Abstract
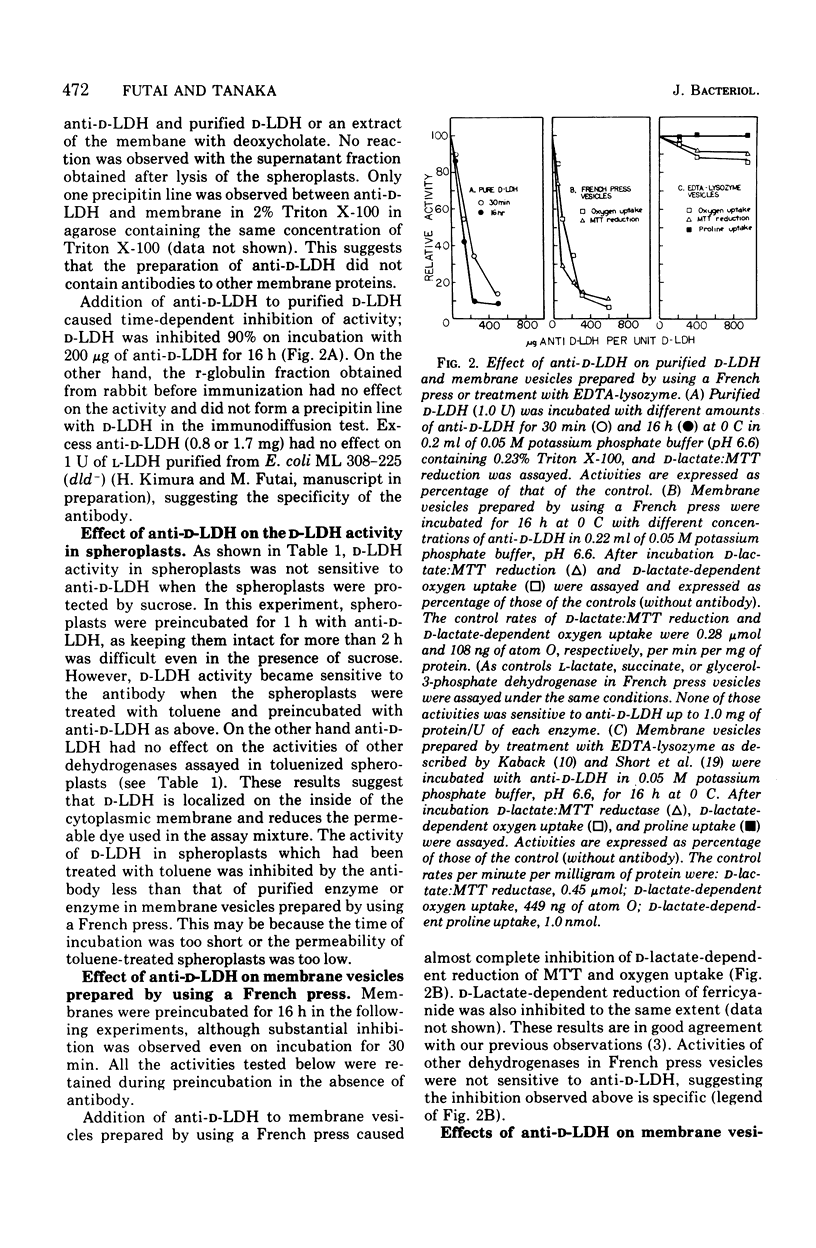
The localization of D-lactate dehydrogenase in membrane vesicles prepared from Escherichia coli was studied using antibody against the purified enzyme. The activity of D-lactate dehydrogenase and D-lactate-dependent oxygen uptake of membrane vesicles prepared by using a French press were completely inhibited by this antibody, suggesting that the enzyme is localized on the outside of these vesicles. This and previous results (Futai, 1974) strongly indicate the inversion of these vesicles. The D-lactate dehydrogenase and D-lactate-dependent oxygen uptake of membrane vesicles prepared by treatment with ethylenediaminetetraacetate-lysozyme were inhibited about 15% by the antibody, whereas proline transport of the vesicles was insensitive to antibody. These results suggest that most of the membrane vesicles have D-lactate dehydrogenase on the inside of the membrane and that such vesicles transport amino acids. This essentially confirms the results of Short, Kaback, and Kohn (1975). However, unlike them we observed that a small but significant portion of activity was sensitive to the antibody as shown above. This portion may represent the completely inverted vesicles in the preparation. Ferricyanide reductase activity cannot be detected in spheroplasts, but about 30 to 50% of the total was detected in membrane vesicles prepared by treatment with ethylenediaminetetraacetate. This confirms our previous findings with membrane prepared by a slightly different procedure. It is concluded that in these vesicles about half the reactive sites for ferricyanide are moved from inside to outside the membrane, whereas 85% of the D-lactate dehydrogenase remains inside the membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Futai M. Reconstitution of transport dependent on D-lactate or glycerol 3-phosphate in membrane vesicles of Escherichia coli deficient in the corresponding dehydrogenases. Biochemistry. 1974 May 21;13(11):2327–2333. doi: 10.1021/bi00708a014. [DOI] [PubMed] [Google Scholar]
- Futai M. Stimulation of transport into Escherichia coli membrane vesicles by internally generated reduced nictotinamide adenine dinucleotide. J Bacteriol. 1974 Nov;120(2):861–865. doi: 10.1128/jb.120.2.861-865.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton M. L., Freese E. Explanation for the apparent inefficiency of reduced nicotinamide adenine dinucleotide in energizing amino acid transport in membrane vesicles. J Bacteriol. 1974 May;118(2):497–504. doi: 10.1128/jb.118.2.497-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCauley R., Racker E. Separation of two monoamine oxidases from bovine bran. Mol Cell Biochem. 1973 May 11;1(1):73–81. doi: 10.1007/BF01659940. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., McClees J. S. Active transport of calcium in inverted membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5042–5046. doi: 10.1073/pnas.71.12.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Hawkins T., Kohn L. D. Immunochemical properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4285–4290. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kaczorowski G., Fisher J., Walsh C. T., Silverstein S. C. Determination of the absolute number of Escherichia coli membrane vesicles that catalyze active transport. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5032–5036. doi: 10.1073/pnas.71.12.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]



