Abstract
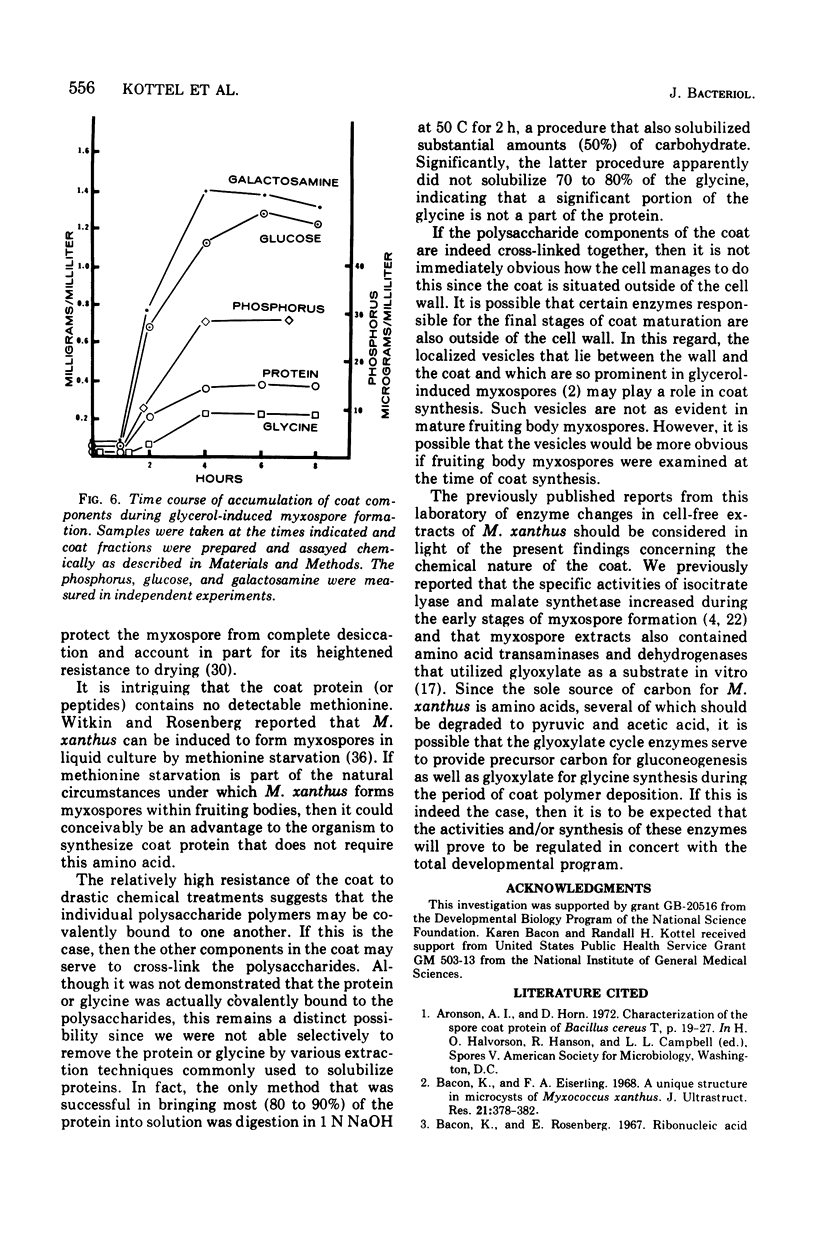
An extracellular coat from glycerol-induced myxospores of Myxococcus xanthus has been isolated and characterized. Coats were examined chemically and by using both transmission and scanning electron microscopy. On a dry weight basis, approximately 75% of the coat is polysaccharide composed entirely of galactosamine and glucose. The remainder of the coat is protein (14%), glycine (8%), and organic phosphorus (less than 1%). Coats remained morphologically intact despite boiling in 10 M urea, sodium lauryl sulfate plus beta-mercaptoethanol, or extraction with warm phenol. Coats also resisted digestion with a variety of proteolytic and polysaccharide degrading enzymes. Synthesis of myxospore coat begins approximately 1 h after the addition of glycerol to a culture. One portion of the coat is complete by 5 to 6 h but additional material consisting primarily of glucose is added after 8 h.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon K., Eiserling F. A. A unique structure in microcysts of Myxococcus xanthus. J Ultrastruct Res. 1967 Dec;21(5):378–382. doi: 10.1016/s0022-5320(67)80147-3. [DOI] [PubMed] [Google Scholar]
- Bacon K., Rosenberg E. Ribonucleic acid synthesis during morphogenesis in Myxococcus xanthus. J Bacteriol. 1967 Dec;94(6):1883–1889. doi: 10.1128/jb.94.6.1883-1889.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland J., Yeh W. K., White D., Hendricks A. Increase in glyoxylate shunt enzymes during cellular morphogenesis in Myxococcus xanthus. Can J Microbiol. 1971 Feb;17(2):209–211. doi: 10.1139/m71-036. [DOI] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. A bacteriophage for Myxococcus xanthus: isolation, characterization and relation of infectivity to host morphogenesis. J Bacteriol. 1966 Mar;91(3):1305–1313. doi: 10.1128/jb.91.3.1305-1313.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., VOELZ H. The formation and germination of microcysts in Myxococcus xanthus. J Gen Microbiol. 1962 Apr;28:81–85. doi: 10.1099/00221287-28-1-81. [DOI] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Dworkin M., Sadler W. Induction of cellular morphogenesis in Myxococcus xanthus. I. General description. J Bacteriol. 1966 Apr;91(4):1516–1519. doi: 10.1128/jb.91.4.1516-1519.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A. E. Separation and identification of monosaccharides from biological materials by thin-layer chromatography. Anal Biochem. 1968 Sep;24(3):452–461. doi: 10.1016/0003-2697(68)90152-8. [DOI] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Hanson C. W., Andreoli A. J. Some aspects of amino acid metabolism in the fruiting myxobacterium, Myxococcus xanthus. Arch Mikrobiol. 1973;92(1):1–10. doi: 10.1007/BF00409506. [DOI] [PubMed] [Google Scholar]
- Kottel R. H., Orlowski M., White D., Grutsch J. Presence of amino acid dehydrogenases and transaminases in Myxococcus xanthus during vegetative growth and myxospore formation. J Bacteriol. 1974 Aug;119(2):650–651. doi: 10.1128/jb.119.2.650-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orlowski M., Martin P., White D., Wong M. C. Changes in activity of glyoxylate cycle enzymes during myxospore development in Myxococcus xanthus. J Bacteriol. 1972 Sep;111(3):784–790. doi: 10.1128/jb.111.3.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Filer D., Zafriti D., Kindler S. H. Aspartokinase activity and the developmental cycle of Myxococcus xanthus. J Bacteriol. 1973 Jul;115(1):29–34. doi: 10.1128/jb.115.1.29-34.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Katarski M., Gottlieb P. Deoxyribonucleic acid synthesis during exponential growth and microcyst formation in Myxococcus xanthus. J Bacteriol. 1967 Apr;93(4):1402–1408. doi: 10.1128/jb.93.4.1402-1408.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder G., Lüderitz O., Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974 May 15;44(2):411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Sadler W., Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966 Apr;91(4):1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L. Comparative aspects of morphogenesis in three prokaryotic genera. Annu Rev Microbiol. 1973;27:133–153. doi: 10.1146/annurev.mi.27.100173.001025. [DOI] [PubMed] [Google Scholar]
- Shimkets L., Seale T. W. Fruiting-body formation and myxospore differentiation and germination in Mxyococcus xanthus viewed by scanning electron microscopy. J Bacteriol. 1975 Feb;121(2):711–720. doi: 10.1128/jb.121.2.711-720.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S. Z., Dworkin M. Comparative biology of prokaryotic resting cells. Adv Microb Physiol. 1973;9:153–224. doi: 10.1016/s0065-2911(08)60378-1. [DOI] [PubMed] [Google Scholar]
- Sudo S. Z., Dworkin M. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1969 Jun;98(3):883–887. doi: 10.1128/jb.98.3.883-887.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H., DWORKIN M. Fine structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962 Nov;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D., Rosenberg E. Deoxyribonucleic acid synthesis during microcyst germination in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):981–986. doi: 10.1128/jb.96.4.981-986.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]







