Abstract
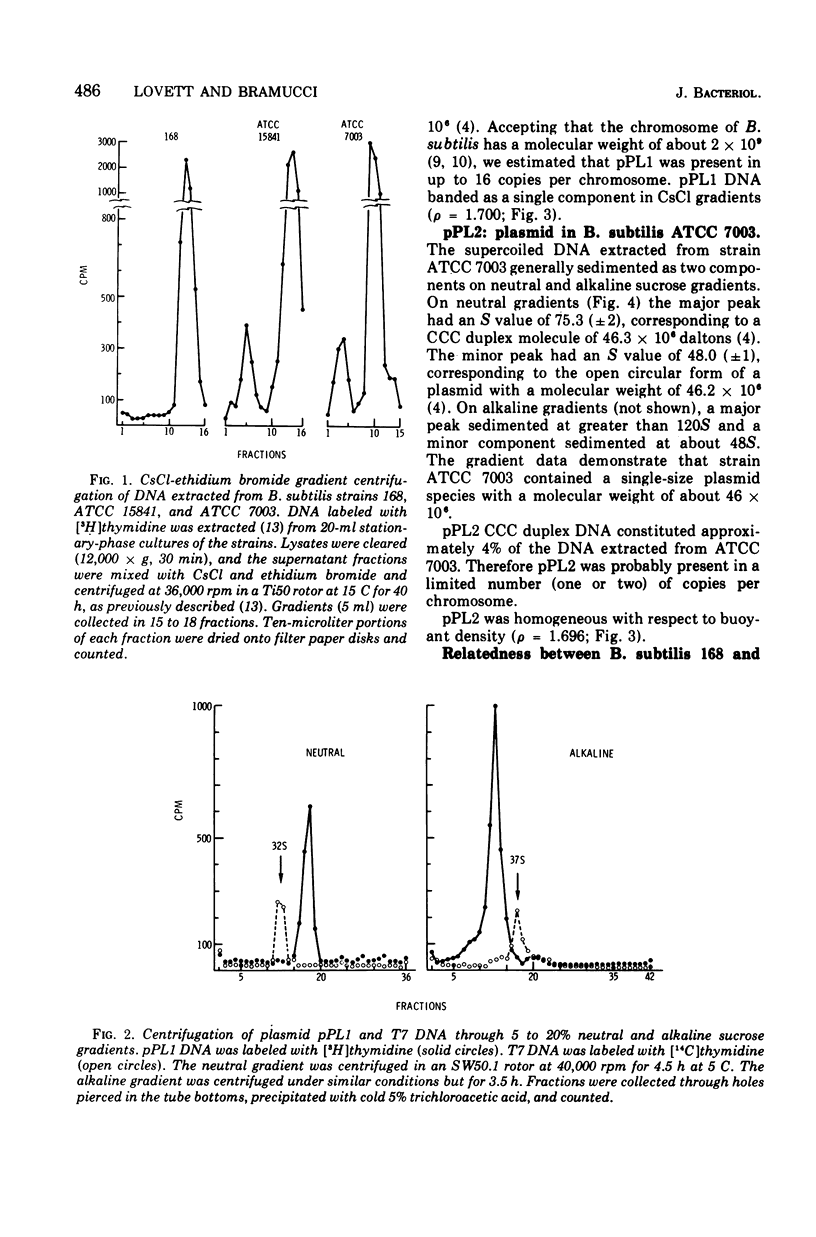
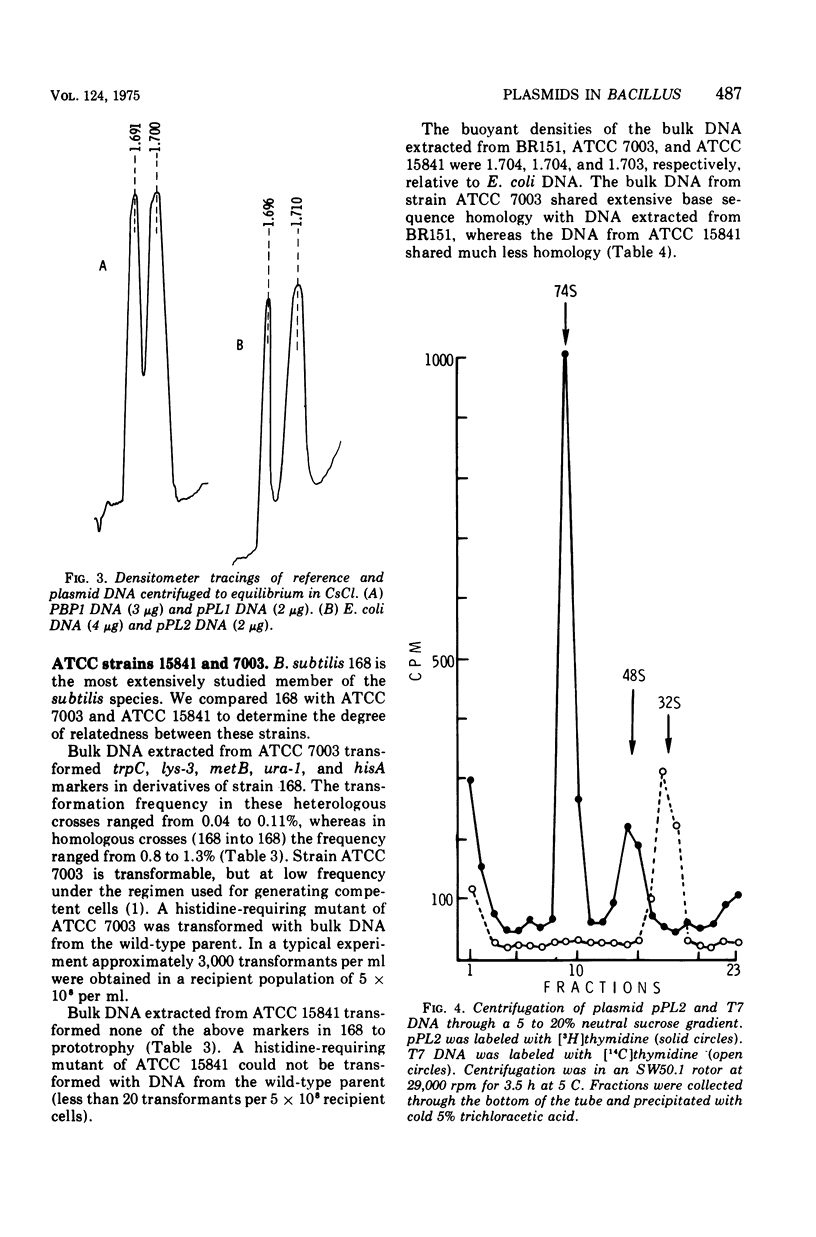
Two of eighteen strains of Bacillus subtilis examined contained covalently closed circular duplex deoxyribonucleic acid (DNA) of homogeneous size and buoyant density. Strain ATCC 15841 contained about 16 copies per chromosome of plasmid pPL1, a circular DNA element having a molecular weight of about 4.7 times 10(6) and a buoyant density of 1.700. Strain ATCC 7003 contained about one to two copies per chromosome of plasmid pPL2. pPL2 had a molecular weight of about 46 times 10(6) and a buoyant density of 1.696. Strain ATCC 7003 appeared to be closely related to B. subtilis 168 by genetic, physiological, and biochemical criteria. Strain ATCC 15841 appeared to be much less closely related. B. pumilus ATCC 12140 contained two size classes of covalently closed circular duplex DNA. The plasmids pMB1 and pMB2 had molecular weights of about 6.8 times 10(6) and 5.3 times 10(6), respectively, and were present in several copies per chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Helinski D. R. Heterogeneous circular DNA elements in vegetative cultures of Bacillus megaterium. Proc Natl Acad Sci U S A. 1969 Oct;64(2):592–599. doi: 10.1073/pnas.64.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C. Selective inhibition of plasmid DNA production in Bacillus megaterium by 6-(p-hydroxy-phenylazo)-uracil: evidence for multiple maintenance systems. Biochem Biophys Res Commun. 1974 Jun 4;58(3):719–727. doi: 10.1016/s0006-291x(74)80477-8. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberry R. C., Carlton B. C. Characterization of the polydisperse closed circular deoxyribonucleic acid molecules of Bacillus megaterium. J Bacteriol. 1973 May;114(2):625–631. doi: 10.1128/jb.114.2.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavenoff R. Characterization of the Bacillus subtilis W23 genome by sedimentation. J Mol Biol. 1972 Dec 30;72(3):801–806. doi: 10.1016/0022-2836(72)90192-1. [DOI] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci D., Bramucci M. G., Burdick B. D. Some properties of the PBP1 transduction system in Bacillus pumilus. J Virol. 1974 Jan;13(1):81–84. doi: 10.1128/jvi.13.1.81-84.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Biochemical studies of two Bacillus pumilus plasmids. J Bacteriol. 1974 Oct;120(1):488–494. doi: 10.1128/jb.120.1.488-494.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Evidence for a nonrandom base sequence in a Bacillus pumilus plasmid: EcoR1 endonuclease digestion of pPL576. J Bacteriol. 1975 Jul;123(1):377–379. doi: 10.1128/jb.123.1.377-379.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Burdick B. D. Cryptic plasmid in Bacillus pumilus ATCC 7065. Biochem Biophys Res Commun. 1973 Sep 5;54(1):365–370. doi: 10.1016/0006-291x(73)90931-5. [DOI] [PubMed] [Google Scholar]
- Lovett P. S. PBPI: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 1972 Mar;47(3):743–752. doi: 10.1016/0042-6822(72)90564-8. [DOI] [PubMed] [Google Scholar]
- Lovett P. S. Plasmid in Bacillus pumilus and the enhanced sporulation of plasmid-negative variants. J Bacteriol. 1973 Jul;115(1):291–298. doi: 10.1128/jb.115.1.291-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]



