Abstract
Purpose:
To compare visual field abnormalities obtained with standard automated perimetry (SAP) to those obtained with the multifocal visual evoked potential (mfVEP) technique in strabismic amblyopes.
Methods:
Humphrey 24-2 visual fields (HVF) and mfVEPs were obtained from each eye of 12 strabismic amblyopes. For the mfVEP, amplitudes and latencies were analyzed and probability plots derived. Multifocal VEP and HVF hemifields were abnormal if they had clusters of 2 or more contiguous points at p<0.01, or 3 or more contiguous points at p<0.05 with at least one at p<0.01. An eye was abnormal if it had an abnormal hemifield.
Results:
On SAP, amblyopic eyes had significantly higher foveal thresholds (p=0.003) and lower mean deviation values (p=0.005) than fellow eyes. For the mfVEP, 11 amblyopic and 6 fellow eyes were abnormal. Of the 11 amblyopic eyes, 6 were abnormal on SAP. The deficits extended from the center to mid-periphery. Monocular mfVEP latencies were significantly decreased for amblyopic eyes compared to control eyes (p<0.0002).
Conclusions:
Both techniques revealed deficits in visual function across the visual field in strabismic amblyopes, but the mfVEP revealed deficits in fellow eyes and in more amblyopic eyes. In addition, mfVEP response latencies for amblyopic eyes were shorter than normal.
Amblyopia is a developmental disorder characterized by decreased vision that cannot be corrected by refractive means and is not accompanied by any overt pathology of the visual system. It is one of the most common causes of decreased vision in childhood. Amblyopia is associated with the presence of strabismus, anisometropia or form deprivation early in life and is characterized by deficits in central visual function such as decreased visual acuity and contrast sensitivity, as well as by visual field deficits. However, there is controversy regarding the extent and depth of these visual field deficits. For example, Phillip and Mayer described visual field deficits in strabismic and ansiometropic amblyopes ranging from central to paracentral scotoma, whereas Sireteanu and Fronius reported finding central defects in strabismic amblyopes with no systematic deficits in the peripheral field.1, 2 Medhorn, who evaluated the central 30 degrees in strabismic amblyopes, found both central and nasal defects in patients with severe amblyopia but found no visual field defects in patients with visual acuity better than 0.2 logMAR.3 A more recent study using standard automated achromatic perimetry (SAP) has challenged these earlier findings and has reported that amblyopia is associated with a slight reduction in sensitivity across the visual field in strabismic and anisometropic amblyopes.4
A relatively new technique, the multifocal visual evoked potential technique (mfVEP) can provide an objective measure of the integrity of local regions of the visual field.5-8 As the initial locus of neural dysfunction in amblyopia is known to occur in the visual cortex (V1) and the mfVEP is largely generated in V1,8-11 this method provides an alternative way to assess visual field abnormalities in amblyopia. Not surprisingly, many earlier studies using the conventional VEP, which reflects activity in V1 and extrastriate cortex, have reported abnormalities in amblyopes. These include increased latency and reduced response amplitudes.12-15 However, the drawback of the conventional VEP is that it does not provide information about the topography of amblyopic deficits. The multifocal VEP (mfVEP), an objective visual field test, provides information about responses from local regions of the field.
The aim of this study is to compare the extent of visual field deficits measured with SAP to those obtained with the mfVEP technique in patients with strabismic amblyopia.
Subjects and Methods
Subjects
Twelve subjects aged 19-70 years with strabismic amblyopia were recruited from the Eye Clinic at Columbia Presbyterian Hospital and from the private practice of one of the authors HE. Informed consent was obtained from all subjects before participation. Procedures adhered to the tenets of the Declaration of Helsinki and the protocol was approved by the Columbia University Medical Center Institutional Review Board for Human Research. In addition the study and data collection complied with the Health Insurance Portability and Accountability Act (HIPAA).
All subjects underwent complete ophthalmological and orthoptic examinations before testing. The criteria for enrollment in the study included the presence of a manifest strabismus on cover test and/or history of strabismus surgery. Amblyopia was defined as an inter-eye difference in visual acuity of ≥2 Snellen lines (≥0.2 logMAR). The clinical characteristics of the 12 subjects are summarized in Table 1. Ten of the subjects had a diagnosis of esotropia, the two subjects (P1 and P3) with exotropia had a prior history of esotropia and developed exotropia following strabismus surgery at 6 and 2 years of age respectively. Four subjects had anisometropia of more than 1.5D. With regard to the age of onset of strabismus, 9 subjects reported an onset of strabismus from 2-3 years of age, and 2 (P3 and P12) an onset at 0-6 months. Only one (P6) was unable to provide this information, he was unaware of a history of strabismus. Three methods were used to assess fixation location: fundus photography with a fixation target, visuoscopy, and recording the location and stability for a period of 30 seconds with the MP-1 microperimeter (Nidek Technologies Srl. Vigonza PD, Italy). The tested eyes had steady or unsteady foveal fixation with the exception of P9 and P12 who had eccentric fixation in the amblyopic eye. For P9, fixation was 1-2° supero-nasal to the fovea and, for P12, fixation was 1-2° infero-nasal to the fovea. None of the patients had latent or manifest nystagmus.
Table 1.
Clinical characteristics
| Subject | Age | Diagnosis Age at onset |
Anisometropia | Log MAR (Snellen acuity) amblyopic eye |
Log MAR (Snellen acuity) fellow eye |
|---|---|---|---|---|---|
| 1 | 43 | Consecutive XT 2-3yrs |
N | 0.10 OS (20/25) |
−0.10 (20/16) |
| 2 | 70 | ET 2-3yrs |
N | 0.30 OS (20/40) |
0.10 (20/25) |
| 3 | 54 | Consecutive XT c DVD 0-6mnths |
N | 0.32 OD (20/40) |
−0.04 (20/20) |
| 4 | 19 | ET with LHT 2-3yrs |
N | 0.40 OS (20/50) |
0.00 (20/20) |
| 5 | 61 | ET 2-3yrs |
Y | 0.62 OS (20/80) |
−0.02 (20/20) |
| 6 | 43 | ET Not known |
N | 0.63 OD (20/80) |
0.00 (20/20) |
| 7 | 62 | ET 2-3yrs |
Y | 0.72 OS (20/100) |
−0.06 (20/16) |
| 8 | 57 | ET with LHT 2-3yrs |
Y | 0.86 OS (20/160) |
−0.12 (20/16) |
| 9 | 37 | ET 2-3 yrs |
N | 1.0 OS (20/200) |
0.00 (20/20) |
| 10 | 45 | ET 2-3yrs |
N | 1.0 OS (20/200) |
−0.14 (20/16) |
| 11 | 22 | ET 2-3yrs |
N | 1.04 OD (20/200) |
−0.06 (20/16) |
| 12 | 62 | ET 0-6mnths |
Y | 1.30 OS (20/400) |
−0.14 (20/16) |
ET esotropia; XT exotropia; DVD dissociated vertical deviation
Standard Automated Perimetry
Standard automated achromatic perimetry was performed on all subjects with the Humphrey Field Analyzer II (model 750, Carl Zeiss Meditec, Inc., Dublin, CA). Humphrey visual fields (HVF 24-2 program SITA standard) were obtained on the same day as the mfVEP. In all cases foveal thresholds were tested twice, once at the beginning and once at the end of the test. Pupil position was continuously monitored and the amblyopic eye was always tested after the fellow eye.
Multifocal VEP
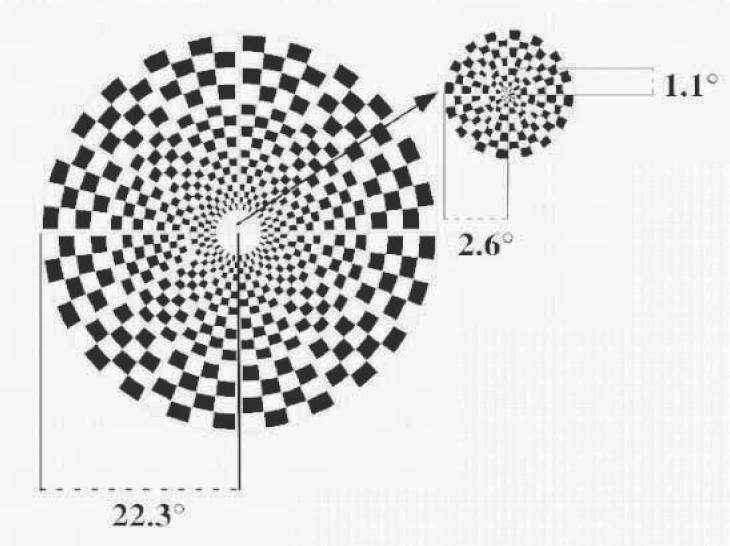
The mfVEP was performed using VERIS 4.3 software (Electro-Diagnostic Imaging, San Mateo, CA). The stimulus is shown in Fig.1. The dartboard pattern consisted of 60 sectors, each with a checkerboard pattern of 16 checks, 8 white (luminance = 200 cd/m2) and 8 black (luminance < 3 cd/m2). The sectors were cortically scaled with eccentricity. The display had a radius of 22.3° and it was displayed on a black and white monitor driven at a frame rate of 75 Hz. The 16-element checkerboard of each sector had a probability of 0.5 of reversing on any pair of frame changes and the pattern of reversals for each sector followed a pseudo-random (m) sequence. On every frame change (every 13.3 ms) each sector could reverse contrast or stay at the same contrast. A refractor/camera was used to refract the subjects and monitor eye position and stability throughout the test. Subjects fixated on the center of a black cross in the middle of the display. Segments contaminated by eye movements, loss of fixation, unsteady fixation and/or external noise were discarded and re-recorded.
Figure 1.
The mfVEP stimulus display: the dartboard pattern consists of 60 sectors. The sectors are cortically scaled with eccentricity. The entire display has a radius of 22.3° and the central 12 sectors have a radius of 2.6°.
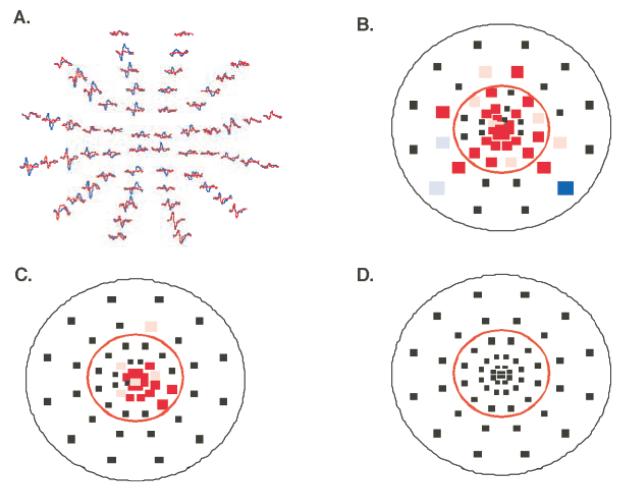
The details of the mfVEP recording and analysis have been previously published [see Hood and Greenstein8 for a review]. Briefly three channels of recording were obtained simultaneously with gold cup electrodes. Recording electrodes were placed at the inion, 4 cm above the inion and at two lateral locations up 1 cm and over 4 cm from the inion. All three channels were filtered with a high and low frequency cutoff of 3 Hz and 100 Hz (Grass Instruments preamplifier P511J, Quincy, MA). An impedance of<5K was achieved for all subjects. The mfVEPs were low-pass filtered using a sharp cutoff at 35 Hz and a fast Fourier transform technique. This and all other analyses were performed with programs written in MATLAB (Math-Works Inc, Natick, MA). The mfVEP records were processed and an array of best channel responses derived as previously described.7, 8, 16,17 The amplitudes of each of the 60 local responses were compared to a normative group18 and interocular (comparison of 2 eyes) and monocular probability plots, analogous to the HVF probability plots, were derived. 7,8 The normative group comprised 100 individuals with visual acuity ≥20/30 in each eye, normal HVFs, and no evidence of any ocular or systemic disease. They ranged in age from 21 to 92 years; the mean age was 49±13.6 years. There was no significant difference in age between the group of 100 normal controls and the 12 subjects with strabismic amblyopia. Figure 2 shows examples of mfVEP responses together with interocular (Fig. 2 B) and monocular probability plots (Fig. 2C and D) obtained from a patient (P8). The points in these plots are positioned in the center of each of the 60 sectors of the display. A colored square indicates that the decrease in the mfVEP response amplitude was statistically significant at either the 5% (>1.96 SD, desaturated color) or 1% (>2.58 SD, saturated color) level. On the interocular plot (Fig. 2B) the color indicates whether it was the response of the left (red) or the right (blue) eye that was significantly smaller than the fellow eye, compared to the mean and standard deviation of the ratios of the root mean square (RMS) values for the 100 normal subjects. In this case the responses for the left eye, the amblyopic eye were significantly smaller than the responses for the right eye particularly within a radius of 10° from the fovea. On the monocular plot the color indicates that the signal-to-noise ratio (SNR) values of the left (red) or right (blue) eye for that location fell below the mean normal values. For P8 the monocular plot for the left eye (Fig. 2C) shows that the responses for the left eye fell below the mean normative values within a radius of 10°.
Figure 2.
An example of mfVEP responses and probability plots from a patient P8. A. The mfVEP responses for the right eye are in blue and for the left eye in red. B. Interocular probability plot derived from the responses in A. The color of each square indicates whether the response of the left (red) or the right (blue) eye was significantly smaller than the fellow eye. The saturated color indicates points with p<0.01 and the desaturated color points with p<0.05. The red circle indicates a radius of 10°. C. Monocular probability plot for the left eye showing a cluster of abnormal points. D. Monocular probability plot for the right eye.
Monocular latencies were also measured using the method described in Hood et al.19 Briefly to obtain a measure of the relative monocular latency of a response, a cross-correlation was calculated between the patient's response and a template. A template was created for each location, eye, and channel, and derived from averaging the responses of the group of 100 normal subjects described above. 18,19 Records with small signal to noise ratios (< 0.23 log unit) or with cross-correlation values of less than zero were excluded as previously described. 19 The relative mfVEP latency values representing the shift in time (ms) that maximized the cross-correlation with the template, with amplitude scaling of the template, were compared to those obtained from each eye of a group of 23 age-similar normal control subjects. The 23 normal controls had visual acuity ≥20/20 in each eye, binocular single vision, no evidence of any ocular disease and ranged in age from 24-72 years, the mean age was 48.3±11.4 years. There was no significant difference in age between the 23 normal controls and the 12 subjects with strabismic amblyopia.
Cluster Criteria for Abnormal mfVEP and HVF
To compare the visual field results obtained with the mfVEP and HVF, the data from both tests were analyzed in terms of hemifields. Each superior and inferior hemifield was subdivided into two regions, a central 10° area (radius of 10°) and the remaining area out to approximately 20° (see Fig. 4). A mfVEP hemifield was considered abnormal if it contained a cluster on the monocular or interocular plot of 2 or more contiguous points at p<0.01, or 3 or more contiguous points at p<0.05 with at least one point at p<0.01. The central 4 points were excluded from this analysis. An eye was defined as abnormal when either the inferior or superior hemifield met the abnormal cluster criteria.8, 20 Similar criteria were used for the HVF. A hemifield was defined as abnormal if a cluster had 2 or more contiguous points at p<0.01, or 3 or more contiguous points at p<0.05 with at least one point at p<0.01. An eye was defined as abnormal when either the inferior or superior hemifield met the abnormal cluster criteria.
Figure 4.
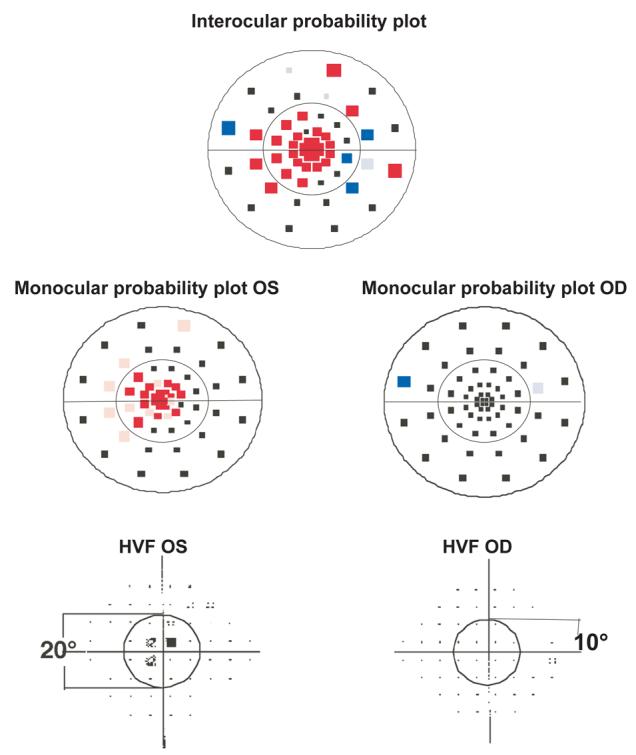
Representative mfVEP probability plots and HVF total deviation plots obtained from one of the patients (P12). Abnormal clusters for the left eye can be seen in both the superior and inferior hemifields on the interocular probability plot and there is an abnormal cluster for the right eye in the inferior hemifield. The monocular probability plot for the left eye also shows abnormal clusters. The HVF total deviation plot for the left eye shows an abnormal cluster in the superior hemifield. The fellow eye or right eye is normal on the HVF.
Results
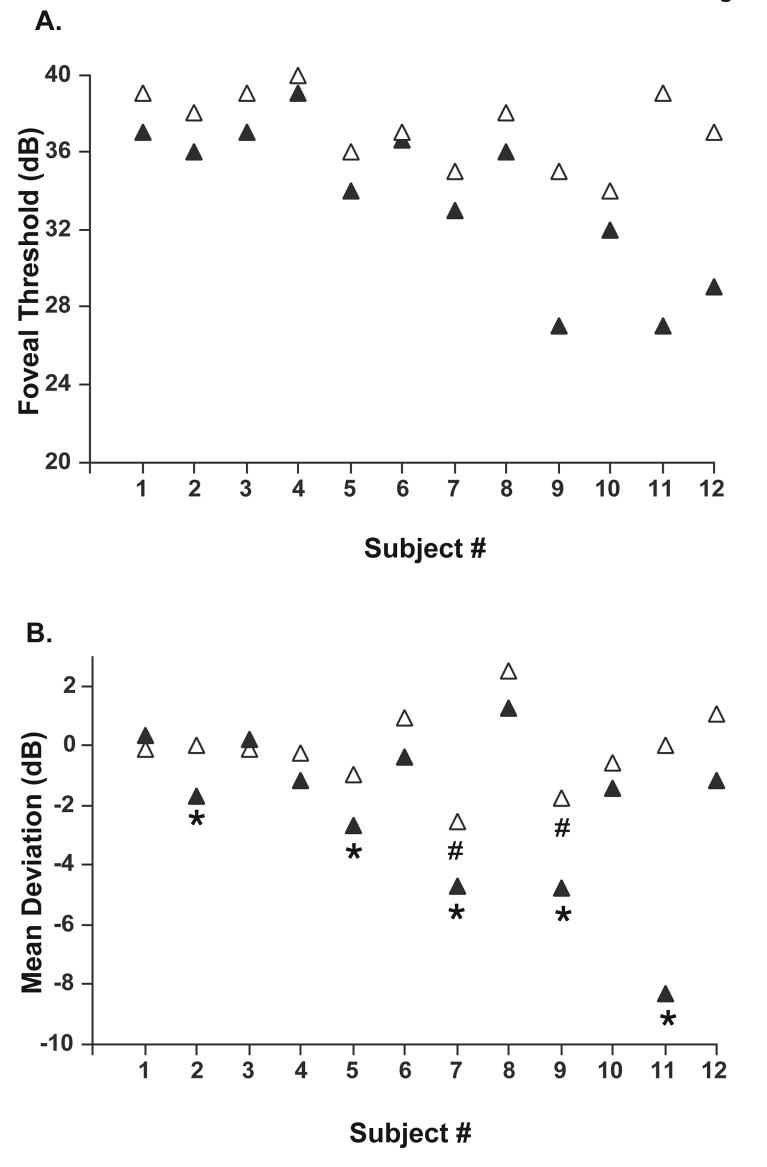
All 12 subjects had reliable SAP results for both amblyopic and fellow eyes. The criteria for reliability were fewer than 20% fixation losses, 33% false positive and false negative responses. For the HVF, foveal thresholds were significantly higher for amblyopic eyes compared to fellow eyes (Wilcoxon matched pairs test p=.003) and mean deviation values were significantly lower (Wilcoxon matched pairs test p=.005). Figure 3A shows the foveal threshold values for the amblyopic and fellow eyes of the 12 patients. Thresholds were higher (i.e. foveal sensitivities were lower) for 11 of the 12 amblyopic eyes (filled triangles) compared to their fellow eyes (open triangles). One patient, P6 had the same threshold values for the amblyopic and fellow eye. The mean deviation (MD) values for the HVF are shown in Fig. 3B. Mean deviation values were lower for the amblyopic eye compared to the fellow eye for 10 patients. For 5 patients, P2, P5, P7, P9 and P11, the mean deviation for the amblyopic eye was significantly decreased compared to Humphrey visual field norms. For P2 and P5, MD was decreased at the p<10% level, and for P7, P9 and P11 at P<0.5% (indicated by * in Fig. 3B). The mean deviation was also decreased for the fellow eyes of P7 and P9 at p<2% and p<10% respectively (both indicated by the # symbol in Fig. 3B).
Figure 3.
A. HVF foveal threshold values for the amblyopic (filled triangles) and fellow eyes (open triangles) of the 12 patients. B. HVF mean deviation values for the amblyopic (filled triangles) and fellow eyes (open triangles). The symbol * indicates that the mean deviation of the amblyopic eye was significantly decreased for P2 and P5 at the P<10% level and for P7, P9, P11 at P<0.5% compared to HVF norms. The symbol # indicates that the mean deviation for the fellow eye of P7 and P9 was decreased at P<2% and P<10% respectively.
Representative mfVEP probability plots and HVF total deviation plots obtained from one of the patients (P12) with an abnormal amblyopic eye on both tests and abnormal fellow eye on the mfVEP are shown in Fig. 4. Abnormal clusters can be seen in both the superior and inferior hemifields on the interocular and monocular probability plots. The responses of the left eye, the amblyopic eye, are significantly smaller than those for the right eye in both hemifields. The HVF total deviation plot (lower panel of Fig. 4) for the left eye shows an abnormal cluster in the superior hemifield. The fellow eye or right eye is normal on the HVF but is abnormal on the mfVEP interocular plot according to our cluster criteria; there is a cluster of 3 abnormal contiguous points in the inferior hemifield. The mfVEP and HVF results for the 12 patients are summarized in Table 2. Based upon our cluster criteria described above 11 amblyopic eyes and 6 fellow eyes were abnormal on the mfVEP, and 6 of the amblyopic eyes were also abnormal on the HVF.
Table 2.
Summary of HVF and mfVEP results
| Amblyopic eyes |
Fellow eyes |
||||||
|---|---|---|---|---|---|---|---|
| 24-2 HVF | 24-2 HVF | ||||||
| Abnormal | Normal | Abnormal | Normal | ||||
| A | 6 | 5 | A | 0 | 6 | ||
| mfVEP | mfVEP | ||||||
| N | 0 | 1 | N | 0 | 6 | ||
| % agreement = 58% | |||||||
| % agreement = 50% | |||||||
A Abnormal; N normal
The extent of the visual field deficit in the amblyopic eye was assessed for both mfVEP and HVF. Nine amblyopic eyes had visual field deficits involving both hemifields on the mfVEP. Five of these eyes also had abnormalities involving both hemifields on the HVF. The deficits were not limited to the central 10°. For 8 of the 12 eyes on the mfVEP, and for all 6 on the HVF, the abnormal clusters extended beyond the central 10 degrees, reaching the outer limits of the tested areas (22.3° and 24°) for 2 eyes.
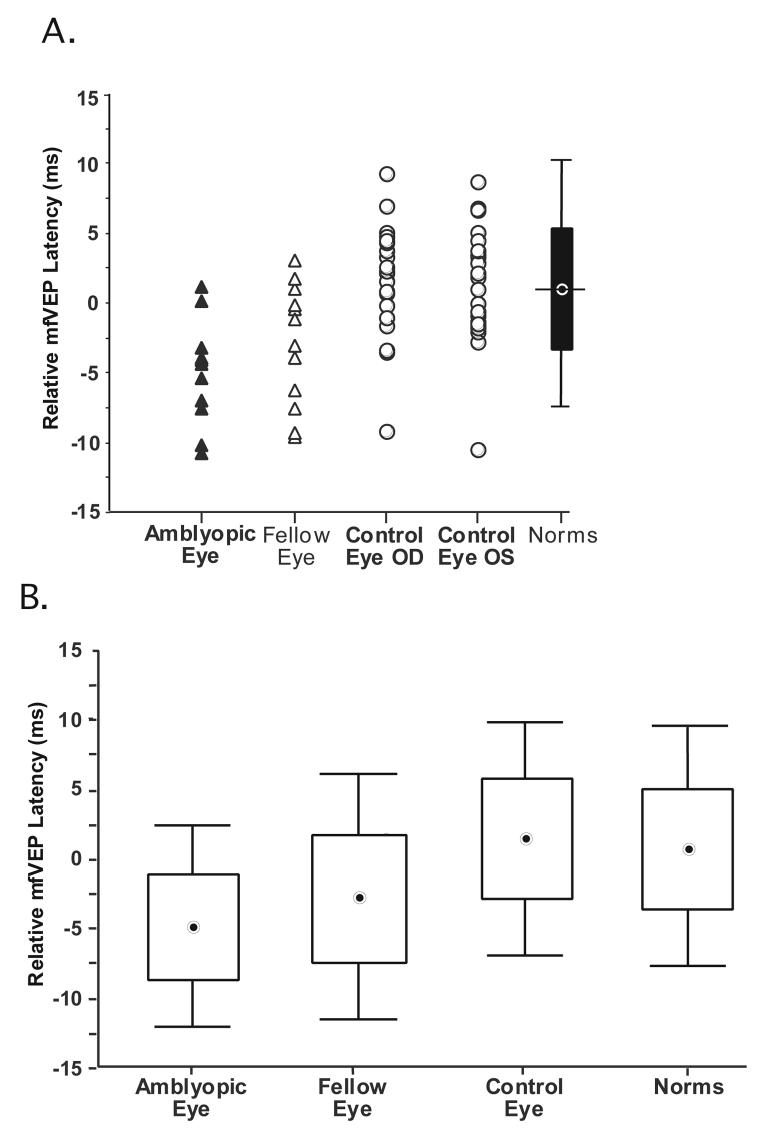
Multifocal VEP latencies were also measured for the 12 amblyopic and fellow eyes and compared with latencies for the right and left eyes of 23 age-similar control subjects. The relative latencies are shown in Fig. 5A. Each data point represents the relative latency for an eye. Figure 5A shows that 10 of the amblyopic eyes and 9 of the fellow eyes have decreased (i.e. shorter) latencies as compared to the mean relative latency of 0.94 ms for the normative group of 100 eyes. Only one of the amblyopic eyes, and 3 of the fellow eyes, showed an increase in latency relative to the mean for the normative group. The mean latency values ±1.96SD for the group of 12 amblyopic eyes, 12 fellow eyes, 23 control eyes (one eye from each of the 23 subjects was randomly selected), and 100 eyes of the normative group can be seen in the form of box and whisker plots in Fig. 5B. The mean latencies for the group of amblyopic and fellow eyes are decreased by 6.34 and 4.24 ms compared to the mean latency of 1.54 ms for the control eyes and the median latencies by 6.45 and 4.43 ms respectively. The mean latency of the control eyes is increased by 0.83 ms and the median by 0.58 ms compared to the values for the normative group. Statistical analysis revealed that monocular latencies were significantly shorter for amblyopic eyes compared to values for the 23 age-similar control eyes and 100 eyes of the normative group (Mann-Whitney U test p<0.0002 and p<0.0001 respectively).
Figure 5.
A. The relative mfVEP latencies for 12 amblyopic eyes (filled triangles), 12 fellow eyes (open triangles), 23 right and 23 left eyes (open circles) of age-similar control subjects. The box plot shows the mean of the normative group (horizontal bar) ±1 SD (box) and ±1.96 SDs (vertical line). B. Box and whisker plots showing the mean latency values ±1.96SD for the group of 12 amblyopic,12 fellow eyes 23 control eyes and 100 eyes of the normative group.
Discussion
We investigated the effects of strabismic amblyopia on visual function across the visual field with conventional perimetry and the mfVEP technique. On SAP foveal sensitivity and the mean deviation were significantly decreased in the amblyopic eye compared to the fellow eye. For 5 patients, the mean deviation was also significantly decreased compared to Humphrey visual field norms. In addition, 50% of the amblyopic eyes had visual field deficits as defined by cluster criteria. These deficits affected the central area and extended into the mid-periphery. The SAP findings are in agreement with previous reports of decreased foveal sensitivity, and central defects or scotomas extending into the periphery using conventional perimetric techniques.1-4
Our main purpose was to compare these conventional perimetric findings to those obtained using the mfVEP technique. We chose the mfVEP technique because it has advantages over SAP. In particular, it provides an objective measure of visual field integrity and the responses reflect activity primarily in V1.8-11 The latter is particularly relevant for investigating patients with amblyopia as current opinion places the earliest functional physiological abnormalities in this cortical area21, 22 and recent fMRI studies have reported that V1 is dysfunctional in amblyopia.23-25 We found that mfVEP responses from amblyopic and fellow eyes were decreased in amplitude and that responses from amblyopic and fellow eyes also showed decreases in latency. When analyzed using a cluster criteria, 11 of the 12 amblyopic eyes had visual field deficits compared to only 6 on SAP. In addition, 6 of the fellow eyes had areas of decreased mfVEP responses. Typically for the amblyopic eyes, abnormal clusters were present in both hemifields and they affected the central 10° extending out towards the limits of the tested area of 22.3°. The extent of these deficits cannot be attributed to unsteady foveal or eccentric fixation in the amblyopic eye. First, care was taken to ensure that fixation was continuously monitored throughout the mfVEP test and any segments of the recording that were contaminated by eye movements, loss of fixation or unsteady fixation were discarded and re-recorded. Second, although a recent study has shown that simulation of unsteady foveal fixation and eccentric fixation in control subjects can result in decreased mfVEP responses, the affected area was not as extensive, and the waveforms showed reversals in polarity.26 Two earlier studies using the mfVEP technique also found that amplitudes were significantly reduced for amblyopic and fellow eyes in the central region.27, 28 However, in contrast to our findings of decreased mfVEP latencies in amblyopic and a similar trend in fellow eyes, both studies reported that latencies were prolonged in amblyopic eyes. In addition the study by Zhang and Zhao28 reported that latencies were prolonged in fellow eyes, but only for those patients with early-onset amblyopia. The latter was defined as amblyopia with a clear history of onset before 18 months. It is possible that the discrepancy between our study and theirs can be attributed to differences in methodology and/or data analysis. For example, Yu et al.27 used a stimulus display consisting of 61 hexagons scaled for retinal cell density. Some of the hexagons cross the horizontal mid-line. These will produce responses of opposite polarity that will result in small responses making it difficult to measure and interpret waveforms, amplitudes and latencies. In addition, a ring analysis was used to measure the amplitudes and latencies. The other study by Zhang and Zhao28 used a dartboard display, but data from the outermost sectors were not analyzed and amplitude and latency values were derived from averaged waveforms.
What could explain our paradoxical finding of shorter rather than prolonged latencies? There is a recent study using a conventional pattern-onset VEP technique by Davis et al., 29 that also reported a shortening of response latencies in amblyopic and fellow eyes. But this occurred only in subjects with early-onset amblyopia. A subject with early-onset amblyopia had to have an unequivocal history of onset of amblyopia before 18 months of age. Although the patients in our study were able to provide a history regarding the age of onset of strabismus, and in some cases the age at which occlusion was started (P1, P5, P8, at age 3-4 years), they were unable to provide an unequivocal age of onset of amblyopia. The drawback of trying to assign subjects to either an early or a late-onset amblyopia group based on recollection of the age of onset of strabismic amblyopia is that both the subject and the family's recollection can be very unreliable. However if one assumes that amblyopia develops soon after the onset of strabismus, then only two of our patients (P3 and P12) who reported an onset at 0-6 months may perhaps fit the category of early-onset amblyopes. The 9 patients who reported an age at onset of strabismus between 2-3 years of age would be categorized as so-called late-onset amblyopes i.e. amblyopia occurring after 18 months of age. In contrast to Davis et al., 29 7 of these late-onset amblyopes had a shortening of response latencies in both the amblyopic and fellow eye. Davis et al 29 suggested that their findings in early-onset amblyopes might result from an enhancement of magnocellular relative to parvocellular responses in strabismic amblyopia. This explanation is based on evidence that experimental esotropia in rhesus monkeys results in shrinkage in the parvocellular layers with no change in the magnocellular layers. 30 In addition monocular deprivation results in an increase in the ratio of magnocellular to parvocellular cell size in the non-deprived and deprived laminae of the LGN.31 Lastly VEPs to achromatic stimuli and motion onset stimuli, both presumably reflecting magnocellular pathway function, are relatively unaffected in strabismic amblyopic eyes.32, 33
In conclusion there are deficits in visual function across the visual field in amblyopic eyes. We found that the deficits were not restricted to the central area; they extended into the mid-periphery. They were present both on conventional perimetry and the mfVEP technique. However, the mfVEP revealed abnormalities in a greater number of amblyopic eyes compared to conventional perimetry and provided evidence that the fellow eye was not normal. In addition it revealed that mfVEP response latencies for strabismic amblyopic eyes were shorter than normal.
Acknowledgments
Supported by a grant from the National Eye Institute EY 02115 Bethesda MD, by unrestricted funds from Research to Prevent Blindness, New York, NY and a grant from The Starr Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Site: Department of Ophthalmology, Columbia University, New York, NY.
Presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, 2005
The authors have no proprietary interests in the development or marketing of any of the materials mentioned in this study.
References
- 1.Phillipp W, Mayer W. Investigation of visual field defects in strabismic and anisometropic amblyopes with the octopus Program G1. Graefes Arch Clin Exp Ophthalmol. 1989;227:448–454. doi: 10.1007/BF02172897. [DOI] [PubMed] [Google Scholar]
- 2.Sireteanu R, Fronius M. Human amblyopia: structure of the visual field. Exp Brain Res. 1990;79:603–614. doi: 10.1007/BF00229328. [DOI] [PubMed] [Google Scholar]
- 3.Medhorn E. Nasal field defects in strabismic amblyopia. Doc Ophthalmol. 1986;45:318–329. [Google Scholar]
- 4.Donahue SP, Wall M, Kutzko KE, et al. Automated perimetry in amblyopia: a generalized depression. Am J Ophthalmol. 1999;127:312–321. doi: 10.1016/s0002-9394(98)90327-0. [DOI] [PubMed] [Google Scholar]
- 5.Baseler HA, Sutter EE, Klein SA, et al. The topography of visual evoked response properties across the visual field. Electroenceph Clin Neurophysiol. 1994;90:65–81. doi: 10.1016/0013-4694(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 6.Klistorner A, Graham SL. Objective perimetry in glaucoma. Ophthalmology. 2000;107:2283–99. doi: 10.1016/s0161-6420(00)00367-5. [DOI] [PubMed] [Google Scholar]
- 7.Hood DC, Zhang X, Greenstein VC, et al. An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci. 2000;41:1580–7. [PubMed] [Google Scholar]
- 8.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–51. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 9.Slotnick SD, Klein SA, Carney T, et al. Using multi-stimulus VEP source localization to obtain a retinotopic map of human primary visual cortex. Clin Neurophysiol. 1999;110:1793–800. doi: 10.1016/s1388-2457(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 10.Fortune B, Hood DC. Conventional pattern-reversal VEPs are not equivalent to summed multifocal VEPs. Invest Ophthalmol Vis Sci. 2003;44:1364–75. doi: 10.1167/iovs.02-0441. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Hood DC. Increasing the sensitivity of the multifocal visual evoked potential (mfVEP) technique: incorporating information from higher order kernels using a principal component analysis method. Doc Ophthalmol. 2004;108:211–22. doi: 10.1007/s10633-004-5323-3. [DOI] [PubMed] [Google Scholar]
- 12.Spekreijse H, Khoe LH, van der Tweel LH. A case of amblyopia. In: Arden GB, editor. the Visual System. Vol. 1972. Plenum Press; New York: pp. 141–156. [Google Scholar]
- 13.Levi DM, Manny RE. The pathophysiology of amblyopia: electrophysiological studies. Ann NY Acad Sci. 1982;388:243–263. doi: 10.1111/j.1749-6632.1982.tb50795.x. [DOI] [PubMed] [Google Scholar]
- 14.Odom JV. Amblyopia and clinical electrophysiology. In: Heckenlively JR, Arden GB, editors. Principles and Practice of Clinical Electrophysiology of Vision. Vol. 1991. Mosby-Year Book; St. Louis: pp. 589–593. [Google Scholar]
- 15.Shawkat FS, Kriss A, Timms C, et al. Comparison of pattern-onset, -reversal and –offset VEPs in treated amblyopia. Eye. 1998;12:863–869. doi: 10.1038/eye.1998.219. [DOI] [PubMed] [Google Scholar]
- 16.Hood DC, Zhang X, Hong JE, et al. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol. 2002;104:303–20. doi: 10.1023/a:1015235617673. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Hood DC, Chen CS, et al. A signal-to-noise analysis of multifocal VEP responses: an objective definition for poor records. Doc Ophthalmol. 2002;104:287–302. doi: 10.1023/a:1015220501743. [DOI] [PubMed] [Google Scholar]
- 18.Fortune B, Zhang X, Hood DC, et al. Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol. 2004;109:87–100. doi: 10.1007/s10633-004-3300-5. [DOI] [PubMed] [Google Scholar]
- 19.Hood DC, Ohri N, Yang EB, et al. Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol. 2004;109:189–99. doi: 10.1007/s10633-004-5512-0. [DOI] [PubMed] [Google Scholar]
- 20.Hood DC, Zhang X, Winn BJ. Detecting glaucomatous damage with multifocal visual evoked potentials: how can a monocular test work? J Glaucoma. 2003 Feb;12(1):3–15. doi: 10.1097/00061198-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9:480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- 22.Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa LM, Werner J, editors. The Visual Neurosciences. MIT Press; Cambridge: 2003. [Google Scholar]
- 23.Barnes GR, Hess RF, Dumoulin SO, et al. The cortical deficit in humans with strabismic amblyopia. J Physiol. 2001;533:281–297. doi: 10.1111/j.1469-7793.2001.0281b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi MY, Lee KM, Hwang JM, et al. Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. Br J Ophthalmol. 2001;8:1052–6. doi: 10.1136/bjo.85.9.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algaze A, Roberts C, Leguire L, et al. Functional magnetic resonance imaging as a tool for investigating amblyopia in the human visual cortex: a pilot study. J AAPOS. 2002;6:300–308. doi: 10.1067/mpa.2002.124902. [DOI] [PubMed] [Google Scholar]
- 26.Winn BJ, Shin E, Odel JG, et al. Interpreting the multifocal visual evoked potential: the effects of refractive errors, cataracts, and fixation errors. Br J Ophthalmol. 2005;89:340–344. doi: 10.1136/bjo.2004.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu M, Brown B, Edwards MH. Investigation of multifocal visual evoked potential in anisometropic and esotropic amblyopes. Invest Ophthalmol Vis Sci. 1998;39:2033–40. [PubMed] [Google Scholar]
- 28.Zhang W, Zhao K. Multifocal VEP difference between early- and late-onset strabismus amblyopia. Doc Ophthalmol. 2005;110:173–180. doi: 10.1007/s10633-005-4312-5. [DOI] [PubMed] [Google Scholar]
- 29.Davis AR, Sloper JJ, Neveu MM, et al. Eletrophysiological and psychophysical differences between early- and late-onset strabismic amblyopes. Invest Ophthalmol Vis Sci. 2003;44:610–617. doi: 10.1167/iovs.02-0240. [DOI] [PubMed] [Google Scholar]
- 30.Crawford ML, von Noorden GK. The effects of short-term experimental strabismus on the visual system in Macaca mulatta. Invest Ophthalmol Vis Sci. 1979;18:496–505. [PubMed] [Google Scholar]
- 31.Sloper JJ. Edrige-Green Lecture: competition and cooperation in visual development. Eye. 1993;7:319–331. doi: 10.1038/eye.1993.70. [DOI] [PubMed] [Google Scholar]
- 32.Kubova Z, Kuba M, Juran J, et al. Is the motion system relatively spared in amblyopia? Evidence from cortical evoked responses. Vision Res. 1996;36:181–190. doi: 10.1016/0042-6989(95)00055-5. [DOI] [PubMed] [Google Scholar]
- 33.Demirci H, Gezer A, Sezen F, et al. Evaluation of the functions of the parvocellular and magnocellular pathways in strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:215–221. doi: 10.3928/0191-3913-20020701-09. [DOI] [PubMed] [Google Scholar]