Abstract
Background
Patients with halitosis contact primary care practitioners, dentists, and gastroenterologists alike.
Objectives
It is unclear whether gastroesophageal reflux disease (GERD) is a risk factor for halitosis.
Design and Patients/Participants
We studied this possible relationship in the general population using the cross-sectional Study of Health in Pomerania (SHIP). Employing structured interviews, self-reported halitosis was assessed among 417 edentulous (toothless) subjects aged 40 to 81 years and among 2,588 dentate subjects aged 20 to 59 years. The presence of heartburn or acid regurgitation (GERD-related symptoms) at 4 levels (absent, mild, moderate, severe) was taken as exposure and used for logistic regression. Analyses were adjusted for relevant confounders, such as age, sex, depressive symptoms, history of chronic gastritis, history of gastric or duodenal ulcer, smoking, school education, and dental status.
Measurements and Main Results
We found a strong positive association between GERD-related symptoms and halitosis (odds ratio 12.94, 95% confidence interval (CI) 2.66–63.09, P = 0.002 for severe compared to no GERD-related symptoms) in denture-wearing subjects and a moderate, positive association between GERD-related symptoms and halitosis (odds ratio 2.24, 95% CI 1.27–3.92, P = 0.005) in dentate subjects with a clear dose–effect relationship.
Conclusions
The present study provides clear evidence for an association between GERD and halitosis. As there are effective treatments for GERD, these results suggest treatment options, such as proton pump inhibitors, for halitosis. These should be studied in randomized controlled trials.
Key words: halitosis, gastroesophageal reflux, epidemiology, periodontal diseases
INTRODUCTION
Halitosis, or bad breath, is a complaint that often creates personal discomfort and social embarrassment.1–3 The epidemiology of halitosis in the general population has been investigated in a few studies, which reported a prevalence of approximately 25%.4,5
Oral conditions, mainly tongue coating and periodontal diseases, 6 have been reported to be the most common causes of halitosis.4,7 In addition, gastroesophageal reflux disease (GERD)3,8–10 has been suggested to be a risk factor for halitosis, but the evidence was not always convincing. This association has typically been examined in combination with other gastrointestinal tract disorders5,11 or as a by-product in analyses of the relationship between oral conditions and halitosis.11 Adjusting GERD for oral conditions, however, has to be done cautiously because oral conditions may mediate or confound the relationship between GERD and halitosis. Moreover, some of these studies have not included detailed oral examinations, which are essential to identifying oral pathology, an acknowledged cause of halitosis. We also note that some of these studies have been small. Large samples may be needed to detect risk factors that are not as influential as oral conditions. The fraction of halitosis attributable to GERD could be relevant because GERD is common, with a prevalence of 10–20% in Western Europe and North America.12
One way to control for the potential roles of oral health conditions is to study edentulous (toothless) subjects. This approach has the advantage that periodontal diseases can be disregarded as a confounder of the relationship between GERD and halitosis. Another approach is to study the effect of treatment of GERD on the presence of halitosis. However, there is only 1 study comparing drug therapy with sucralfate or rabeprazole to placebo. Although treatment significantly reduced halitosis, this study was small and was performed among persons who had postcholecystectomy alkaline gastritis rather than GERD.13
An additional challenge when studying the relationship between GERD and halitosis is the so-called “bad breath paradox.” That is, in studies of clinical populations, people with objective halitosis have been unaware of the problem, whereas others are convinced that they have halitosis when no objective evidence can be found.1,7,14 Psychological conditions, such as depressive disorders, are thought to contribute to “false-positive” self-assessed halitosis in such studies.14 Fortunately, self report appears to be substantially more accurate in studies conducted in the general population.5,15–17
Therefore, we used data from a large population–based study that includes data on both oral health and psychologic symptoms to test the hypotheses that (1) an association between GERD and self-reported halitosis exists in edentulous subjects and (2) in dentate subjects, the strength of the association between GERD and self-reported halitosis is not altered by including dental variables in multivariable analyses.
MATERIALS AND METHODS
Participants
The Study of Health in Pomerania (SHIP) is a cross-sectional population-based survey in West Pomerania, a northeastern region of Germany with a total population of 212,157 inhabitants.18 A two-stage cluster sampling method using population registries was used to select German citizens residing in the study area who were 20 to 79 years of age at the time of invitation. Seven thousand eight persons were invited, including 292 of each gender in each of 12 5-year age strata. To minimize losses caused by migration or death, subjects were selected in 2 waves. The net sample (without migrated or deceased persons) comprised 6,267 eligible subjects, of whom 4,310 (68.8%) agreed to participate. The study was approved by the local ethics committee. All participants gave informed written consent. Data collection was conducted from October 1997 until May 2001 and included 4 parts: a medical examination, an oral health examination, a health-related interview, and a self administered health- and risk-factor-related questionnaire.
Outcome and Exposure Measures
We assessed the outcome variable, halitosis, using responses to the interview question: “Do you often suffer from a bad taste in your mouth or from bad breath?” The trained interviewers offered the options “yes” or “no.” “I do not know” was not offered as an option and was registered silently in the precoded format. We identified GERD-related symptoms using the single item “presence of heartburn or acid regurgitation” from the modified version of the von Zerssen’s complaints scale from the questionnaire.19This complaints scale assesses distressing mental and physical symptoms on 38 items. The scale was introduced to the subject by the following sentence: “You will find a number of complaints in the following list. Please rate the degree (absent, mild, moderate or severe) to which you suffer from each of the complaints.”
Design Options Used to Prevent Confounding
Because of the important relationship between dental health and halitosis, we stratified the population by dental status – edentulous (no teeth) versus dentate (at least 1 tooth) – and excluded the 20 individuals who did not have an oral exam (Fig. 1). To enhance homogeneity within these groups, we required dentate subjects to be less than 60 years of age and edentulous subjects to be at least 40 years old. This left 2,837 subjects (1,506 women) in the dentate sample and 498 subjects (240 women) in the edentulous sample. We excluded subjects who took drugs for acid-related disorders or who had missing data on drug use because it would be impossible to separate the effect of treatment from the opposite effect of GERD in this cross-sectional data set.20,21 We also excluded patients who did not respond to the question we used to define halitosis or the complaints scale item regarding GERD symptoms. Finally, we excluded subjects who had missing data for potential confounders including dental history, depressive symptoms, chronic gastritis, or periodontal pockets. Our final dentate sample included 1,388 women and 1,200 men. Our final edentulous sample included 206 women and 211 men.
Figure 1.
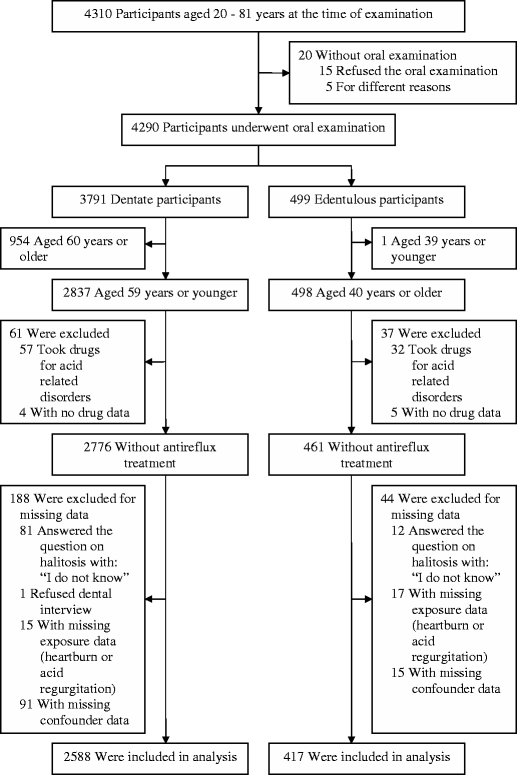
Description of the study population.
Measurement of Confounders
Putative confounders for the association between GERD and halitosis were selected based on the literature. From the interview, we used the following as confounders: age (5-year age groups), sex, chronic gastritis, history of gastric or duodenal ulcer, cigarette smoking status (never, former, and current smokers), total alcohol consumption (beer, wine, and spirits) during the past weekend (continuous scale; g alcohol), consumption of spirits during the past weekend (>/≤40 g), coffee consumption (cups per day), living together (with or without marriage), school education (<10, 10, >10 years), menopause status, history of chronic esophagitis, and gingival bleeding while tooth brushing (never, sometimes, often).
From the questionnaire, we used depressive symptoms defined by 5 items on the von Zerssen’s complaints scale: nervousness, poor concentration, inner tension, depression, and rumination (sum score with a range from 0 to 15). Difficulty in swallowing was considered as a confounder and used as an item of its own.
Eight calibrated licensed dentists performed the entire examination. Each half-year to year, calibration exercises 22 were performed on a subset of persons not connected with the study, yielding an intraclass correlation of 0.82 to 0.91 per examiner and an interrater correlation of 0.84 relative to attachment loss. Bleeding on probing, dental plaque, and calculus were dichotomously evaluated at a maximum of 6 teeth and were evaluated at 4 sites for each tooth (mesiobuccal, midbuccal, distobuccal, and midlingual). For bleeding on probing, dental plaque, and calculus, the percentage of affected sites was calculated. The status of the periodontal tissues was recorded by probing depth (distance from the gingival margin to the pocket base) and attachment loss (distance from the cemento-enamel junction to the pocket base) half-mouth using the periodontal probe PCP11 (Hu-Friedy, Chicago, IL, USA) at the 4 sites mentioned above. Increased probing depths and attachment loss indicate periodontal diseases. The number of decayed, missing, and filled tooth surfaces was registered (DMF-S). All fully erupted teeth, except third molars, were assessed, resulting in a maximum of 14 teeth per subject. The number of teeth was determined full-mouth on a maximum of 28 teeth. The presence of removable partial or complete dentures was ascertained.
Alternative Variable Definitions in Sensitivity Analyses
In alternative models, instead of depressive symptoms, we used factor scores of subscales for anxiety/depression (nervousness, depression, anxiety, rumination, inner tension, irritability, sleeplessness) and exhaustion (fatigue, excessive need of sleep, loss of energy, faintness, poor concentration, weakness) that had been used in a previous SHIP report.23 Similarly, we substituted a 3-item subscale for digestive complaints (heartburn or acid regurgitation, abdominal feeling of fullness, stomach ache) instead of the single-item heartburn or acid regurgitation.
Statistical Analysis
Data on quantitative characteristics are expressed as a mean and standard deviation. Data on qualitative characteristics are expressed as percent values or absolute numbers as indicated. For continuous data, comparisons between groups were done using the Mann–Whitney U-Test for nominal data with the chi-squared test. Logistic regression analyses were performed to test the relationship between GERD-related symptoms and halitosis, controlling for potential confounders. To reduce the effect of misclassification in halitosis, we additionally analyzed the relationship between GERD and halitosis using only persons in the lower tertile of depressive symptoms. We used the criterion of the change in the coefficient of interest to estimate the effect of a confounder or, in the case of dental variables, to estimate the masking effect on the relationship between GERD and halitosis. A substantial change was considered present if inclusion in the model led to ≥10% change in the coefficient of the GERD-related symptoms. A value of P < 0.05 was considered statistically significant. Analyses were conducted with SPSS software for Windows, version 13.0 (SPSS, Chicago, IL, USA).
RESULTS
Participant Characteristics
In dentate subjects, 565 subjects (21.8%) responded affirmatively to the question on halitosis. The frequency of GERD-related symptoms of moderate or greater severity was 15.3%. Subjects complaining of halitosis were older, more often female, and had GERD-related symptoms and esophagitis more often than subjects without complaints of halitosis (Table 1). Subjects complaining of halitosis had higher scores for depressive symptoms. They exhibited a higher percentage of chronic gastritis, as well as of history of gastric or duodenal ulcers, and were more often found to have difficulty in swallowing. They were more often former smokers and spirits-drinkers. Subjects complaining of halitosis more often had gingival bleeding while tooth brushing, more commonly had bleeding on probing, had a higher number of periodontal pockets ≥4 mm, had more attachment loss ≥3 mm, had a higher DMF-S, and had a lower number of teeth.
Table 1.
Baseline Characteristics of Dentate (n = 2588)
| Variable | Halitosis: no | Halitosis: yes |
|---|---|---|
| Number of participants | 2023 (78.2) | 565 (21.8) |
| Age, years* | 40.3 ± 11.2 | 41.6 ± 10.7 |
| Sex, female‡ | 1046 (51.7) | 342 (60.5) |
| Symptoms of gastroesophageal reflux disease‡ | ||
| Absent | 1116 (55.2) | 239 (42.3) |
| Mild | 635 (31.4) | 203 (35.9) |
| Moderate | 234 (11.6) | 96 (17.0) |
| Severe | 38 (1.9) | 27 (4.8) |
| Chronic esophagitis† | 6 (0.3) | 9 (1.6) |
| Depressive symptoms, sum score‡ | 3.8 ± 2.9 | 4.9 ± 3.1 |
| Chronic gastritis* | 89 (4.4) | 40 (7.1) |
| History of gastric or duodenal ulce‡ | 16 (0.8) | 16 (2.8) |
| Difficulty in swallowing‡ | ||
| Absent | 1712 (84.6) | 422 (74.7) |
| Mild | 271 (13.4) | 113 (20.0) |
| Moderate | 38 (1.9) | 25 (4.4) |
| Severe | 2 (0.1) | 5 (0.9) |
| Cigarette smoking status* | ||
| Never smoker | 684 (33.8) | 173 (30.6) |
| Former smoker | 552 (27.3) | 185 (32.7) |
| Current smoker | 787 (38.9) | 207 (36.6) |
| Alcohol consumption during the past weekend, g | 48.8 ± 61.5 | 53.0 ± 72.7 |
| >40 g spirits consumption during the past weekend, g† | 76 (3.8) | 37 (6.5) |
| Coffee consumption, cups per day | 2.9 ± 2.4 | 3.1 ± 2.5 |
| Living together (with or without marriage)† | 1547 (76.5) | 565 (81.9) |
| School education | ||
| <10 years | 412 (20.4) | 127 (22.5) |
| 10 years | 1191 (58.9) | 339 (60.0) |
| >10 years | 420 (20.8) | 99 (17.5) |
| Gingival bleeding while tooth brushing‡ | ||
| Never | 1215 (60.1) | 246 (43.5) |
| Sometimes | 724 (35.8) | 250 (44.2) |
| Often | 84 (4.2) | 89 (12.2) |
| Bleeding on probing, % (n = 2584)† | 33.8 ± 26.2 | 37.8 ± 27.7 |
| Dental plaque, % (n = 2579) | 48.4 ± 30.2 | 50.6 ± 29.8 |
| Calculus, % (n = 2579) | 13.8 ± 18.9 | 14.9 ± 19.8 |
| Number of periodontal pockets ≥4 mm‡ | 3.8 ± 5.5 | 4.7 ± 5.9 |
| Attachment loss ≥3 mm, % (n = 2523)‡ | 35.4 ± 32.2 | 40.6 ± 32.5 |
| Number of teeth† | 22.5 ± 5.9 | 21.9 ± 5.9 |
| DMF-S (n = 2586)† | 28.5 ± 15.7 | 30.6 ± 15.4 |
| Partial denture, upper jaw | 330 (16.3) | 106 (18.8) |
| Partial denture, lower jaw | 246 (12.2) | 62 (11.0) |
Values are number (percentage), or mean ± standard deviation
*P < 0.05
†P < 0.01
‡P < 0.001
Among the edentulous subjects, 38 subjects (9.1%) reported having halitosis. The frequency of GERD-related symptoms of moderate or greater severity was 17.5%. Subjects complaining of halitosis were more likely to have GERD-related symptoms and had more depressive symptoms than subjects without halitosis (Table 2).
Table 2.
Baseline Characteristics of Edentulous Subjects (n = 417)
| Variable | Halitosis: no | Halitosis: yes |
|---|---|---|
| Number of participants | 379 (90.9) | 38 (9.1) |
| Age, years | 69.7 ± 7.3 | 67.1 ± 9.3 |
| Sex, female | 189 (49.9) | 17 (44.7) |
| Symptoms of gastroesophageal reflux disease† | ||
| Absent | 228 (60.2) | 15 (39.5) |
| Mild | 90 (23.7) | 11 (28.9) |
| Moderate | 53 (14.0) | 8 (21.1) |
| Severe | 8 (2.1) | 4 (10.5) |
| Chronic esophagitis | 5 (1.3) | 0 (0) |
| Depressive symptoms, sum score† | 3.4 ± 3.1 | 4.6 ± 2.8 |
| Chronic gastritis | 28 (7.4) | 1 (2.6) |
| History of gastric or duodenal ulcer | 9 (2.4) | 0 (0) |
| Difficulty in swallowing | ||
| Absent | 319 (84.2) | 30 (78.9) |
| Mild | 45 (11.9) | 6 (15.8) |
| Moderate | 15 (4.0) | 2 (5.3) |
| Severe | 0 (0) | 0 (0) |
| Cigarette smoking status | ||
| Never smoker | 129 (34.0) | 12 (31.6) |
| Former smoker | 174 (45.9) | 16 (42.1) |
| Current smoker | 76 (20.1) | 10 (26.3) |
| Total alcohol consumption during the past weekend, g | 25.0 ± 41.6 | 31.2 ± 57.9 |
| >40 g spirits consumption during the past weekend, g | 11 (2.9) | 3 (7.9) |
| Coffee consumption, cups per day | 2.4 ± 1.7 | 2.3 ± 1.8 |
| Living together (with or without marriage) | 244 (64.4) | 29 (76.3) |
| School education | ||
| <10 years | 317 (83.6) | 29 (76.3) |
| 10 years | 42 (11.1) | 7 (18.4) |
| >10 years | 20 (5.3) | 2 (5.3) |
| Complete denture, upper jaw | 366 (96.6) | 36 (94.7) |
| Complete denture, lower jaw | 342 (90.2) | 33 (86.8) |
Values are number (percentage), or mean ± standard deviation
*P<0.05
†P < 0.01
Main Analyses
The results of the logistic regression in dentate subjects are given in Table 3. A dose–response relationship between GERD-related symptoms and halitosis was observed (Table 3). Depressive symptoms, but no other single variable, met our criterion for a confounder of this relationship (Table 3). In particular, the inclusion of dental variables did not reduce the coefficient of interest.
Table 3.
Association Between Gastroesophageal Reflux Related Symptoms and Halitosis in Dentate Subjects (n = 2588)
| Model | Symptom: heartburn or acid regurgitation; referent: absent | ||
|---|---|---|---|
| Mild (n = 838) OR (95%-CI) | Moderate (n = 330) OR (95%-CI) | Severe (n = 65) OR (95%-CI) | |
| 1 | 1.49 (1.21–1.84) | 1.92 (1.45–2.52) | 3.32 (1.99–5.54) |
| 2 | 1.53 (1.24–1.90) | 1.94 (1.47–2.57) | 3.33 (1.98–5.59) |
| 3 | 1.40 (1.12–1.74) | 1.75 (1.32–2.32) | 2.71 (1.60–4.60) |
| 4 | 1.37 (1.10–1.70) | 1.70 (1.27–2.26) | 2.67 (1.57–4.55) |
| 5 | 1.31 (1.05–1.64) | 1.62 (1.21–2.17) | 2.47 (1.44–4.23) |
| 6 | 1.31 (1.05–1.63) | 1.60 (1.19–2.14) | 2.49 (1.44–4.31) |
| 7 | 1.29 (1.03–1.61) | 1.56 (1.16–2.09) | 2.45 (1.42–4.25) |
| 8 | 1.28 (1.02–1.60) | 1.48 (1.09–1.99) | 2.24 (1.27–3.92) |
Logistic regression analysis (dependent variable halitosis). Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: adjusted for variables in model 2 and depressive symptoms. Model 4: adjusted for variables in model 3, chronic gastritis, and history of gastric or duodenal ulcer. Model 5: adjusted for variables in model 4 and difficulty in swallowing. Model 6: adjusted for variables in model 5, spirits consumption during the past weekend, coffee consumption, and smoking status. Model 7: adjusted for variables in model 6, living together, and school education. Model 8: adjusted for variables in model 7, number of periodontal pockets, and gingival bleeding while tooth brushing
OR = odds ratio, CI = confidence interval
The results of the logistic regression in edentulous subjects are given in Table 4. A similar dose-dependent relationship was found between increasing severity of GERD-related symptoms and halitosis after controlling for putative confounders. Based on the age- and gender-adjusted model, disorders of the gastrointestinal tract, but not oral health, met our statistical criterion for a confounder.
Table 4.
Association Between Gastroesophageal Reflux Related Symptoms and Self-reported Halitosis in Edentulous Subjects (n = 417)
| Model | Symptom: heartburn or acid regurgitation referent: absent | ||
|---|---|---|---|
| Mild (n = 101) OR (95%-CI) | Moderate (n = 61) OR (95%-CI) | Severe (n = 12) OR (95%-CI) | |
| 1 | 1.86 (0.82–4.20) | 2.29 (0.92–5.69) | 7.60 (2.05–28.14) |
| 2 | 2.09 (0.89–4.90) | 2.87 (1.10–7.48) | 11.22 (2.71–46.49) |
| 3 | 1.79 (0.75–4.29) | 2.45 (0.92–6.54) | 10.44 (2.48–43.96) |
| 4 | 2.02 (0.84–4.87) | 2.71 (1.01–7.32) | 14.19 (3.09–65.13) |
| 5 | 2.08 (0.86–5.02) | 2.73 (1.01–7.39) | 15.03 (3.26–69.38) |
| 6 | 2.01 (0.83–4.90) | 2.68 (0.98–7.31) | 13.75 (2.93–64.55) |
| 7 | 2.04 (0.83–5.01) | 2.78 (1.00–7.65) | 13.58 (2.84–64.91) |
| 8 | 2.06 (0.84–5.08) | 2.74 (0.99–7.62) | 12.94 (2.66–63.09) |
Logistic regression analysis (dependent variable: self-reported halitosis). Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: adjusted for variables in model 2 and depressive symptoms. Model 4: adjusted for variables in model 3, chronic gastritis, and history of gastric or duodenal ulcer. Model 5: adjusted for variables in model 4 and difficulty in swallowing. Model 6: adjusted for variables in model 5, spirits consumption during the past weekend, coffee consumption, and smoking status. Model 7: adjusted for variables in model 6, living together, and school education. Model 8: adjusted for variables in model 7, complete denture upper jaw, and complete denture lower jaw
OR = odds ratio, CI = confidence interval
Ancillary Analyses
Although oral conditions were not identified as confounders for the association between GERD-related symptoms and halitosis, they were major determinants of halitosis in dentate subjects. Compared to subjects without bleeding while tooth brushing, the odds ratio for subjects who experienced bleeding often was 3.42 (95% confidence interval: 2.35–4.98) in the final model. The results were similar when the sample was restricted to participants who had at least 6 teeth (n = 2,502).
The relationship between GERD-related symptoms and halitosis was not weakened when the analysis was restricted to dentate subjects in the lower tertile of depressive symptoms (i.e., with a maximum of 2 mild depressive symptoms or 1 moderate depressive symptom, n = 878; data not shown). Our results were similar when we used the anxiety/depression and exhaustion subscales instead of depressive symptoms. When we used the expanded set of digestive complaints instead of the single heartburn or acid regurgitation question, we still found a positive association between these symptoms and halitosis. However, unlike the single heartburn and acid regurgitation item, there was no dose–response relationship between halitosis and these additional digestive symptoms (abdominal feeling of fullness, stomach ache).
DISCUSSION
We found a positive association between GERD-related symptoms and self-reported halitosis in both dentate and edentulous subjects. This association was lower in magnitude among dentate subjects but still quite clear. The dose–response relationship we observed suggests that the risk of halitosis rises with the severity of the GERD-related symptoms. Thus, the present study suggests a relationship between GERD and halitosis in the general population.
An association between GERD and halitosis is biologically plausible for 3 reasons. First, it has been proposed that odor from the posterior tongue dorsum derives mainly from postnasal drip accumulating there.24 In GERD, acidic contents of the stomach can reach the nasopharynx and cause irritation of its walls, resulting in postnasal drip. Thus, postnasal drip and tongue coating may be mediators of the pathway between GERD and halitosis. Second, impaired lower esophageal sphincter function in subjects with GERD allows intestinal gas and stomach contents to reflux into the esophagus,10 which might produce malodor. Third, halitosis may be produced by direct acid-peptic injury to susceptible supraesophageal tissue.
A relationship between GERD and halitosis has previously been suggested,10,11 but a dose–response relationship could not be demonstrated. In addition, the link was not always convincing or corrected for confounding factors such as medication or dental status. Most studies investigating halitosis have focused on the relationship with oral conditions and hygiene.4,25 For oral conditions, we assessed periodontal diseases but not tongue coating, which we consider as a mediator in the relationship between GERD and halitosis (see above). In concordance with other reports,4,7,26 we found a strong relationship between periodontal diseases and halitosis in dentate subjects. However, controlling for periodontal diseases did not weaken the relationship between GERD-related symptoms and halitosis.
In line with other studies, the proportion of dentate subjects with halitosis was close to 25%.4,5 In contrast, the proportion of edentulous subjects with halitosis was clearly lower than 25%. There are at least 3 explanations for this finding: First, periodontal diseases cannot contribute to halitosis. Second, several studies have found impairments in taste and smell acuity in older persons.27 Third, older persons may be more concerned with conditions other than halitosis.
A limitation of our study is the self-assessment of halitosis. Contradicting results have been reported regarding the agreement between objective evaluation and self-perceived halitosis. Previous studies in clinical populations suggest poor agreement,1,4 leading to the concept that there is a “bad breath paradox—people suffering from breath odor being completely unaware of their problem, whereas others are convinced that they have a problem where nothing was found”7 Conversely, self-assessment of halitosis appears to be both more reliable and more valid in a general population than in highly selected patients.4,5,14 A recent study 15 suggested that self-estimated halitosis is a fair to good predictor of the presence of objective halitosis, which was considered present if the average level of volatile sulfur compounds was ≥125 ppb and the organoleptic measurement using a 0–5-point scale was ≥2 (sensitivity = 89.0%; specificity = 61.4%). Moreover, “objective measurement [sulphide monitoring or gas chromatography] of breath components is rarely used in routine clinical practice, as it is expensive and time consuming.”28 Indeed, from a public health point of view, the subjective perception of a health problem might be more relevant than an objective measure because awareness is one of the key processes that have to be activated before people reach a sufficient motivational stage to change behavior and accept treatment.29
In our study, this possible limitation was diminished by 2 strategies. First, we controlled for depressive symptoms using a 5-item scale derived from the von Zerssen’s complaints scale. Second, we examined the relationship between GERD and halitosis in nondepressive subjects. The relationship between halitosis and GERD-related symptoms persisted in this homogeneous group, which further supports the relationship between GERD and halitosis.
A second potential source of bias is our definition of halitosis. Subjects may experience GERD as bad taste, which might generate a positive response to the question we used to define halitosis—“Do you often suffer from a bad taste in your mouth or from bad breath?” According to recent reviews regarding the neurocognitive aspects of oral sensation, taste and smell interact like a synesthesia rather than in isolation.30,31 Perceptual confusion between the senses of smell and taste was reported.32 Additionally, certain odors may be described in terms of taste.31 Thus, it is appropriate that a halitosis question also covers bad taste to avoid missing halitosis. Conversely, subjects with GERD may answer our question on halitosis affirmatively more frequently than subjects without GERD. This so-called differential measurement error in halitosis with respect to GERD may lead to an over- or underestimation of the association of interest.33 Provided there exists an odor–taste synesthesia,31 GERD would be assumed to be a risk factor for halitosis, even if the association between halitosis and the exposure was based only on bad taste as a symptom of GERD. Bad taste’s characteristic as a symptom for a disease (GERD) does not exclude its relationship to another disease (halitosis).
A further limitation of our study was the use of a nonspecific questionnaire to assess GERD rather than a specific questionnaire, endoscopy, or direct-function test with pH measurements. Thus, from the von Zerssen’s complaints scale, we chose a priori heartburn and acid regurgitation as the main symptom of GERD to increase specificity. Several studies have demonstrated that questions covering heartburn and acid regurgitation exhibit good validity and reliability in identifying subjects with GERD.34,35 More recently, it was recognized that additional upper gastrointestinal symptoms such as abdominal pain and distension could also be indicative of GERD12 and we therefore used those symptoms in ancillary analysis. Their associations with halitosis, however, were less conclusive than that for the better defined GERD-related symptoms heartburn and acid regurgitation. As abdominal pain and distension could also indicate non-GERD abdominal disorders, such as ulcer disease, irritable bowel syndrome, or functional dyspepsia, we believe that GERD, rather than non-GERD diseases represents a risk factor for halitosis. Residual confounding from other disorders of the gastrointestinal tract cannot completely be excluded. However, our results were not changed by adjusting for several such putative confounders, including chronic gastritis, history of gastric or duodenal ulcer, and difficulty in swallowing.34
Finally, we did not use a validated depression scale to measure depression. In the main analyses, we used a prespecified depression scale that we derived using complaints that we considered likely to be depressive symptoms. We believe this scale is a reasonable proxy for depressed mood. The limitations of a proxy remain, but we note that our results did not change when we included the scores of 2 statistically derived factors (anxiety and depression; exhaustion) from the von Zerssen’s complaints scale in sensitivity analyses.
Notwithstanding these limitations, several strengths of our study merit consideration. We used a large population-based sample, allowing for a high degree of generalizability of the present findings. We confirmed the relationship between GERD-related symptoms and halitosis in both edentulous and dentate subjects. By excluding subjects with antireflux treatment, we investigated the association between GERD-related symptoms and halitosis on a subclinical level of GERD rather than for a clinically manifest disease. We believe that the association of interest would be stronger for the latter.
In summary, our findings suggest that GERD increases the risk of halitosis in both edentulous and dentate subjects. Because of the increasing prevalence of overweightness and obesity, which are associated with GERD,36 this may be of increasing public health relevance. Moreover, GERD is a treatable condition, suggesting that antireflux therapy for halitosis should be studied.
ACKNOWLEDGMENTS
This work is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant no. ZZ9603), the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg-West Pomerania.
Conflicts of Interest None disclosed.
Abbreviations
- DMF-S
number of decayed, missing and filled surfaces
- GERD
gastroesophageal reflux disease
- SHIP
Study of Health in Pomerania
REFERENCES
- 1.Rosenberg M, Kozlovsky A, Gelernter I, et al. Self-estimation of oral malodor. J Dent Res. 1995;74:1577–82. [DOI] [PubMed]
- 2.Delanghe G, Ghyselen L, Feenstra L, van Steenberghe D. Experiences of a Belgian multidisciplinary breath odour clinic. In: van Steenberghe D, Rosenberg M, eds. Bad Breath. A Multidisciplinary Approach. Leuven: Leuven University Press; 1996:199–208.
- 3.Bosy A. Oral malodor: philosophical and practical aspects. J Can Dent Assoc. 1997;63:196–201. [PubMed]
- 4.Miyazaki H, Fujita C, Soh I, Takehara T. Relationship between volatile sulphur compounds and oral conditions in the general Japanese population. In: van Steenberghe D, Rosenberg M, eds. Bad Breath. A Multidisciplinary Approach. Leuven: Leuven University Press, 1996.
- 5.Al-Ansari JM, Boodai H, Al-Sumait N, Al-Khabbaz AK, Al-Shammari KF, Salako N. Factors associated with self-reported halitosis in Kuwaiti patients. J Dent. 2006;34:444–9. [DOI] [PubMed]
- 6.Philstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. [DOI] [PubMed]
- 7.Delanghe G, Ghyselen J, van Steenberghe D, Feenstra L. Multidiscliplinary breath-odour clinic. Lancet. 1997;350:187. [DOI] [PubMed]
- 8.Tonzetich J. Oral malodour: an indicator of health status and oral cleanliness. Int Dent J. 1978;28:309–19. [PubMed]
- 9.Napierkowski J, Wong RKH. Extraesophageal manifestations of GERD. Am J Med Sci. 2003;326:285–99. [DOI] [PubMed]
- 10.van Steenberghe D. Breath Malodor: A step by step approach. Copenhagen: Quintessence Publishing; 2004.
- 11.Ben-Aryeh H, Horowitz G, Nir D, Laufer D. Halitosis: an interdisciplinary approach. Am J Otolaryngology. 1998;19:8–11. [DOI] [PubMed]
- 12.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones , Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. [DOI] [PubMed]
- 13.Santarelli L, Gabrielli M, Candelli M, et al. Post-cholecystectomy alkaline reactive gastritis: a randomized trial comparing sucralfate versus rabeprazole or no treatment. Eur J Gastroenterol Hepatol. 2003;15:975–9. [DOI] [PubMed]
- 14.Eli I, Baht R, Koriat H, Rosenberg M. Self-perception of breath odor. J Am Dent Assoc. 2001;132:621–6. [DOI] [PubMed]
- 15.Iwanicka-Grzegorek E, Michalik J, Kepa J, Wierzbicka M, Aleksinski M, Pierzynowsk E. Subjective patients` opinion and evaluation of halitosis using halimeter and organoleptic scores. Oral Dis. 2005;11:86–8. [DOI] [PubMed]
- 16.Greenstein RBN, Goldberg S, Marku-Cohen S, Sterer N, Rosenberg M. Reduction of oral malodor by oxidizing lozenges. J Periodontol. 1997;68:1176–81. [DOI] [PubMed]
- 17.Rosenberg M, Kozlovsky A, Wind Y, Mindel E. Self-assessment of oral malodor 1 year following initial consultation. Quintessence Int. 1999;30:324–7. [PubMed]
- 18.John U, Greiner B, Hensel E, et al. Study of health in Pomerania (SHIP): a health related examination survey in an east German region: objectives and design. Soz Präventivmed. 2001;46:186–94. [DOI] [PubMed]
- 19.Grabe HJ, Völzke H, Lüdemann J,et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand. 2005;112:286–93. [DOI] [PubMed]
- 20.D’Agostino RB Jr, D’Agostino RB Sr. Estimating treatment effects using observational data. JAMA. 2007;297:314–6. [DOI] [PubMed]
- 21.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–85. [DOI] [PMC free article] [PubMed]
- 22.Hensel E, Gesch D, Biffar R, et al. Study of health in Pomerania (SHIP): a health survey in an east German region. Objectives and design of the oral health section. Quintessence Int. 2003;34:370–8. [PubMed]
- 23.Konerding U, Kohlmann T, Alte D, John U. Subjective health complaints, health-related quality of life and physician visits: results of the study of health in Pomerania (SHIP). Soz Präventivmed. 2006;51:162–73. [DOI] [PubMed]
- 24.Rosenberg M, Leib E. Experiences of an Israeli Malodor Clinic. In: Rosenberg M, ed. Bad Breath. Research Perspectives. Tel Aviv: Ramot; 1997:137–48.
- 25.Liu XN, Shinada K, Chen XC, Zhang BX, Yaegaki K, Kawaguchi Y. Oral malodour-related parameters in the Chinese population. J Clin Periodontol. 2006;33:31–6. [DOI] [PubMed]
- 26.Morita M, Wang H-L. Association between oral malodor and adult periodontitis: a review. J Clin Periodontol. 2001;28:813–9. [DOI] [PubMed]
- 27.Finkelstein JA, Schiffman SS. Workshop on taste and smell in the elderly. Physiol Behav. 1999;66:173–6. [DOI] [PubMed]
- 28.Porter SR, Scully C. Oral malodour (halitosis). BMJ. 2006;333:632–5. [DOI] [PMC free article] [PubMed]
- 29.Prochaska JO, Velicer WF. The transtheoretical model. Am J Health Prom. 1997;12:6–7. [DOI] [PubMed]
- 30.Duffy VB. Variation in oral sensation: implications for diet and health. Curr Opin Gastroenterol. 2007;23:171–7. [DOI] [PubMed]
- 31.Verhagen JV, Engelen L. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev. 2006;30:613–50. [DOI] [PubMed]
- 32.Stevenson RJ, Prescott J, Boakes RA. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem Senses. 1999;24:627–35. [DOI] [PubMed]
- 33.Kelsey JL, Whittemore AS, Evans AS, Thompson WD. Methods in observational epidemiology. New York: Oxford University Press; 1996:348–9.
- 34.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–47. [DOI] [PubMed]
- 35.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–50. [DOI] [PubMed]
- 36.Jacobsen BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–8. [DOI] [PMC free article] [PubMed]


