Abstract
The highly-conserved, commonly used MAP kinase signaling cascade plays multiple integral roles in germline development in C. elegans. Using a functional proteomic approach, we found that the transcription factor DPL-1, a component of the LIN-35(Rb)/EFL-1(E2F)/DPL-1(DP) pathway, is a candidate phosphorylation substrate of MAP kinase. Moreover, dpl-1 genetically interacts with mpk-1(MAP kinase) to control chromosome morphology in pachytene of meiosis I, as does lin-35. However, EFL-1, the canonical DPL-1 heterodimeric partner, does not have a role in this process. Interestingly, we find that DPL-1 and EFL-1, but not LIN-35, promote the expression of a negative regulator of MPK-1, the MAP kinase phosphatase LIP-1. Two E2F consensus motifs are present upstream of the lip-1 open reading frame. Therefore the Rb/E2F/DP pathway intersects with MAP kinase signaling at multiple points to regulate different aspects of C. elegans germ cell development. These two highly conserved pathways with major regulatory roles in proliferation and differentiation likely have multiple mechanisms for cross-talk during development across many species.
Keywords: C. elegans, germline, MAP kinase, LIP-1 phosphatase, E2F/DP
INTRODUCTION
The receptor tyrosine kinase (RTK)/mitogen-activated protein kinase (MAPK) signaling cascade is an evolutionarily conserved signaling module used by cells to transduce extracellular signals into intracellular responses. The canonical ERK (extracellular signal-regulated kinase) MAPK cascade is composed of three sequentially acting protein kinases, RAF, MEK and MAP kinase (MAPK). This signaling module has a myriad of effector functions and regulates diverse cellular processes including chromatin remodeling, cytoskeletal reorganization, cell proliferation, cell differentiation and apoptosis (Lewis et al., 1998). One distinguishing characteristic of MAP kinase signaling in metazoans is that, even when the core signaling components are the same, the outcome of the signaling can differ depending on cell type (Tan et al., 1998; Szewczyk et al., 2002; Marenda et al., 2005; Mogila et al., 2006). One reason for this functional diversity is that MAP kinase can have distinct phosphorylation substrates in different cell types, which in turn promote a cell-specific response (e.g. Tan et al., 1998). These substrates have been difficult to identify through traditional genetic screens, likely due to functional redundancy.
During C. elegans development, ERK-like MAP kinase signaling plays an important role in multiple aspects of somatic development, including vulval induction, sex myoblast migration, and apoptosis (Wu and Han 1994; Sundaram et al., 1996; Gumienny et al., 1999) MAP kinase signaling also acts at multiple stages of germline development. Germ cells require MAP kinase signaling to proceed through the pachytene stage into diakinesis in meiotic prophase I (Church et al., 1995). A strong loss-of-function mutation in any of the core signaling components, let-60 (RAS), lin-45 (RAF), mek-2 (MAPKK), and mpk-1 (MAPK) causes germ cells to arrest in pachytene and exhibit an aberrant, condensed DNA morphology. Additionally, a cue originating from sperm activates MAP kinase signaling to promote ovulation of the maturing oocyte in the proximal gonad (Miller et al., 2001).
Activated, di-phosphorylated MAP kinase is detectable in a dynamic pattern consistent with these two functions in wild type hermaphrodites. An antibody specific for the activated form of MAP kinase is first detectable at the mid- to late-pachytene region of the gonad, where pachytene arrest occurs in mpk-1 mutants (Hajnal and Berset 2002). Staining of di-phosphorylated MAP kinase rapidly disappears as germ cells exit the pachytene stage and enter the diplotene/diakinesis phase of meiotic prophase I. This disappearance is mediated by the action of the dual-specificity (serine and threonine) phosphatase LIP-1 (Hajnal and Berset 2002). Activated MAP kinase becomes detectable again in the most proximal oocytes undergoing maturation prior to ovulation (Hajnal and Berset 2002). MAP kinase is once again inactivated in the zygote immediately following fertilization (Miller et al., 2001). Thus MAP kinase signaling is both positively and negatively regulated at distinct locations in the gonad during different stages of germ cell development.
Another pathway that acts in germ cells is the Rb/E2F pathway. In C. elegans, LIN-35(Rb) binds to the heterodimeric transcription factor E2F, which is composed of two subunits EFL-1 and DPL-1 (Ceol and Horvitz 2001). In somatic tissues, these three proteins appear to act coordinately to repress expression of target genes (reviewed in Fay and Yochem 2007). The Rb/E2F pathway clearly has a different role in the germ line, as EFL-1 and DPL-1 mostly promote gene expression, and do so independently of LIN-35(Rb), which appears to act primarily to repress gene expression (Chi and Reinke 2006). DPL-1 and EFL-1 promote oogenesis and early embryogenesis by increasing the expression of a set of genes required for these developmental programs (Chi and Reinke 2006). DPL-1 is widely expressed in germ cells (Ceol and Horvitz 2001), while its binding partner EFL-1 is restricted to the mid-pachytene stage of meiosis I (Page et al., 2001), close to the location of activated, di-phosphorylated MAP kinase (Leacock and Reinke 2006). Thus, the relationship of the Rb/E2F transcriptional regulatory pathway to MAP kinase signaling in the gonad is of interest because both of these major regulatory pathways appear to act in the same general region of the germ line. Comparisons of genes regulated by E2F have little overlap with those regulated by MAP kinase activity, suggesting that the pathways are independent (Leacock and Reinke 2006). However, EFL-1 and DPL-1 are required for downregulation of active MAP kinase in diplotene and early diakinesis (Page et al., 2001), suggesting an antagonistic relationship, although the mechanism underlying this downregulation is unknown.
To obtain a broad view of the functions of MAP kinase in the development of the germ line, we performed a functional proteomic screen to identify the phosphorylation substrates of MAP kinase that mediate meiotic progression of germ cells. We focused on transcription factors because they are typically primary phosphorylation targets of MAP kinase in the nucleus (Treisman 1996). Our biochemical studies indicate that one of the phosphorylation targets of MAP kinase is DPL-1, a subunit of the E2F heterodimer. We demonstrate that the activity of dpl-1, but not efl-1, is required for the aberrant pachytene nuclear morphology seen in mpk-1(MAP kinase) mutants, suggesting that dpl-1 functions independently of efl-1 at this stage of germ cell development. We also find that dpl-1 and efl-1 act together at a slightly later stage of germ cell development to promote the expression of lip-1, which encodes a MAP kinase phosphatase, providing a likely mechanism for the ability of E2F to downregulate MAP kinase activity in the proximal germ line. Thus, the MAP kinase and Rb/E2F pathways intersect at multiple points to regulate different aspects of germ cell development.
MATERIALS AND METHODS
Strains, alleles and genetic analysis
Nematode strain maintenance and genetic manipulation were carried out as described (Brenner 1974). The following mutations were used: LG II: unc-4(e120)/mnC1, dpy-10(e128), unc-52(e444), dpl-1(n3316), dpl-1(n3643), dpl-1(n2994), dpl-1(n3380), lip-1(zh15); LG III: unc-79(e1068), mpk-1(ga111); LG V: efl-1(n3318). The Bristol strain N2 was used as the wild type. Strains were maintained at 20°C unless stated otherwise. To synchronize worms, 8 to 12 well-fed young adult hermaphrodites were picked to a seeded NGM plate (60-mm diameter) and allowed to lay eggs for 2-hours at room temperature. The hermaphrodites were dissected and eggs were transferred to experimental temperature conditions. Unless specified otherwise, all phenotypic characterizations were performed on hermaphrodites 24 hour post L4/adult molt.
Protein expression and MAP kinase assay
The 181 candidate targets are listed in Supplementary Table 1. Gateway cloning was used throughout this project (Invitrogen, Carlsbad, CA, USA). Approximately half of the cDNA clones were provided in the Gateway Entry vector pDONR201 by the lab of Marc Vidal from the ORFeome project (Walhout et al., 2000), and the rest were amplified by RT-PCR from mixed stage hermaphrodite worm RNA using gene specific primers containing Gateway recombination sites, and then cloned into the pENTRY-D vector according to protocols from Invitrogen. Confirmed cDNAs were recombined into a destination vector pLBQ28 (derivative of pDEST10, Invitrogen) that permitted expression in baculovirus-containing Sf9 cells (map of pLBQ28 available upon request). These destination vectors were then recombined into a bacmid according to provided protocol from Invitrogen. Recombinant bacmids were used to transfect cultured Sf9 cells with Cellfectin (Invitrogen). Virus titration, duration of infection, and protein expression levels were monitored and optimized in an attempt to ensure that the majority of target proteins being expressed and harvested. Crude cell lysate was prepared and supernatant was collected according to protocols from Invitrogen.
The in vitro kinase assay was performed in a 25-μl final volume containing 40 mM HEPES (pH7.5), 25 mM magnesium acetate, 100 mM potassium glutamate, 2.5 mM EGTA, 2 mM DTT, 0.01% NP-40 and 10% glycerol. We used 5 μl supernatant (up to 500 ng of protein) from crude lysate preparation as a substrate in the initial kinase assay. ERK2 kinase (25 ng) was used according to supplier’s protocols (NEB, Ispwich, MA). Reactions were carried out at 30°C and stopped after 1 hour by the addition of SDS-PAGE loading buffer.
The preliminary candidates were interpreted according to SDS-PAGE profiles as described in the text. Preliminary positive candidate proteins were then purified from cell lysate via the 6x HIS tag through Ni-NTA resin, and 100 ng purified protein was used in a kinase assay. For validation of the putative targets in the E. coli expression system, we cloned the cDNA into pGEX4T, a vector that fuses 6xHIS and GST to the C-terminus of the target protein (GE Healthcare, Piscataway, NJ). We then performed tandem affinity purification of the fusion protein first via the GST tag interaction with glutathione agarose, followed by the 6x HIS tag. The kinase assay was then performed as described above using approximately 100 ng of purified protein as substrate.
Immunofluorescence, in situ mRNA hybridization and imaging
Extruded gonads were used for mRNA in situ hybridization and immunochemistry. in situ hybridization was performed as described (Jones et al., 1996). DNA was amplified from full-length lip-1 cDNA cloned into the pCR2.1 vector (Invitrogen). PCR products were purified twice from agarose gels and used as templates (0.8 μg) to generate digoxigenin-labeled probes. Sense and antisense probes were prepared using single primer extension and then ethanol precipitated and resuspended in 400 μl of hybridization buffer. Probes were diluted 2x, and hybridized to dissected gonads at 48° C for 24 h. Alkaline-phosphatase-conjugated anti-digoxigenin was used to visualize the mRNA.
For immunostaining, gonads were attached to microscope slides by liquid nitrogen immersion followed by freeze-crack removal of the coverslip, fixed with 3.7% formaldehyde in 0.1M phosphate buffer (pH7.2) for 5 minutes followed by immersion in (−20 °C) methanol for 1 minute. Antisera α-ERK2 (1:100, Sigma) and α-LIP-1 (1:100, gift from J. Kimble), incubation and washes were performed as described (Kelly et al., 2002). Measurement of the distance from the distal tip to the first evidence of expression of lip-1 mRNA or LIP-1 protein was performed using Axiovision (Zeiss) software measurement tools. The first evidence of LIP-1 protein expression was defined as the occurrence of cell membrane staining (hexagonal shapes) punctuated with small foci. The diffuse background staining seen in the distal tip of wild type animals persists in the lip-1 mutant background and is non-specific.
RNAi
RNAi was performed essentially as described (Tabara et al., 1999). efl-1 cDNA was cloned into pL4440 vector and T7 promoter sequence was used as primer for PCR. PCR products were purified from agarose gels and used as templates for in vitro transcription using T7 RNA polymerase. The dsRNAs were precipitated by ethanol and pellet was resuspended to 2μg/μl. The multiple cloning site of the pL4440 vector was similarly amplified and transcribed and used as control dsRNA. Injected young adult hermaphrodites were recovered, and eggs laid for the first 16 hours after recovery were discarded. Then F1 eggs were collected at 12 hour intervals and cultured at the experimental temperature. F1 progeny were used at young adult stage for in situ hybridization and immunochemistry.
RT-PCR
Fifty gonads were dissected in 1x egg salts and 0.1% Tween 20. To prevent RNA degradation, 1 μL of RNase inhibitor (GE) was added to the dissection buffer before dissection. Total RNA was isolated in TRIzol (Invitrogen) and precipitated with isopropranol. Each 25 μL RT reaction contained 0.2 μg of total RNA, anchored polyT oligonucleotide, dNTPs and the reverse transcriptase OmniScriptase(Qiagen). PCR was conducted on 1 μL of RT reaction as template in a 50-μL PCR reaction. Primers used to amplify 405 bp lip-1sequence are: forward, CCAACTCCGATTGGAAGGTGAA; reverse, GAACCCATTCGTAGGCAGAATCA. Hexokinase was used as internal control.
RESULTS
The C-terminus of DPL-1 is phosphorylated by MAP kinase in vitro
The C. elegans MAP kinase MPK-1 becomes highly active in mid-pachytene, where it enables the germ cell transition from the pachytene stage to diakinesis (Church et al., 1995; Supplementary Figure 1). To identify candidate phosphorylation targets of MAP kinase that might play a role in this process, we employed a functional proteomic screen. We selected 181 putative candidate factors from the C. elegans genome (Supplementary Table 1), using three criteria. First, the expression levels of these genes are enriched in the germ line based on prior expression profiling experiments (Reinke et al., 2001).
Second, proteins encoded by these genes likely bind DNA and/or regulate gene expression, based on sequence identity. Third, the encoded proteins have one or more MAP kinase consensus motifs, [P]X[S/T]P (Clark-Lewis et al., 1991). We chose LIN-1 and LIN-31 as positive controls (Tan et al., 1998) and 16 factors that meet the first two criteria but not the third as negative controls. To effectively express these 199 proteins, we cloned the corresponding open reading frames into a Gateway vector designed to express His-tagged factors in a baculovirus system (Walhout et al., 2000; see Materials and Methods). We succeeded in cloning 179 of the 199 factors into bacteria (90%), of which we achieved detectable expression for 137 (76%) in the baculovirus expression system, including 121 candidates, 15 negative controls, and the positive control LIN-1 (Supplementary Table 1).
To rapidly identify candidate MAP kinase phosphorylation substrates, we independently transfected insect Sf9 cells with each candidate, and subjected crude cell lysates to an in vitro kinase assay using the murine MAP kinase ERK2. ERK2 can functionally rescue the mpk-1 mutant phenotype in transformation experiments (Wu and Han 1994), and has been used previously to define MPK-1 phosphorylation substrates in C. elegans (Tan et al., 1998). After subjecting the phosphorylated lysates to SDS-PAGE, we then examined each lane for the presence of a band close to the expected size of the candidate protein that was not present in untransfected Sf9 cell lysate. Using this assay, we narrowed down our candidate proteins from 121 to 25 (Supplementary Table 1). The corresponding crude lysates were then subjected to affinity purification via a 6xHIS tag and re-tested in the in vitro kinase assay. Thirteen proteins were still phosphorylated by ERK2 after purification. Finally, we expressed 12 of the 13 proteins in an E. coli expression system and successfully purified nine through N-terminal 6xHIS and C-terminal GST tags. We repeated the in vitro kinase assay using these bacterially-expressed and purified proteins to validate our previous baculovirus expression results. Six of the nine successfully retested as in vitro substrates of MAP kinase. Throughout these assays, the negative control(s) never exhibited ERK2-dependent phosphorylation, whereas LIN-1 was always positive.
One of these six targets, DPL-1, the C. elegans ortholog of the mammalian E2F heterodimerization partner DP, was of particular interest (Ceol and Horvitz 2001). We expressed and purified the C-terminal amino acids (294 to 598) of DPL-1, which contains four putative MAP kinase phosphorylation sites, from E. coli via the HIS and GST tags (Figure 1, A, B). This recombinant protein can be phosphorylated in vitro by activated murine ERK2, but not a kinase-dead version (Figure 1C, lanes 2 and 3). Moreover, the phosphorylation can be removed by PP1, a phosphoserine/phosphothreonine-specific phosphatase (Figure 1C, lane 4), suggesting that phosphorylation likely happens at one or more serine or threonine residues in DPL-1.
Figure 1. DPL-1 is phosphorylated by ERK2 in vitro.
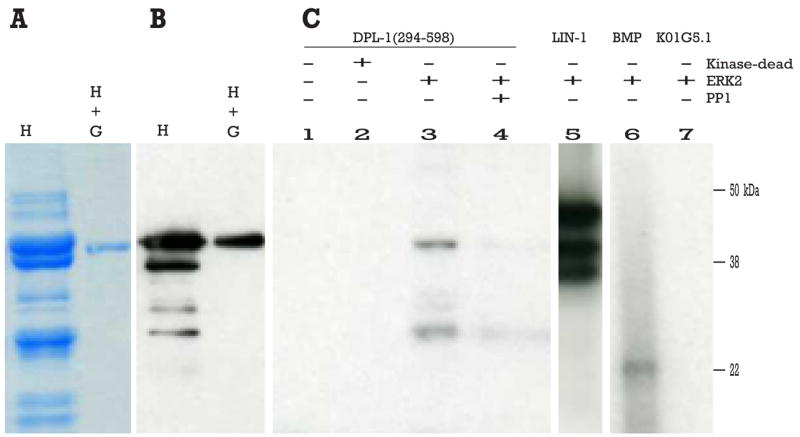
(A) SDS-PAGE of DPL-1 expressed in E. coli, first affinity purified by 6x-HIS tag (H), and then successively purified using a GST tag (H + G). (B) Corresponding western blot with an anti-6xHIS antiserum. (C) SDS-PAGE of in vitro kinase assay. DPL-1 does not auto-phosphorylate (lane 1), is not phosphorylated by inactivated ERK2 (lane 2), but is phosphorylated by activated ERK2 (lane 3). The serine/threonine phosphatase PP1 removes the phosphate(s) from DPL-1 (lane 4). The lower band in these lanes is likely to be a degradation product of DPL-1, as it does not appear in lanes lacking DPL-1, is dependent on ERK2 activity, and can be reversed by PP1 phosphatase. Positive controls include LIN-1(lane 5) and myelin basic protein (MBP) (lane 6); K01G5.1 (a protein lacking MAP kinase phosphorylation consensus site and likely expressed in the germ line) is used as negative control (lane 7).
The pachytene nuclear morphology phenotype of mpk-1 mutants requires dpl-1
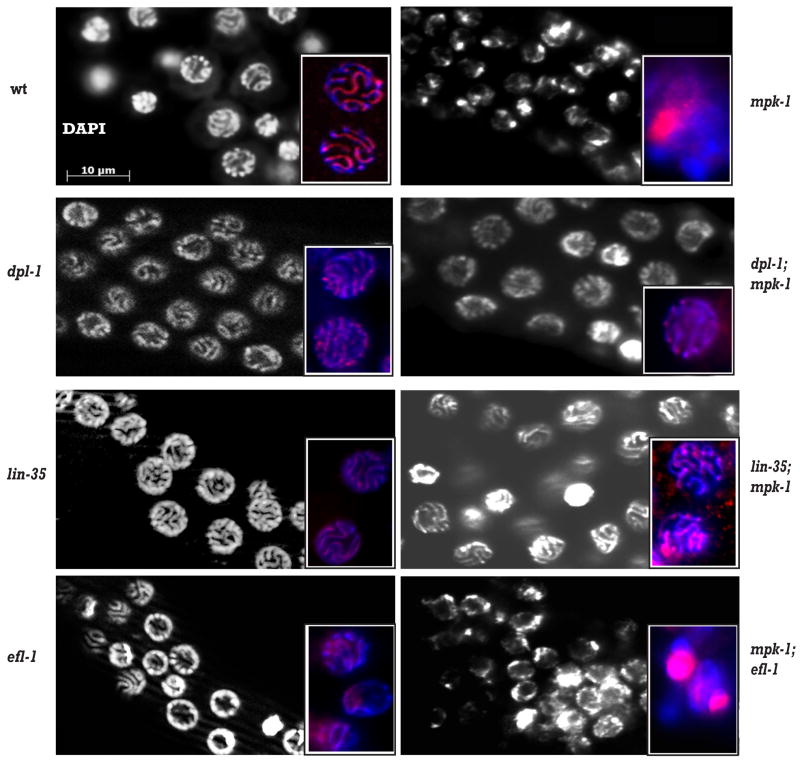
To determine the potential biological relevance of the in vitro phosphorylation of DPL-1 by MAP kinase in germ cells, we investigated whether the individual phenotypes of mpk-1 or dpl-1 mutants were affected by altering the activity of both genes simultaneously. The mpk-1(ga111) mutant is temperature-sensitive. mpk-1(ga111) worms raised at the restrictive temperature of 25°C are completely sterile due to a failure to exit from the pachytene stage of meiosis I (Lackner and Kim 1998; Leacock and Reinke 2006). dpl-1(n3316) mutant animals do not have obvious defects in progression through pachytene, but are sterile because oocyte maturation, ovulation and fertilization are defective (Ceol and Horvitz 2001; Chi and Reinke 2006). When we examined dpl-1(n3316); mpk-1(ga111) double mutant animals, we found that they exhibited a pachytene arrest similar to that of mpk-1(ga111) single mutants, and were still sterile. However, we noticed a striking change in the appearance of chromosomes in the pachytene stage. In wild type, pachytene germ nuclei are well-organized in rows along the long axis of the gonad, and aligned chromosome homologs are clearly visible as long tracks at the surface of the nuclei when stained with DAPI (Figure 2). The pachytene chromosome morphology of dpl-1(n3316) mutants appeared similar to wild type, while >90% of mpk-1(ga111) mutants raised at 25°C had abnormal pachytene morphology, with clumped chromosomes and aggregated, possibly degenerating, nuclei that stained intensely with DAPI. In dpl-1(n3316); mpk-1(ga111) double mutants, chromosome morphology was greatly improved relative to mpk-1(ga111) mutants alone, and resembled that seen in dpl-1(n3316) single mutant animals (Figure 2).
Figure 2. Loss of dpl-1 or lin-35, but not efl-1, improves the chromosome morphology defect of mpk-1 mutants.
Wild type, single mutant and double mutant hermaphrodites were raised at 25°C and gonads dissected and stained with DAPI and anti-HIM-3. Large panels show DAPI staining of the chromosomes; insets show both DAPI (blue) and HIM-3 (red) staining.
To further investigate the chromosome morphology of these mutants, we examined the expression and localization of the synaptonemal complex (SC) protein HIM-3 (Zetka et al., 1999; insets in Figure 2). In wild type, HIM-3 colocalized along the length of paired chromosomes in pachytene nuclei. In dpl-1(n3316) mutant gonads, HIM-3 is still aligned with the chromosomes in most pachytene nuclei, although its distribution along the chromosomes was not as even as in wild type. By contrast, HIM-3 is no longer associated with the aggregated chromosomes of mpk-1(ga111) gonads. Strikingly, concomitant loss of dpl-1 activity greatly ameliorates this phenotype: HIM-3 staining of dpl-1(n3316); mpk-1(ga111) double mutant animals is very similar to dpl-1(n3316) alone, consistent with the improved DNA morphology, and suggesting that the chromosomes of the double mutant are now undergoing synapsis. Thus, while the pachytene exit phenotype of mpk-1(ga111) mutants is not affected by loss of dpl-1 activity, the aberrant chromosome morphology phenotype is, suggesting that dpl-1 is only required for a specific aspect of the mpk-1 mutant phenotype.
lin-35, but not efl-1, also regulates meiotic chromosome morphology
dpl-1 has been shown to act in the same genetic pathway as both efl-1 and lin-35. Additionally, the DPL-1 protein can physically interact with both EFL-1 and LIN-35 proteins (Ceol and Horvitz 2001). Therefore, we asked whether either efl-1 and/or lin-35 are also required for the chromosome morphology defects seen in mpk-1(ga111) mutants. We first investigated whether LIN-35(Rb) has any role in the chromosome morphology defects seen in mpk-1(ga111) mutants. lin-35(n2242) is likely to be a null allele (Lu and Horvitz 1998) and lin-35(n2242) animals raised at 25°C display incompletely penetrant sterility. Dissected gonads from lin-35(n2242) animals stained with HIM-3 and DAPI showed that the pachytene nuclear morphology of the mutant appears similar to wild type (Figure 2). In the lin-35(n2242); mpk-1(ga111) double mutant, the chromosomes displayed greatly improved morphology relative to the mpk-1(ga111) single mutant, and appeared similar to the lin-35(n2242) mutant. Notably, lin-35(n2242); mpk-1(ga111) animals exhibit the same pachytene exit phenotype seen in mpk-1(ga111) single mutants. This result suggests that LIN-35 may also act downstream or independent of the MAP kinase pathway in regulating pachytene chromosome architecture, potentially through an interaction with DPL-1.
We next used RNAi to reduce efl-1 transcript abundance in wild type and in mpk-1(ga111) animals. efl-1(RNAi) mimics the efl-1(n3318) mutant, displaying a similar phenotype of sterility and embryonic lethality at >70% penetrance (Ceol and Horvitz 2001). Injection of control dsRNA showed no effects, indicating that efl-1(RNAi) is effective and specific. In addition, antibody staining for activated MAP kinase demonstrated elevated di-phosphorylated MAP kinase activity in the proximal gonads from progeny of efl-1(RNAi) hermaphrodites but not in control RNAi animals (Page et al., 2001; data not shown).
We examined the morphology of pachytene germ nuclei in young adults via HIM-3 and DAPI staining in F1 progeny of wild type and mpk-1(ga111) animals injected with efl-1 or control dsRNA. Unlike dpl-1(n3316);mpk-1(ga111) double mutants, the pachytene chromosome morphology of mpk-1(ga111);efl-1(RNAi) hermaphrodites is very similar to that of control mpk-1(ga111) animals (Figure 2). This result indicates that EFL-1 activity is not required for the chromosome morphology phenotype seen in the mpk-1(ga111) mutant, suggesting that DPL-1 functions independently of EFL-1 in this process.
To ensure that the failure of efl-1(RNAi) to rescue the mpk-1(ga111) chromosome morphology defect was not due to residual efl-1 activity, we examined the ability of the efl-1(n3318) mutant to rescue the mpk-1(ga111)-mediated chromosome morphology defect. The mpk-1(ga111); efl-1(n3318) double mutant cannot be distinguished at early adulthood from siblings that are mpk-1(ga111); efl-1(n3318)/+, so we examined the offspring from mpk-1(ga111); efl-1(n3318)/+ hermaphrodites as a population. If rescue of the mpk-1 chromosome defect occurs upon loss of efl-1, then 25% of the offspring should exhibit improved chromosome morphology, corresponding to the proportion of the population that is mpk-1(ga111); efl-1(n3318). However, we found that only 5% (4/77) of the offspring of mpk-1(ga111); efl-1(n3318)/+ hermaphrodites exhibited greatly improved, pachytene chromosome morphology. This finding is comparable to what is seen in mpk-1(ga111) single mutants, which had a background level of “normal” chromosome morphology of 6% (4/63) in this experiment. These findings are consistent with what we observed using efl-1(RNAi), and further support the likelihood that loss of efl-1 fails to rescue the mpk-1 chromosome morphology defect.
Full expression of lip-1 mRNA in the gonad requires dpl-1 activity
Our cytological experiments suggest that DPL-1 and LIN-35, but not EFL-1, work downstream of or in parallel to MAP kinase signaling to regulate chromosome morphology at the pachytene stage of germ cell development. However, previous work has demonstrated a role for both dpl-1 and efl-1 upstream of MAP kinase, in which dpl-1 and efl-1 negatively regulate MAP kinase activity during oogenesis in the proximal gonad (Page et al., 2001). To further clarify the relationship between DPL-1 and MPK-1, we investigated the mechanism for how DPL-1 and EFL-1 negatively regulate MPK-1 in the proximal gonad. We hypothesized that, as transcriptional regulators, EFL-1 and DPL-1 might promote the expression of a negative regulator of MAP kinase. One of the best described negative regulators of MAP kinase in C. elegans is the MAP kinase phosphatase, LIP-1 (Berset et al., 2001). LIP-1 has been shown to attenuate MAP kinase signaling in the proximal germ line, and reduction of mpk-1 activity can rescue the sterility of a lip-1 null mutant, lip-1(zh15) (Hajnal and Berset 2002, and Supplementary Figure 2). In the distal portion of the gonad, lip-1 expression is promoted by Notch signaling, but kept at low levels by FBF (Lee et al., 2006). However, the mechanisms controlling lip-1 expression in the pachytene region or proximal gonad, where it is known to inhibit MAP kinase, are unknown.
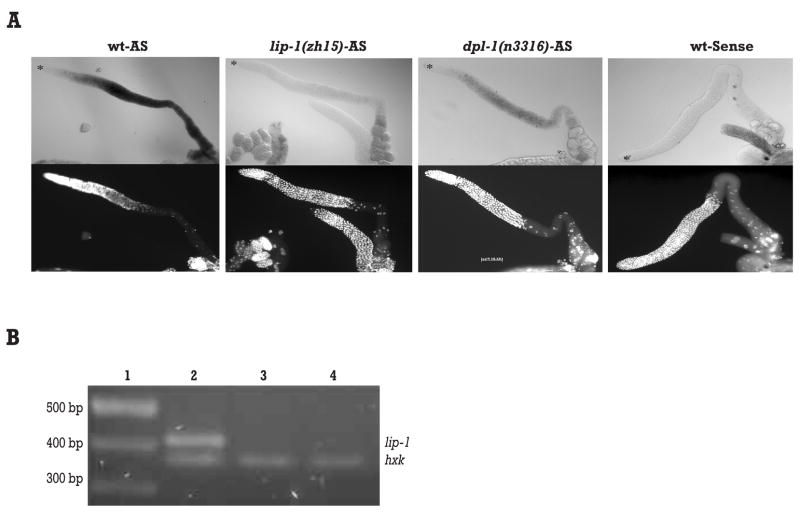
To test whether lip-1 is regulated by dpl-1, we employed in situ hybridization to examine the overall levels of lip-1 mRNA in dissected gonads of wild type and dpl-1(n3316) mutant animals. In the wild type germ line, lip-1 mRNA became detectable at the transition zone, increased sharply at mid-pachytene, and persisted into maturing oocytes before reducing slightly in ovulating oocytes (Figure 3A; Lee et al., 2006). This pattern is similar to that of genes known to be targets of EFL-1 and DPL-1 (Chi and Reinke 2006). In the dpl-1(n3316) null mutant, lip-1 expression levels were significantly reduced, although the spatial expression pattern of the residual lip-1 transcript is unchanged (Figure 3A). The residual lip-1 staining in the dpl-1(n3316) mutant was not background, because all detectable signal was abolished in lip-1(zh15) mutants or when a sense probe was used (Figure 3A). We also found that lip-1 expression in a series of dpl-1 mutants decreased with increasing severity of the dpl-1 mutation (Supplementary Figure 3). Thus we concluded that DPL-1 is required for normal lip-1 mRNA levels in the germ line. This result suggests that lip-1 mRNA levels might not be exclusively regulated by DPL-1, because lip-1 expression is not totally abolished in the dpl-1 null mutant. This residual expression could be due to transcriptional activation by the GLP-1/Notch pathway in the distal germ line (Lee et al., 2006). Alternatively, maternally deposited DPL-1 protein from the heterozygous mother might contribute to lip-1 mRNA levels in the dpl-1(n3316) background.
Figure 3. DPL-1 is required for normal lip-1 mRNA levels.
(A) In situ hybridization of lip-1 in dissected gonads. A probe antisense to the lip-1 mRNA was used to stain wild type, lip-1(zh15) and dpl-1(n3316) dissected gonads. A sense probe was also used to stain wild type to detect background hybridization. All genotypes were stained in parallel with identical hybridization, color development, and image exposure times. DAPI staining is included below corresponding in situ images to distinguish gonad morphology. Asterisk indicates the distal end of gonad. (B) Semi-quantitative RT-PCR of lip-1 mRNA levels using total RNA prepared from 50 gonads for each genotype. Molecular marker (lane 1), wild type (lane 2), dpl-1(n3316) (lane 3), lip-1(zh15) (lane4). Hexokinase (hxk) was used as internal control.
Finally, to confirm the in situ findings, we used semi-quantitative RT-PCR to compare lip-1 mRNA levels in dissected gonads from wild type and dpl-1(n3316) mutants (Figure 3B). Consistent with our in situ data, the relative amount of lip-1 mRNA present in dpl-1(n3316) gonads was about 25% of that of wild type, while lip-1(zh15) gonads displayed even lower lip-1 mRNA levels, about 10% of that in wild type. Together, these results strongly suggest that DPL-1 regulates lip-1 transcripts levels in the germ line.
LIP-1 protein is absent in the germ line of dpl-1 mutants
To assess whether LIP-1 protein levels were also lower in dpl-1 mutants, we stained dissected gonads with an affinity-purified LIP-1 antiserum (Lee et al., 2006). In wild type gonads, LIP-1 staining was detected as membrane-associated puncta from the transition zone throughout the entire pachytene region, becoming weaker in diakinesis (Figure 4 and Hajnal and Berset 2002). In dpl-1(n3316) mutant gonads, LIP-1 levels were reduced dramatically, to levels indistinguishable from that seen in lip-1(zh15) (Figure 4). The reduction of LIP-1 protein levels in the dpl-1 null mutant further supports the hypothesis that DPL1 is required for full LIP-1 activity. LIP-1 protein is also not detectable in the gonads of three non-null dpl-1 mutants, even though the lip-1 mRNA levels are not reduced as severely as in the null mutant (Supplementary Figure 4), suggesting that translation efficiency of LIP-1 might be decreased if the mRNA level falls below a certain threshold. Alternatively, the marked reduction of LIP-1 protein even in the presence of intermediate lip-1 mRNA levels could reflect the difference in the sensitivity of detection between the two experimental approaches.
Figure 4. LIP-1 protein is reduced in dpl-1 mutant gonads.
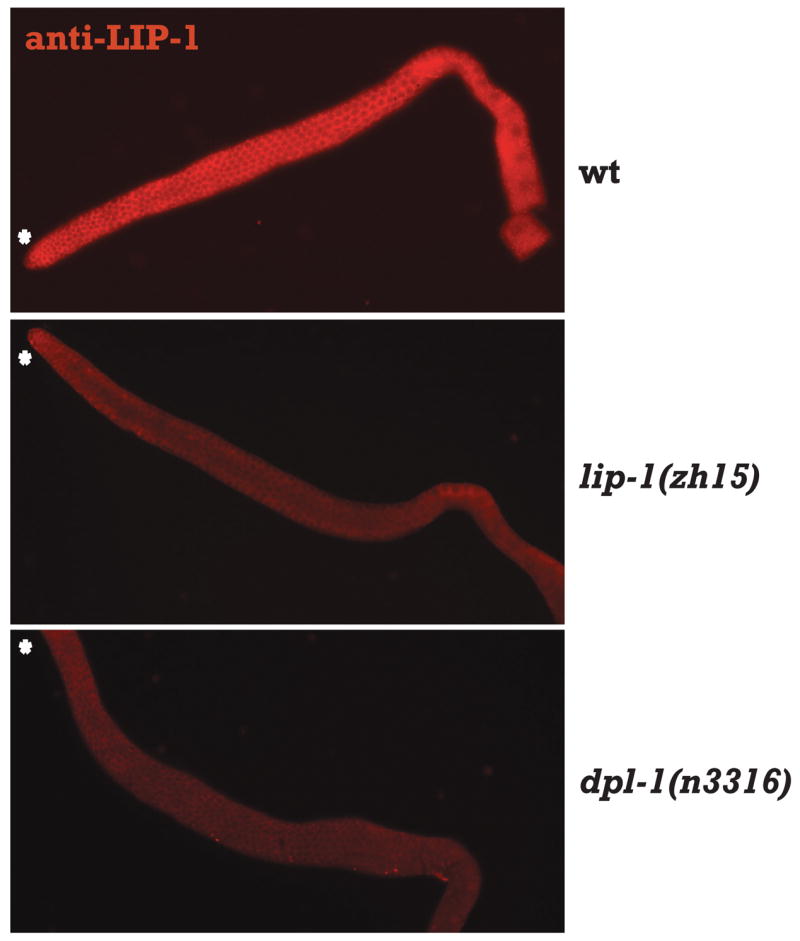
Representative fluorescent micrographs of wild type, lip-1(zh15), and dpl-1(n3316) hermaphrodite gonads stained with anti-LIP-1 antibody. Asterisk indicates the distal end of gonad. Asterisk indicates the distal end of gonad.
EFL-1 and LIN-35 have opposite effects on lip-1 mRNA levels
Next, we asked if EFL-1 is also required for normal lip-1 mRNA levels by performing in situ hybridization of gonads from efl-1(RNAi) animals. We found that severely affected efl-1(RNAi) animals have morphologically smaller gonad with fewer germ cells, but still contained germ cells of all stages, including oocytes and sperm. In these severely affected gonads, the in situ hybridization exhibited little to no detectable lip-1 staining (Figure 5A). More mildly affected gonads exhibited a milder reduction in lip-1 mRNA levels (Supplementary Figure 5). This result suggests that the EFL-1 activity is required for lip-1 mRNA levels. Because we used RNAi to reduce efl-1 activity, we most likely decreased both maternal and zygotic efl-1 function, causing a stronger effect on lip-1 transcript levels than observed in the dpl-1 mutant background, which retains maternally-provided DPL-1 activity.
Figure 5. EFL-1 and LIN-35 have opposite effects on lip-1 mRNA levels.
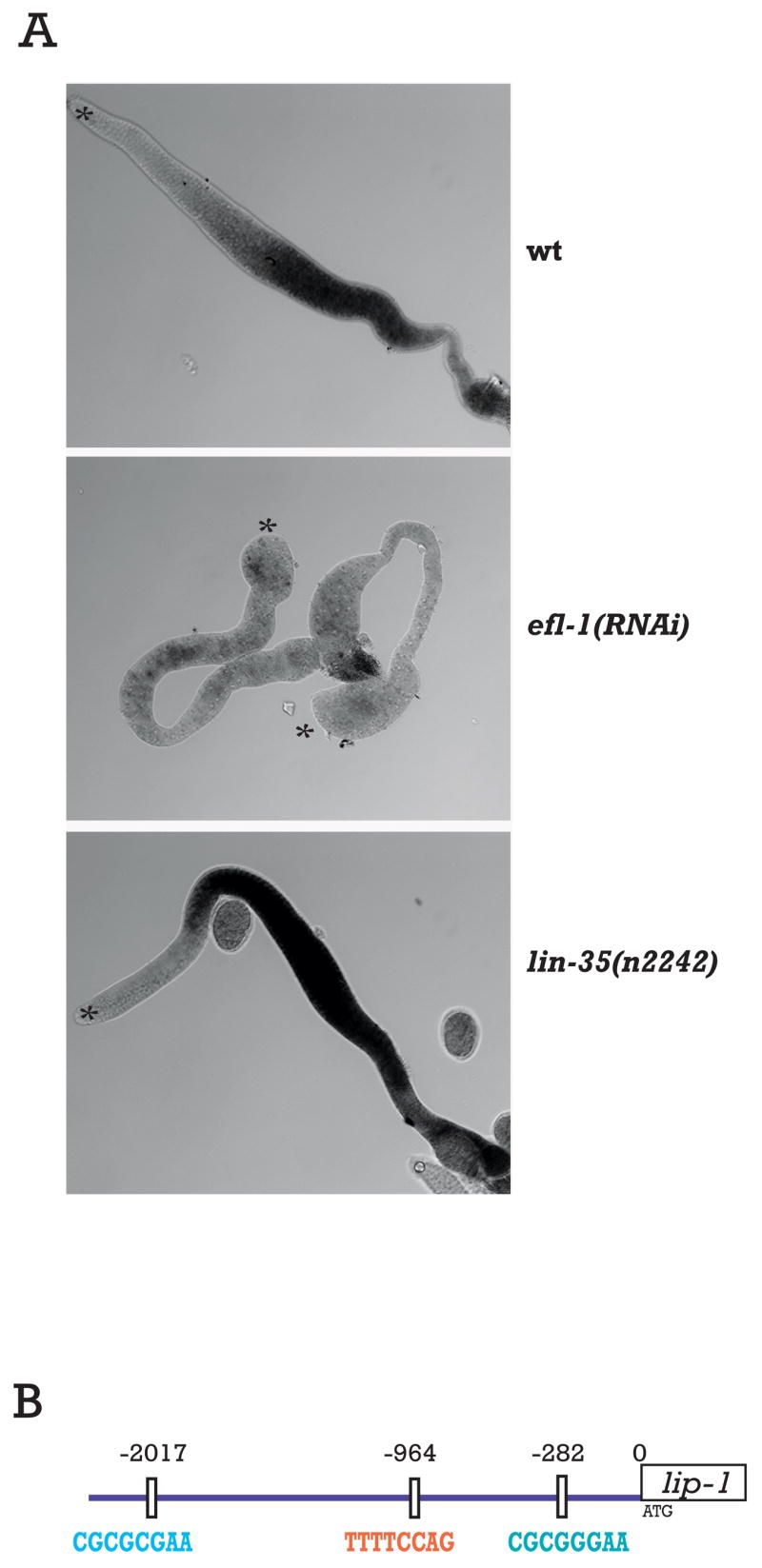
lip-1 antisense RNA probes were used for in situ hybridization of dissected gonads. Two gonads are shown in the efl-1(RNAi) panel. Asterisk indicates the distal end of gonad. All genotypes were stained in parallel with identical hybridization, color development, and image exposure times. (B) Two EFL-1/DPL-1 consensus motifs are present upstream of the lip-1 translational start site, at −282 bp and −2017 bp, and one candidate motif previously found upstream of LIN-35 downregulated genes is located at −964 bp.
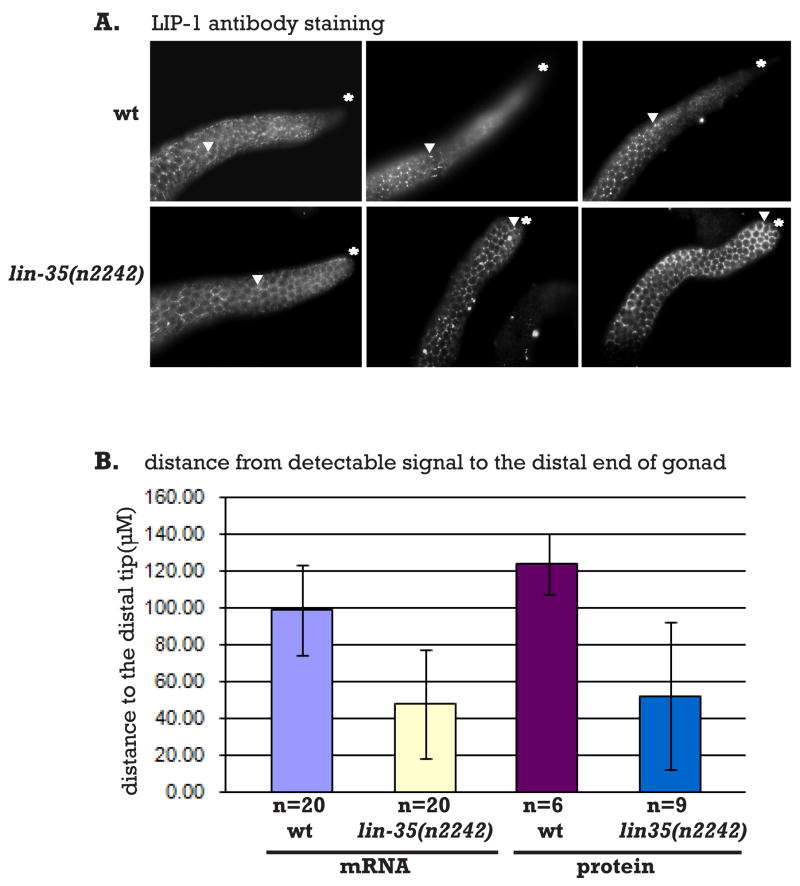
Finally, we tested whether LIN-35(Rb) has a role in regulating lip-1 mRNA levels by performing lip-1 in situ hybridization on gonads from lin-35(n2242) animals. We found that in the lin-35(n2242) mutant, lip-1 mRNA levels appeared higher than wild type (Figure 5A). Moreover, about one-third of the dissected gonads examined showed detectable lip-1 mRNA more distally than seen in wild type. This result suggests that LIN-35 functions to downregulate lip-1 expression in the whole gonad. We therefore extended our analysis to examine LIP-1 protein expression in lin-35 mutant gonads (Figure 6A). We found that lin-35 mutant gonads displayed a more distal expression of LIP-1 protein, with the characteristic hexagonal lattice staining of LIP-1 becoming apparent in the very distal tip of some gonads. We quantified this effect for both the in situ and antibody staining experiments (Figure 6B). This antagonistic function between LIN-35 and EFL-1/DPL-1 is consistent with the demonstrated role for Rb in repressing the expression of genes regulated by E2F in other systems (Nevins 1998).
Figure 6. Loss of lin-35 activity results in ectopic LIP-1 expression.
A. Gonads dissected from wild type and lin-35(n2242) animals were stained with anti-LIP-1. Three examples of each genotype are shown. The asterisk indicates the distal end of the gonad; arrowhead indicates the border at which the distal-most LIP-1 puncta are detected. B. Measurements of the distance from the distal gonad to the border of lip-1 expression (mRNA) or LIP-1 localization as punctae (protein). Error bars indicate standard deviation.
The upstream sequence of lip-1 contains both E2F and LIN-35(Rb) candidate regulatory motifs
E2F binds to a specific DNA consensus motif commonly found in the regulatory regions of responsive genes (Lees et al., 1993). A similar motif has been identified in C. elegans genes found to be regulated by EFL-1 and DPL-1 in expression profiling experiments (Chi and Reinke 2006, Kirienko and Fay 2007). We examined the candidate regulatory region upstream of lip-1 coding sequences to determine if there are potential EFL-1 binding sites. We identified two such motifs upstream of lip-1. The first motif is located at −282 bp upstream of the start codon, consistent with the previous observation that many EFL-1-responsive genes contain this site within 500 bp of the translation start site (Chi and Reinke 2006). The second motif is located about 2kb upstream of the start codon (Figure 5B).
Interestingly, we also identified a motif in the lip-1 upstream sequences that is commonly found in genes regulated by LIN-35 in the gonad (Chi and Reinke 2006). This DNA motif is located at -964 bp from the start codon of the lip-1 gene, midway between the two candidate E2F binding motifs (Figure 5B). Although we cannot assign biological significance to the motifs in the lip-1 promoter in the absence of experimental evidence, their presence suggests that the regulation of lip-1 mRNA levels by EFL-1/DPL-1 and/or a complex containing LIN-35 could be direct.
DISCUSSION
In this report we provide data supporting two new functions for the DPL-1(DP) transcriptional regulator in C. elegans germline development. First, we place dpl-1 in a genetic pathway with mpk-1 to regulate chromosome morphology in pachytene of meiosis I. Our biochemical data demonstrates that DPL-1 can serve as a MAP kinase phosphorylation substrate, suggesting that the genetic relationship between mpk-1 and dpl-1 could be direct. Moreover, our genetic results indicate that LIN-35(Rb) also regulates pachytene chromosome morphology in the same pathway as DPL-1, whereas EFL-1(E2F) does not. Second, we find that expression of the MAP kinase phosphatase LIP-1 requires DPL-1 and EFL-1, but not LIN-35, activity. Indeed, LIN-35 could be acting in opposition to DPL-1/EFL-1 to repress lip-1 expression. Together the data presented here shed light on the relationship between two major regulatory pathways that control many aspects of development in many species.
The role of mpk-1 and dpl-1 in regulating meiotic chromosome morphology
In mid-pachytene, chromosomes undergo a series of dynamic events critical for the completion of meiosis, including synapsis and recombination. In mpk-1 mutants, progression beyond pachytene is halted and chromosomes become hypercondensed and no longer display normal hallmarks of synapsis. Previously it has not been clear whether the chromosome morphology phenotype is a downstream consequence of the pachytene arrest, or whether it is a direct result of the loss of MPK-1 activity. Our results indicate that these two phenotypes are separable because the absence of dpl-1 activity in the mpk-1 background improves the aberrant chromosome morphology phenotype, but does not affect the pachytene arrest phenotype. Therefore, mpk-1 might regulate chromosome morphology independently of progression through pachytene through dpl-1 and lin-35.
Chromosome morphology is fairly normal in a dpl-1(n3316) mutant, indicating that DPL-1 activity is dispensable for normal chromosome architecture of germ cell at the mid-pachytene stage. However, functional DPL-1 protein in the mid-pachytene region of gonad is required for the mpk-1(ga111) condensed chromosome phenotype. One interpretation for this result is that DPL-1 plays a non-essential role in promoting some form of chromosome condensation during pachytene (Figure 7). MPK-1 acts at a specific time to block this activity, possibly through phosphorylation of DPL-1, to control the amount of condensation and permit normal pachytene chromosome morphology and function. Thus, in the absence of activated MPK-1, continued DPL-1 activity would result in the compact, aberrant morphology seen in mpk-1 mutants.
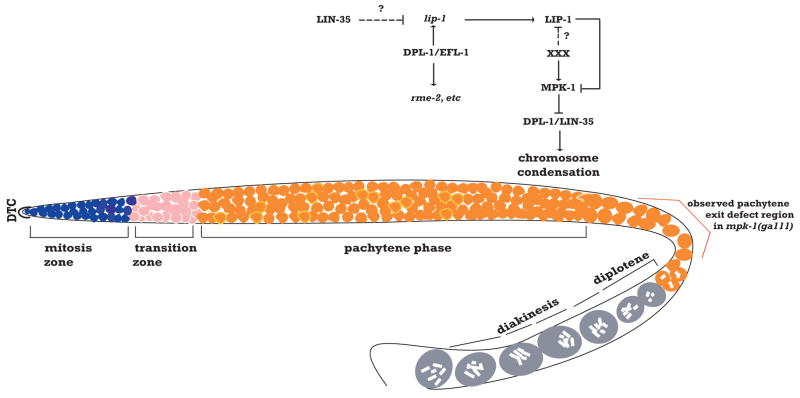
Figure 7. Model for mutual regulatory relationship between the Rb/E2F and MAP kinase pathways in the C.elegans germ line.
In this model, depending on the place and time in germ cell development, MAP kinase either controls DPL-1 function or vice versa. DPL-1 can exist in a regulatory complex with EFL-1 to promote lip-1 expression in mid-pachytene. XXX represents an unknown external signal that results in spatially-restricted activation of MAP kinase, possibly through blocking LIP-1 function. Once cells move beyond reach of the signal, LIP-1 inactivates MPK-1 in the proximal gonad. DPL-1 might also exist in a separate complex with LIN-35 to promote chromosome condensation. This complex is inhibited by MAP kinase in late pachytene through phosphorylation of DPL-1 to promote normal chromosome morphology.
The fact that loss of lin-35 activity also has the ability to improve the pachytene chromosome morphology of mpk-1 mutants, combined with the fact that DPL-1 and LIN-35 have been shown to physically interact, suggests that DPL-1 and LIN-35 might be in a complex together that regulates chromosome morphology. Conversely, loss of efl-1 has little effect on chromosome morphology in mpk-1 mutants, suggesting that it is not involved in this process with dpl-1 and lin-35. Our findings are consistent with the fact that DPL-1 expression is found in all germ nuclei in the oogenic germ line, whereas EFL-1 expression is restricted to a brief window in pachytene (Ceol and Horvitz 2001; Page et al., 2001). Supporting this possibility, recent data shows that dpl-1 and lin-35 work together to control DNA damage-induced germ cell apoptosis, whereas efl-1 is dispensable (Schertel and Conradt 2007). Moreover, dpl-1 and lin-35, but not efl-1, are required to prevent mis-expression of germ cell determinants in the somatic tissue of C. elegans (Wang et al., 2005). Therefore, DPL-1 is likely not acting as part of a sequence-specific transcriptional regulator to modulate chromosome morphology, but instead has a novel function whose mechanism is currently poorly understood, but might involve LIN-35.
The MAP kinase phosphatase LIP-1 is regulated by E2F
C. elegans LIP-1 belongs to a family of dual-specificity phosphatases (DUSP) that are known to inactivate MAP kinase signaling through the dephosphorylation of key serine and threonine residues in the MAP kinase protein (Berset et al., 2001). LIP-1 has been previously shown to inhibit MAP kinase signaling in the developing vulva (Berset et al., 2001), and in the distal germ line to modulate the extent of proliferation, despite being at low levels in that region relative to the proximal germ line (Lee et al., 2006). The high LIP-1 levels in the medial and proximal regions of the germ line correspond to the ability of LIP-1 to negatively regulate MAP kinase in germ cells in diplotene/early diakinesis and permit a brief G2/M meiotic arrest in oocytes (Hajnal and Berset 2002). We have found that lip-1 expression is regulated in the medial germ line at the mRNA level by the action of EFL-1 and DPL-1. By in situ hybridization, the downregulation of lip-1 message was more pronounced in efl-1(RNAi) gonads than in dpl-1(n3316) mutant gonads, likely because RNAi was able to remove both maternal and zygotic activity of efl-1, whereas dpl-1(n3316) homozygous mutants are always born from heterozygous mothers and carry the maternal contribution of dpl-1 activity. In our previous expression profiling experiments to identify regulatory targets of DPL-1 and EFL-1 in the C. elegans gonad (Chi and Reinke 2006), we did not detect significant changes in lip-1 mRNA levels, probably because of the lingering effects of the maternal contribution of gene activity in the mutant strains we used. Additionally, the regulation of lip-1 by Notch signaling in the distal gonad (Lee et al., 2006) might also have dampened the effect of loss of dpl-1 or efl-1 activity. This observation suggests that DPL-1 and EFL-1 likely regulate additional genes like lip-1 in the gonad that remain to be identified.
We found two instances of the worm E2F consensus motif in the upstream regulatory sequence of lip-1, indicating that its regulation by EFL-1/DPL-1 is likely to be direct. In particular, the motif at −282nt, closest to the first exon of lip-1, matches the consensus sequence defined in Chi and Reinke (2006) perfectly. Intriguingly, recent work suggests that our observations are not restricted to the gonad of C. elegans. In mammals, the E2F transcription factor regulates the expression of several phosphatases that act to inhibit MAP kinase family members via E2F consensus motifs present upstream of the open reading frames (Wu et al., 2007; Wang et al., 2007).
Our in situ hybridization experiments indicate that lin-35(Rb) activity has a role in limiting lip-1 expression. Although in situ hybridization is difficult to quantitate, lip-1 levels appear elevated in lin-35 mutants compared to wild type, and the domain of expression of lip-1 is detectable more distally. This observation suggests that LIN-35 has a unique and opposing function to the EFL-1/DPL-1 transcriptional complex. This possibility is consistent with extensive data in mammalian systems, in which Rb binds to E2F and represses its ability to activate transcription (Nevins 1998). We do not know whether LIN-35 directly binds to EFL-1/DPL-1 to limit lip-1 expression in the C. elegans gonad, or whether the action of LIN-35 is independent of an interaction with EFL-1/DPL-1, but two observations suggest that the latter is more likely. First, EFL-1 expression is normally limited to the pachytene stage of meiosis I, whereas the repressive effects of LIN-35 are likely to occur in more distal germ cells. Second, a separate motif associated with LIN-35-downregulated genes (Chi and Reinke 2006) is present in the lip-1 upstream regulatory regions, suggesting that regulation through this site, rather than through the E2F consensus motif, is potentially responsible for the LIN-35-mediated downregulation of lip-1.
Coordinating regulation of germ cell development by two major pathways
Superficially, our results suggest a scenario in which MPK-1 negatively regulates DPL-1, a protein that promotes expression of the MPK-1 inhibitor LIP-1. In this case, where MPK-1 activity results in the suppression of its inhibitor, how is a runaway feed-forward loop avoided? We suggest that DPL-1 exists in two discrete complexes, one in which DPL-1 is phosphorylated by MPK-1, and one in which it is not. The DPL-1 complex regulated by MPK-1 is responsible for controlling chromosome condensation, possibly with LIN-35(Rb), while the MPK-1-independent complex regulates lip-1 expression, in conjunction with its heterodimeric transcription factor partner, EFL-1. Thus, the DPL-1-containing complex regulated by MPK-1 has a different composition from that regulating lip-1. Moreover, these two complexes are not likely to be functioning at the same time during germ cell development. How might the activities of these two complexes be coordinated? EFL-1 expression precedes MPK-1 activation, and also target genes of EFL-1 and DPL-1 are expressed more distally than are those of MPK-1 (Leacock and Reinke 2006). These facts suggest that the presence of EFL-1 drives the formation of the transcriptional regulatory EFL-1/DPL-1 complex to promote expression of lip-1 and other target genes in early- to mid-pachytene. Once EFL-1 expression decreases in mid-pachytene, DPL-1 is then free to function in chromosome condensation, and be regulated by MPK-1 (Figure 6). Alternatively, DPL-1 acts in chromosome condensation for an extended period of germ cell development, and a subset of DPL-1 interacts with EFL-1 briefly within this period.
Conclusion
The proper spatial and temporal regulation of MAP kinase activity is very important for successful germ cell development in C. elegans. As demonstrated in this report, the restricted domain of activated MAP kinase in the pachytene region is able to inactivate the function of DPL-1 in chromosome condensation, while leaving its ability to act as a transcriptional regulator with EFL-1 intact. Our work has revealed an underlying mechanism for how a portion of the pattern of activated MAP kinase is established: EFL-1/DPL-1-mediated activation of lip-1 leads to the inhibition of MAP kinase in the proximal gonad. However, the signal that first activates MAP kinase in the pachytene stage is still mysterious. Further investigation of the interface between the conserved MAP kinase signaling pathway and Rb/E2F regulatory pathway during germ cell development in C. elegans is likely to shed light on common mechanisms governing the coordination of diverse developmental decisions.
Supplementary Material
Supplementary Figure 1 MPK-1 expression pattern in the germ line of Caenorhabditis elegans.
Within the gonad, activated, di-phosphorylated MAP kinase (MPK-1) is first detectable at the mid-pachytene stage of the meiotic prophase I. The ligand that stimulates this activation has yet been identified. The activated form of MAP kinase disappears as germ cells pass the pachytene stage and enter the diplotene/diakinesis phase of meiotic prophase I. LIP-1 (lateral induced phosphatase I) is thought to attenuate the MAP kinase activity during this phase. Activated MAP kinase become detectable again in the most proximal oocyte(s) that are undergoing maturation, prior to fertilization. This proximal MAP kinase activity is induced by a cue from sperm.
Supplementary Figure 2 LIP-1 antagonizes the activity of MPK-1 in the germ line of Caenorhabditis elegans
A. Activated MPK-1 levels are elevated in lip-1 mutants. In lip-1(zh15) mutant gonads, diP-MAP kinase levels were elevated throughout the proximal gonad, from mid-pachytene region to the most proximal end of the gonad, similar to previously published results (Hajnal and Berset, 2002). Refer to Supplementary Figure 1 for wild type MPK-1 antibody staining pattern. B. Reduction of MPK-1 activity can suppress the sterility of lip-1 null mutation. The brood size defect of lip-1(zh15) mutant hermaphrodites is rescued in lip-1(zh15);mpk-1(ga111) double mutants at 20°C. Twelve synchronized animals from each genotype were used for for each experiment, which was repeated three times; error bars represent the standard deviation. These results mirror those previously described in Hajnal and Berset, 2002.
Supplementary Figure 3 in situ hybridization of lip-1 mRNA in unc-4 and non-null dpl-1 mutants
A. The reduction of lip-1 mRNA signal observed in in situ hybridization null mutant animals of dpl-1(n3316) is not due to the presence of a linked mutation, unc-4(e120), because unc-4(e120) single mutant animals do not exhibit a reduction in lip-1 staining. In addition, the reduction is not allele-specific, as multiple non-null dpl-1 mutants, dpl-1(n2294), dpl-1(n3643), and dpl-1(n3380), exhibit reduced lip-1 mRNA levels relative to wild type. Asterisk indicates distal tip of gonad. B. Schematic drawing of the dpl-1 gene structure and the nature of the non-null mutant alleles. dpl-1(n2294), dpl-1(n3643), and dpl-1(n3380) are all single nucleotide substitutions that are predicted to result in a truncated DPL-1 protein.
Supplementary Figure 4 LIP-1 protein level is reduced in non-null dpl-1 mutants
In the non-null dpl-1 mutants, dpl-1(n2994) and dpl-1(n3643), LIP-1 protein levels are reduced drastically compared to wild type. This result is consistent with the in situ hybridization of lip-1 mRNA in these mutants (see Supplementary Figure 3). The significant reduction of LIP-1 protein in these non-null mutants indicates that translation efficiency of LIP-1 is substantially decreased once lip-1 mRNA level fall below a certain threshold. An alternative explanation for the drastic reduction of LIP-1 protein in mutants with moderate lip-1 mRNA levels could be a consequence of a difference in the sensitivity of detection between the in situ hybridization of mRNA and protein immunostaining.
Supplementary Figure 5 lip-1 mRNA in situ hybridization in mildly affected efl-1(RNAi) gonads efl-1(RNAi) gonads that exhibited less dramatic effects when viewed under the microscope still display mild reduction of lip-1 mRNA expression by in situ hybridization. The major effect seems to be a slightly delayed onset of expression in the pachytene region. Asterisks mark distal tip of gonad.
Acknowledgments
The authors would like to thank Marc Vidal and David Hill for providing many of the Gateway clones that were used in our study. We also appreciate the gifts of anti-LIP-1 from Judith Kimble and anti-HIM-3 from Monique Zetka, as well as several strains from HR Horvitz. We thank Te-Wen Lo for assistance with injection of efl-1 dsRNA. Some of the strains used in this study were provided by the CGC. This work was covered by grants from the NIH (GM65682, VJR) and from the Pew Foundation (VJR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berset T, Hoier EF, Battu G, Canevascini S, Hajnal A. Notch inhibition of RAS signalling through MAP kinase phosphatase LIP-1 during C elegans vulval development. Science. 2001;291:1055–1058. doi: 10.1126/science.1055642. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceol C, Horvitz HR. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–473. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Chi W, Reinke V. Promotion of oogenesis and embryogenesis in the C. elegans gonad by EFL-1/DPL-1 (E2F) does not require LIN-35 (pRB) Development. 2006;133:3147–3157. doi: 10.1242/dev.02490. [DOI] [PubMed] [Google Scholar]
- Church DL, Kuan K-L, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I, Sanghera JS, Pelech SL. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis activated myelin basic protein kinase. J Biol Chem. 1991;266:15180–15184. [PubMed] [Google Scholar]
- Dynlacht BD, Brook A, Dembski M, Yenush L, Dyson N. DNA-binding and trans-activation properties of Drosophila E2F and DP proteins. Proc Natl Acad Sci U S A. 1994;91:6359–6363. doi: 10.1073/pnas.91.14.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay DS, Yochem J. The Synmuv genes of Caenorhabditis elegans in vulval development and beyond. Dev Biol. 2007;306:1–9. doi: 10.1016/j.ydbio.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Berset T. The C. elegans MAPK phosphatase LIP-1 is required for the G(2)/M meiotic arrest of developing oocytes. EMBO J. 2002;21:4317–4326. doi: 10.1093/emboj/cdf430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Schaner CE, Dernburg AF, Lee MH, Kim SK, Villeneuve AM, Reinke V. X-chromosome silencing in the germline of C elegans. Development. 2002;129:479–492. doi: 10.1242/dev.129.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko N, Fay D. Transcriptome profiling of the C. elegans Rb ortholog reveals diverse developmental roles. Dev Biol. 2007;305:674–684. doi: 10.1016/j.ydbio.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden S. Control of germline stem cells, entry into meiosis, and the sperm/oocyte decision in C. elegans. Ann Review Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Jones AR, Francis R, Schedl T. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol. 1996;180:165–183. doi: 10.1006/dbio.1996.0293. [DOI] [PubMed] [Google Scholar]
- Lackner MR, Kim SK. Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics. 1998;150:103–117. doi: 10.1093/genetics/150.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leacock SW, Reinke V. Expression profiling of MAP kinase-mediated meiotic progression in Caenorhabditis elegans. PLoS Genet. 2006;2:e174. doi: 10.1371/journal.pgen.0020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Hook B, Lamont LB, Wickens M, Kimble J. LIP-1 phosphatase controls the extent of germline proliferation in Caenorhabditis elegans. EMBO J. 2006;25:88–96. doi: 10.1038/sj.emboj.7600901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13:813–825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Lu X, Horvitz HR. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development. 2005;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee M-H, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Magila V, Xia F, Li WX. An intrinsic cell cycle checkpoint pathway mediated by MEK and ERK in Drosophila. Mol Cell. 2006;11:575–582. doi: 10.1016/j.devcel.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster DL, Bonnette PC, Duronio RJ. A role for the DP subunit of the E2F transcription factor in axis determination during Drosophila oogenesis. Development. 2000;127:3249–3261. doi: 10.1242/dev.127.15.3249. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- Page B, Guedes S, Waring D, Priess JR. The C. elegans E2F- and DP- related proteins are required for embryonic asymmetry and negatively regulate Ras/MAPK signaling. Mol Cell. 2001;7:451–460. doi: 10.1016/s1097-2765(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Anderson EC, Huang MC, Horvitz HR. DPL-1 (DP), LIN-35 (Rb) and EFL-1 (E2F) act with the MCD-1 zinc-finger protein to promote programmed cell death in Caenorhabditis elegans. Genetics. 2007;175:1719–1733. doi: 10.1534/genetics.106.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2001;6:605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Schertel C, Conradt B. C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development. 2007;134:3691–3701. doi: 10.1242/dev.004606. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Yochem J, Han M. A Ras-mediated signal transduction pathway is involved in the control of sex myoblast migration in Caenorhabditis elegans. Development. 1996;122:2823–2833. doi: 10.1242/dev.122.9.2823. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, Peterson BK, Jocobson LA. Activation of ras and the mitogen-activated protein kinase pathway promote protein degradation in muscle cells of Caenorhabditis elegans. Mol Cel Biol. 2002;22:4181–4188. doi: 10.1128/MCB.22.12.4181-4188.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Tan PB, Lackner MR, Kim SK. MAP kinase signaling specificity mediated by the LIN-1 Ets/LIN-31 WH transcription factor complex during C. elegans vulval induction. Cell. 1998;93:569–580. doi: 10.1016/s0092-8674(00)81186-1. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Walhout AJ, Temple GF, Brasch MA, Hartley JL, Lorson MA, van den Heuvel S, Vidal M. GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol. 2000;328:575–592. doi: 10.1016/s0076-6879(00)28419-x. [DOI] [PubMed] [Google Scholar]
- Wang D, Kennedy S, Conte D, Jr, Kim JK, Gabel HW, Kamath RS, Mello CC, Ruvkun G. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–597. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- Wang J, Yin DP, Liu Y-X, Baer R, Yin Y. Dual specificity phosphatase 1/CL100 is a direct transcriptional taget of E2F-1 in the apoptotic response to oxidative stress. Cancer Res. 2007;67:6737–6744. doi: 10.1158/0008-5472.CAN-06-4402. [DOI] [PubMed] [Google Scholar]
- Wu J, Jin YJ, Calaf GM, Huang W-L, Yin Y. PAC1 is a direct transcription target of E2F-1 in apoptotic signaling. Oncogene. 2007;26:6526–6535. doi: 10.1038/sj.onc.1210484. [DOI] [PubMed] [Google Scholar]
- Wu Y, Han M. Suppression of activated let-60 ras protein defines a role of Caenorhabditis elegans sur-1 MAP kinase in vulval differentiation. Genes Dev. 1994;8:147–159. doi: 10.1101/gad.8.2.147. [DOI] [PubMed] [Google Scholar]
- Zetka M, Kawasaki I, Strome S, Muller F. Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev. 1999;13:2258–2270. doi: 10.1101/gad.13.17.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 MPK-1 expression pattern in the germ line of Caenorhabditis elegans.
Within the gonad, activated, di-phosphorylated MAP kinase (MPK-1) is first detectable at the mid-pachytene stage of the meiotic prophase I. The ligand that stimulates this activation has yet been identified. The activated form of MAP kinase disappears as germ cells pass the pachytene stage and enter the diplotene/diakinesis phase of meiotic prophase I. LIP-1 (lateral induced phosphatase I) is thought to attenuate the MAP kinase activity during this phase. Activated MAP kinase become detectable again in the most proximal oocyte(s) that are undergoing maturation, prior to fertilization. This proximal MAP kinase activity is induced by a cue from sperm.
Supplementary Figure 2 LIP-1 antagonizes the activity of MPK-1 in the germ line of Caenorhabditis elegans
A. Activated MPK-1 levels are elevated in lip-1 mutants. In lip-1(zh15) mutant gonads, diP-MAP kinase levels were elevated throughout the proximal gonad, from mid-pachytene region to the most proximal end of the gonad, similar to previously published results (Hajnal and Berset, 2002). Refer to Supplementary Figure 1 for wild type MPK-1 antibody staining pattern. B. Reduction of MPK-1 activity can suppress the sterility of lip-1 null mutation. The brood size defect of lip-1(zh15) mutant hermaphrodites is rescued in lip-1(zh15);mpk-1(ga111) double mutants at 20°C. Twelve synchronized animals from each genotype were used for for each experiment, which was repeated three times; error bars represent the standard deviation. These results mirror those previously described in Hajnal and Berset, 2002.
Supplementary Figure 3 in situ hybridization of lip-1 mRNA in unc-4 and non-null dpl-1 mutants
A. The reduction of lip-1 mRNA signal observed in in situ hybridization null mutant animals of dpl-1(n3316) is not due to the presence of a linked mutation, unc-4(e120), because unc-4(e120) single mutant animals do not exhibit a reduction in lip-1 staining. In addition, the reduction is not allele-specific, as multiple non-null dpl-1 mutants, dpl-1(n2294), dpl-1(n3643), and dpl-1(n3380), exhibit reduced lip-1 mRNA levels relative to wild type. Asterisk indicates distal tip of gonad. B. Schematic drawing of the dpl-1 gene structure and the nature of the non-null mutant alleles. dpl-1(n2294), dpl-1(n3643), and dpl-1(n3380) are all single nucleotide substitutions that are predicted to result in a truncated DPL-1 protein.
Supplementary Figure 4 LIP-1 protein level is reduced in non-null dpl-1 mutants
In the non-null dpl-1 mutants, dpl-1(n2994) and dpl-1(n3643), LIP-1 protein levels are reduced drastically compared to wild type. This result is consistent with the in situ hybridization of lip-1 mRNA in these mutants (see Supplementary Figure 3). The significant reduction of LIP-1 protein in these non-null mutants indicates that translation efficiency of LIP-1 is substantially decreased once lip-1 mRNA level fall below a certain threshold. An alternative explanation for the drastic reduction of LIP-1 protein in mutants with moderate lip-1 mRNA levels could be a consequence of a difference in the sensitivity of detection between the in situ hybridization of mRNA and protein immunostaining.
Supplementary Figure 5 lip-1 mRNA in situ hybridization in mildly affected efl-1(RNAi) gonads efl-1(RNAi) gonads that exhibited less dramatic effects when viewed under the microscope still display mild reduction of lip-1 mRNA expression by in situ hybridization. The major effect seems to be a slightly delayed onset of expression in the pachytene region. Asterisks mark distal tip of gonad.