Abstract
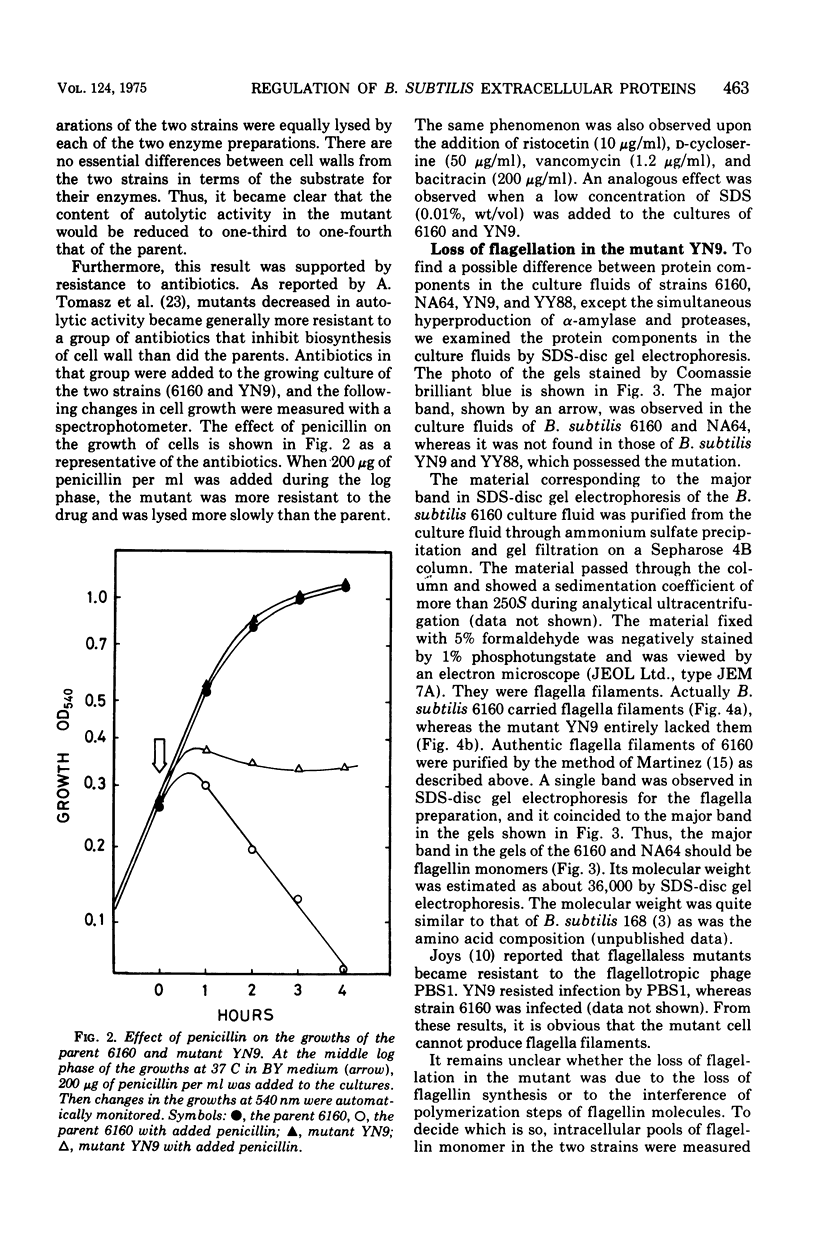
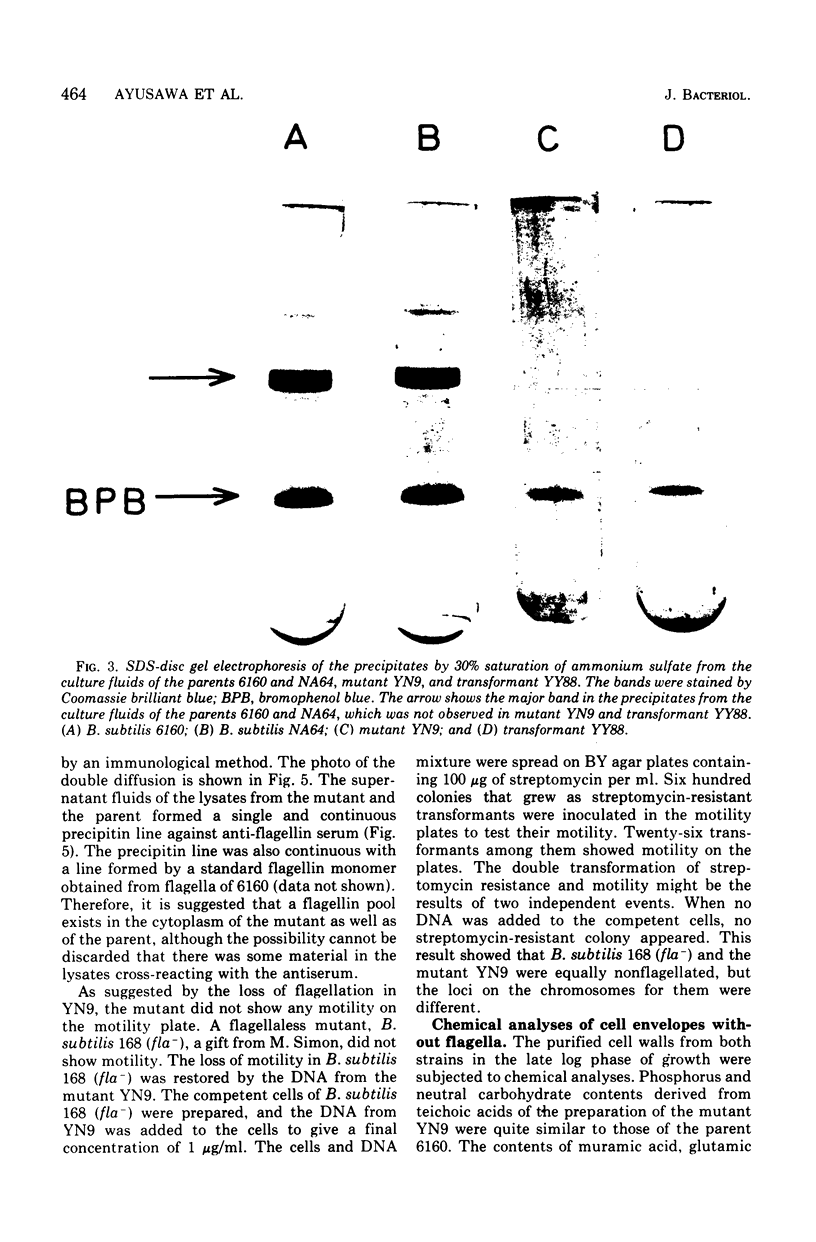
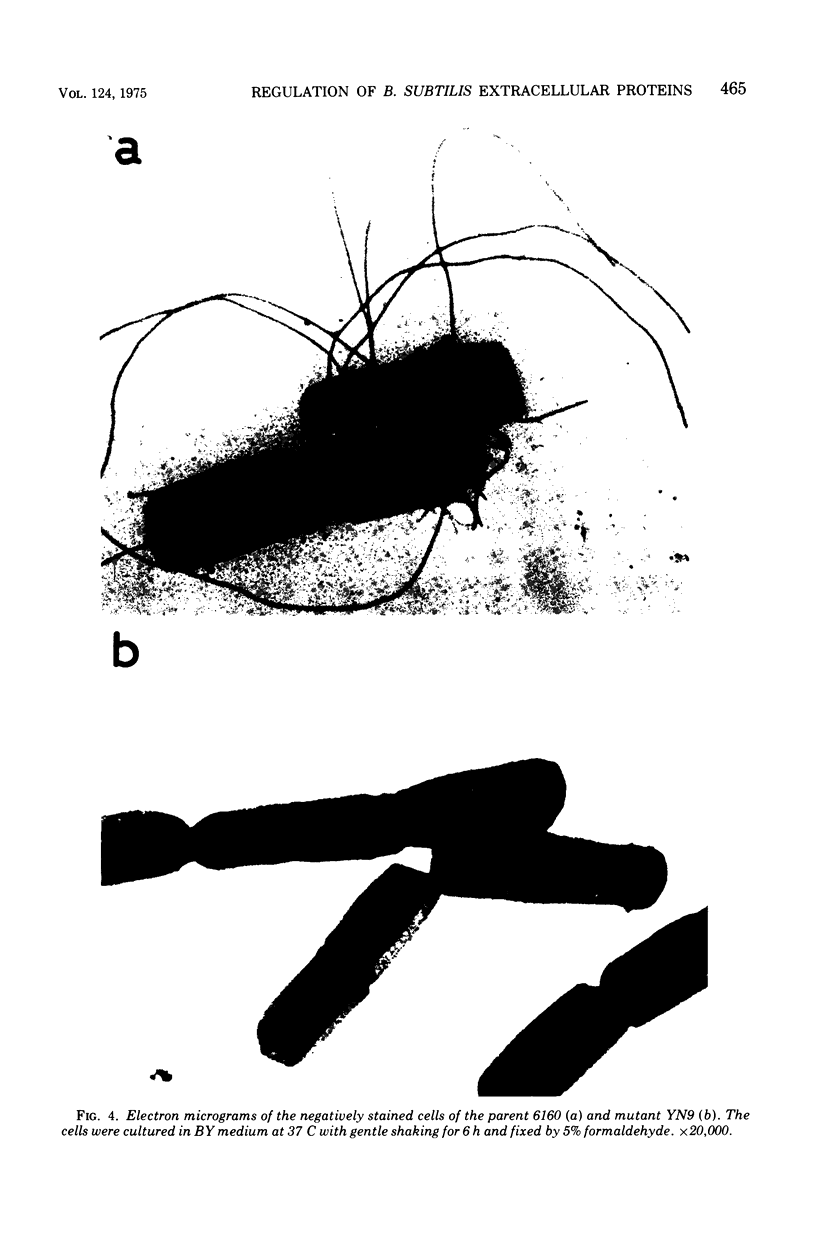
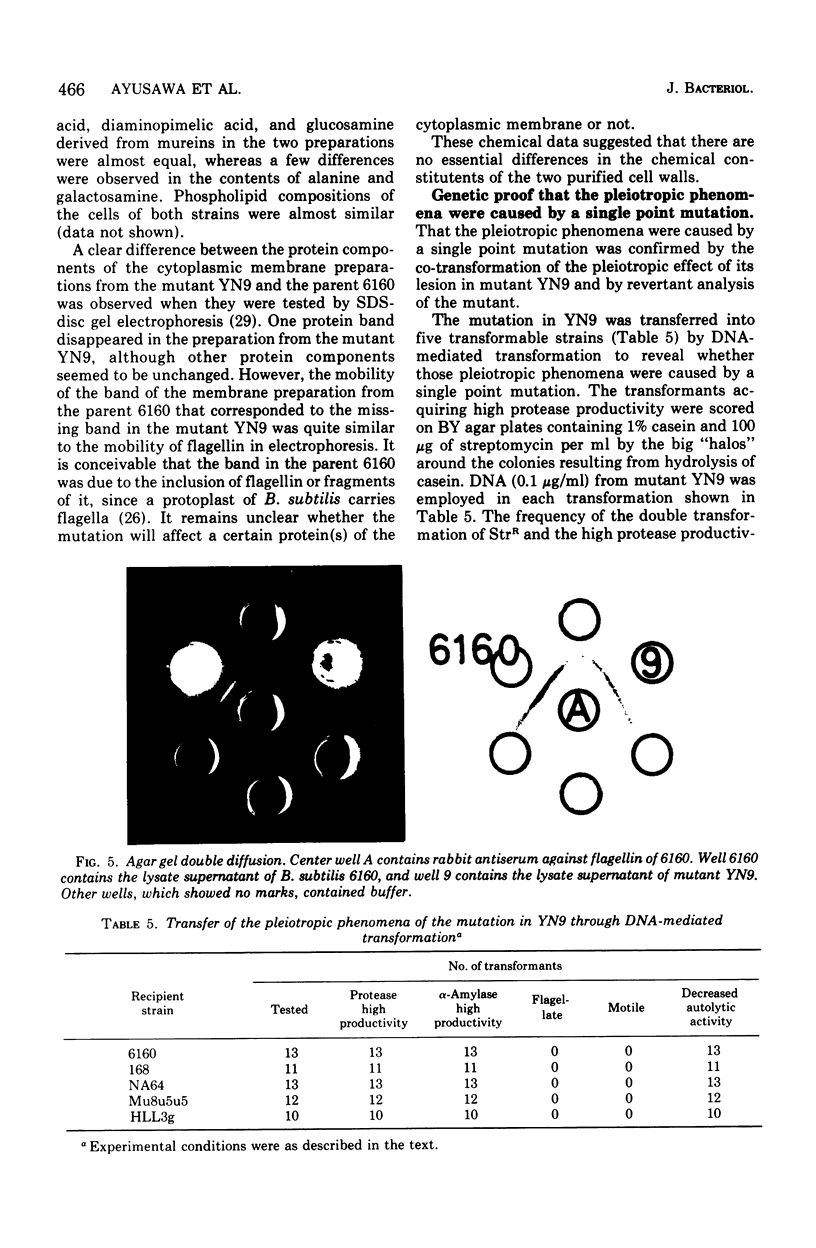
A mutant of Bacillus subtilis 6160 that had been isolated by its hyperproduction of alpha-amylase and protease lacked flagella and motility, and its content of autolytic enzyme(s) was reduced to one-third to one-fourth that of the parent. These phenotypic differences were completely co-transferred by the deoxyribonucleic acid (DNA) of the mutant when five DNA recipient strains of B. subtilis were transformed. The revertants, isolated by motility with a frequency of approximately 10(-7), recovered a normal level of autolytic activity and showed reduced productivity of alpha-amylase and protease. This point mutation allowed normal flagellin synthesis, spore formation, and rate of growth. The comparison of cell envelope of the mutant with that of the parent indicated that there was no significant difference except loss of flagella. Therefore the association at the cell surface of a group of extracellular proteins consisting of alpha-amylase, proteases, flagellin, and autolytic enzymes(s) seem to be coordinately regulated by the gene or seem to be affected coordinately by certain undetected alterations of the cell envelope.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Delange R. J., Chang J. Y., Shaper J. H., Martinez R. J., Komatsu S. K., Glazer A. N. On the amino-acid sequence of flagellin from Bacillus subtilis 168: comparison with other bacterial flagellins. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3428–3431. doi: 10.1073/pnas.70.12.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Simon M. I. Variation in the primary structure of Bacillus subtilis flagellins. J Bacteriol. 1971 Jun;106(3):949–954. doi: 10.1128/jb.106.3.949-954.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISCH-NIGGEMEYER W., REDDI K. K. Studies on ribonuclease in tobacco leaves. I. Purification and properties. Biochim Biophys Acta. 1957 Oct;26(1):40–46. doi: 10.1016/0006-3002(57)90051-3. [DOI] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Cell wall alterations associated with the hyperproduction of extracellular enzymes in Neurospora crassa. J Bacteriol. 1972 Aug;111(2):443–446. doi: 10.1128/jb.111.2.443-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslewood S. R., Smith J. T. Envelope alterations produced by R factors in Proteus mirabilis. J Gen Microbiol. 1974 Nov;85(1):146–152. doi: 10.1099/00221287-85-1-146. [DOI] [PubMed] [Google Scholar]
- Joys T. M. Correlation between susceptibility to bacteriophage PBS1 and motility in Bacillus subtilis. J Bacteriol. 1965 Dec;90(6):1575–1577. doi: 10.1128/jb.90.6.1575-1577.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRIDGE D. FLAGELLAR SYNTHESIS IN SALMONELLA TYPHIMURIUM: THE INCORPORATION OF ISOTOPICALLY-LABELLED AMINO ACIDS INTO FLAGELLIN. J Gen Microbiol. 1963 Oct;33:63–76. doi: 10.1099/00221287-33-1-63. [DOI] [PubMed] [Google Scholar]
- Kurn N., Ammer S., Shapiro L. A pleiotropic mutation affecting expression of polar development events in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3157–3161. doi: 10.1073/pnas.71.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTINEZ R. J. A METHOD FOR THE PURIFICATION OF BACTERIAL FLAGELLA BY ION EXCHANGE CHROMATOGRAPHY. J Gen Microbiol. 1963 Oct;33:115–120. doi: 10.1099/00221287-33-1-115. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- McGroarty E. J., Koffler H., Smith R. W. Regulation of flagellar morphogenesis by temperature: involvement of the bacterial cell surface in the synthesis of flagellin and the flagellum. J Bacteriol. 1973 Jan;113(1):295–303. doi: 10.1128/jb.113.1.295-303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Ishida M., Kitahara K. Chemical composition of the protoplast membrane of Bacillus megaterium. J Biochem. 1966 Apr;59(4):374–381. doi: 10.1093/oxfordjournals.jbchem.a128312. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of a defective alkaline phosphatase subunit by a mutant of Escherichia coli. J Biol Chem. 1967 Apr 10;242(7):1604–1611. [PubMed] [Google Scholar]
- Sekiguchi J., Takada N., Okada H. Genes affecting the productivity of alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1975 Feb;121(2):688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H., Yoneda Y., Yamane K., Maruo B. Regulation of neutral protease productivity in Bacillus subtilis: transformation of high protease productivity. J Bacteriol. 1974 Jul;119(1):82–91. doi: 10.1128/jb.119.1.82-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAITUZIS Z., DOETSCH R. N. FLAGELLA OF SALMONELLA TYPHIMURIUM SPHEROPLASTS. J Bacteriol. 1965 Jun;89:1586–1593. doi: 10.1128/jb.89.6.1586-1593.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIAME J. M., STORCK R., VANDERWINKEL E. Biosynthèse induite d'arabokinase dans les protoplastes de Bacillus subtilis. Biochim Biophys Acta. 1955 Nov;18(3):353–357. doi: 10.1016/0006-3002(55)90097-4. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Genetic control of the rate of alpha-amylase synthesis in Bacillus subtilis. J Bacteriol. 1974 Aug;119(2):410–415. doi: 10.1128/jb.119.2.410-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Maruo B. Mutation of Bacillus subtilis causing hyperproduction of alpha-amylase and protease, and its synergistic effect. J Bacteriol. 1975 Oct;124(1):48–54. doi: 10.1128/jb.124.1.48-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. Temperature-sensitive mutants of Bacillus subtilis. I. Multiforked replication and sequential transfer of DNA by a temperature-sensitive mutant. Proc Natl Acad Sci U S A. 1970 Jan;65(1):206–213. doi: 10.1073/pnas.65.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki S. On the gene controlling the rate of amylase production in Bacillus subtilis. Biochem Biophys Res Commun. 1968 Apr 19;31(2):182–187. doi: 10.1016/0006-291x(68)90727-4. [DOI] [PubMed] [Google Scholar]