriol. 1968 May;95(5):1813–1827. doi: 10.1128/jb.95.5.1813-1827.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E. Blocking of bacteriophage receptor sites by Concanavalin A. J Gen Microbiol. 1972 Dec;73(3):581–585. doi: 10.1099/00221287-73-3-581. [DOI] [PubMed] [Google Scholar]
- Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- Breakefield X. O., Landman O. E. Temperature-sensitive divisionless mutant of Bacillus subtilis defective in the initiation of septation. J Bacteriol. 1973 Feb;113(2):985–998. doi: 10.1128/jb.113.2.985-998.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Membrane lipoteichoic acid is not a precursor to wall teichoic acid in pneumococci. J Bacteriol. 1975 Apr;122(1):335–337. doi: 10.1128/jb.122.1.335-337.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clive D., Landman O. E. Reversion of Bacillus subtilis protoplasts to the bacillary form induced by exogenous cell wall, bacteria and by growth in membrane filters. J Gen Microbiol. 1970 May;61(2):233–243. doi: 10.1099/00221287-61-2-233. [DOI] [PubMed] [Google Scholar]
- Coley J., Duckworth M., Baddiley J. The occurrence of lipoteichoic acids in the membranes of gram-positive bacteria. J Gen Microbiol. 1972 Dec;73(3):587–591. doi: 10.1099/00221287-73-3-587. [DOI] [PubMed] [Google Scholar]
- Doyle R. J. Modification of bacteriophage phi 25 adsorption to Bacillus subtilis by concanavalin A. J Bacteriol. 1973 Jan;113(1):198–202. doi: 10.1128/jb.113.1.198-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., PUCK T. T. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951 Sep;94(3):177–189. doi: 10.1084/jem.94.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Hurst A., Stubbs J. M. Electron microscopic study of membranes and walls of bacteria and changes occurring during growth initiation. J Bacteriol. 1969 Mar;97(3):1466–1479. doi: 10.1128/jb.97.3.1466-1479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Abortive infection of lysogenic Bacillus subtilis 168(SPO2) by bacteriophage phi 1. J Virol. 1974 Apr;13(4):870–880. doi: 10.1128/jvi.13.4.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of a pyrimidine replacing thymine in bacteriophage DNA. Biochem Biophys Res Commun. 1964 Jun 1;16(2):106–110. doi: 10.1016/0006-291x(64)90344-4. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. An Escherichia coli mutant which inhibits the injection of phage lambda DNA. Virology. 1974 Apr;58(2):504–513. doi: 10.1016/0042-6822(74)90084-1. [DOI] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsigray R. M., Miss A. L., Landman O. E. Penetration of a bacteriophage into Bacillus subtilis: blockage of infection by deoxyribonuclease. J Virol. 1973 Jan;11(1):69–77. doi: 10.1128/jvi.11.1.69-77.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]