Summary
Objective
Little is known about associations between psychiatric comorbidity and hospital mortality for acute medical conditions. This study examined if associations varied according to the method of identifying psychiatric comorbidity and agreement between the different methods.
Patients/Participants
The sample included 31,218 consecutive admissions to 168 Veterans Affairs facilities in 2004 with a principle diagnosis of congestive heart failure (CHF) or pneumonia. Psychiatric comorbidity was identified by: (1) secondary diagnosis codes from index admission, (2) prior outpatient diagnosis codes, (3) and prior mental health clinic visits. Generalized estimating equations (GEE) adjusted in-hospital mortality for demographics, comorbidity, and severity of illness, as measured by laboratory data.
Measurements and Main Results
Rates of psychiatric comorbidities were 9.0% using inpatient diagnosis codes, 27.4% using outpatient diagnosis codes, and 31.0% using mental health visits for CHF and 14.5%, 33.1%, and 34.1%, respectively, for pneumonia. Agreement was highest for outpatient codes and mental health visits (κ = 0.51 for pneumonia and 0.50 for CHF). In GEE analyses, the adjusted odds of death for patients with psychiatric comorbidity were lower when such comorbidity was identified by mental health visits for both pneumonia (odds ratio [OR] = 0.85; P = .009) and CHF (OR = 0.70; P < .001) and by inpatient diagnosis for pneumonia (OR = 0.63; P ≤ .001) but not for CHF (OR = 0.75; P = .128). The odds of death were similar (P > .2) for psychiatric comorbidity as identified by outpatient codes for pneumonia (OR = 1.04) and CHF (OR = 0.93).
Conclusions
The method used to identify psychiatric comorbidities in acute medical populations has a strong influence on the rates of identification and the associations between psychiatric illnesses with hospital mortality.
KEY WORDS: psychiatric comorbidity, hospital mortality, heart failure, pneumonia
INTRODUCTION
The 12-month period prevalence rates for psychiatric disorders in the United States have been recently estimated as high as 26%.1 Prior studies demonstrate that patients with psychiatric illnesses have lower long-term survival,2 higher rates of various medical comorbidities,3 and worse short-term outcomes after Surgery.4 Additional research has found adverse long-term risks in patients with acute coronary syndromes, who also have with depression.5,6 Such patients may also be at risk for lower quality of care7 and may be less likely to undergo coronary revascularization after acute myocardial infarction.8–10
Much of the prior research on the impact of psychiatric comorbidities on outcomes of hospitalization has relied on secondary data sources, including administrative data.1,3,4,7,11 These studies have used several approaches for identifying patients with psychiatric comorbidities, such as secondary diagnosis codes from inpatient hospitalizations and diagnosis codes from ambulatory encounters. However, no studies have compared these approaches for identifying psychiatric comorbidities or how these approaches may impact associations between the comorbidities and outcomes of hospital care.
To address these limitations, we conducted a study of patients admitted to VA hospitals for treatment of 2 common conditions—pneumonia and congestive heart failure (CHF). We capitalized on the rich infrastructure of VA databases available for analysis, including inpatient and outpatient encounter data and laboratory data, and identified patients with psychiatric comorbidity using 3 distinct approaches. This study specifically sought to understand how the different approaches affected rates of identification of psychiatric illness and associations between psychiatric comorbidity and hospital mortality.
METHODS
Data Sources
Data for the study was drawn from 3 VA databases: (1) the patient treatment file (PTF), (2) the outpatient care files, and (3) the decision support system (DSS) laboratory files. The PTFs contain data on all VA hospitalizations. Data elements include: demographic and socioeconomic information, presence of a disability related to military service, primary and secondary diagnoses and procedures, as defined by International Classification of Diseases, ninth Clinical Modification (ICD-9-CM) codes, admission source (e.g., transfer from outside facility, emergency department), admission and discharge dates, and disposition at discharge. The outpatient care files include claim information for all outpatient encounters. Data elements include: dates of visits, type of clinic (e.g., primary care, mental health), and associated ICD-9-CM codes. The DSS laboratory files contain the results of selected laboratory tests for all patient encounters. Unique identifiers allowed merging of patient-level information across the databases.
Patient Population
The PTF was used to identify 43,305 consecutive admissions with a principle diagnosis of CHF (ICD-9-CM 428; n = 21,794) or pneumonia (ICD-9-CM 480–487; n = 21,511) to 168 VHA facilities during fiscal year 2004 (October 1, 2003 to September 30, 2004). For patients with more than 1 hospitalization, we randomly selected a single admission for analysis and excluded 4,849 admissions for CHF and 2,226 pneumonia admissions that represented repeat admissions. We then excluded patients without any VA outpatient visits during months 24–36 before admission (n = 1,799 for CHF and n = 2,358 for pneumonia), leaving the final study samples of 16,927 pneumonia patients and 15,146 CHF patients. This ensured that each patient was eligible for VA care during the 2 years before admission.
Study Variables
The primary endpoint was in-hospital death. The primary independent variable was the presence of a psychiatric comorbidity, which was defined using 3 different approaches: (1) ICD-9-CM codes recorded as secondary diagnosis codes from the index hospitalization, (2) ICD-9-CM codes captured on 1 or more outpatient encounters to primary care or other clinics during the 2 years preceding admission, and (3) 1 or more visits to a mental health clinic (e.g., psychiatry, psychology) during the 2 years preceding admission, without regard to the diagnoses recorded during these visits. We chose these 3 approaches as each has been used by prior investigators. For the first 2 approaches, diagnosis codes identified patients with 5 major categories of psychiatric illness: (1) depressive disorders (ICD-9-CM 296.20–36, 311, 300.4), (2) anxiety disorders (ICD-9-CM 300.00-02, 293.84, 309.28, 309.21–23), (3) posttraumatic stress disorder (PTSD; ICD-9-CM 309.81), (4) bipolar disorders (296.0–19, 296.37–89), and (5) psychotic disorders, including schizophrenia and schizoaffective disorders (ICD-9-CM 295). The 5 categories were not mutually exclusive.
Other variables used to describe the study population and adjust for differences in risk included: age, race (categorized as white, black, Hispanic, or missing), gender, admission source, comorbid medical conditions, as defined using the diagnostic algorithms of Elixhauser,12 which define the presence of conditions that are likely to have existed before hospitalization, and the results of 8 selected laboratory tests (serum sodium, blood urea nitrogen, creatnine, glucose, albumin, and total bilirubin, hemoglobin, and white blood cell count) obtained within a 48-hour window of hospital admission. These laboratory tests were selected because each is commonly obtained on hospitalized patients at the time of admission. For each laboratory test, we selected the most abnormal value, based on weightings used in the Acute Physiology and Chronic Disease Health Evaluation (APACHE) III methodology.13 Weights (i.e., point values) associated with the APACHE III categorizations were then summed to create an overall laboratory severity score. Missing laboratory values were considered to be normal. For patients with severity scores of 0, a minimum of 4 laboratory values were required to calculate a score. Patients with a score of 0 and who were missing more than 4 laboratory values were considered to have missing laboratory severity scores.
Data Analysis
The analysis involved several steps. First, we assessed the agreement between the 3 methods of identifying psychiatric illness using the kappa statistic. Kappa statistics for each possible pairwise comparison of methods were determined. Second, we compared patient characteristics and unadjusted in-hospital mortality rates in patients with and without psychiatric comorbidity, as identified by each of the 3 methods, using chi-square and t tests. Third, we identified demographic and clinical characteristics that were independently associated (P < .05) with mortality for pneumonia and CHF using stepwise logistic regression. Continuous variables (e.g., age, laboratory severity score) were examined as continuous variables, as quadratic functions, and as a series of indicator variables. The form of the variable that maximized model discrimination, as measured by the c statistic,14 and model calibration, as measured by the Hosmer–Lemeshow (HL) statistic, was retained in the final model. In the models, missing laboratory severity scores were analyzed as an indicator variable.
c statistics of the models for CHF and pneumonia were 0.782 and 0.781, respectively. HL statistics were nonsignificant (chi-square = 6.0 [P = .64, 10 df] for CHF and 7.3 [P = .51, 10 df] for pneumonia). These models were then used to generate a predicted probability of death for each patient.
Finally, we determined the relationships between the presence of psychiatric comorbidity and in-hospital mortality using generalized estimating equations (GEEs) with an exchangeable working correlation matrix. The GEE models included the logit of the predicted probability of death for each patient, based on the risk-adjustment models, and indicator variables for the presence of a psychiatric illness and accounted for clustering of patients within individual hospitals. Separate GEE analyses were conducted for each of the 3 methods of identifying psychiatric comorbidity. The adjusted odds of death associated with the presence of a psychiatric illness were estimated by taking the exponent of the regression coefficients for each of the indicator variables. Additional GEE analyses were conducted that included separate indicator variables for the 5 individual categories of psychiatric comorbidity. All analyses were performed using SAS statistical software version 9.1 (Cary, NC). The study was approved by the both the University of Iowa Institutional Review Board and the Research and Development Committee at the Iowa City VA Medical Center.
RESULTS
Demographic and clinical characteristics of study patients are shown in Table 1. For the 2 conditions in aggregate, patients had a mean age of 70.4 years, and 98% were male. Sixty-one percent of patients were white and 16% were black; race was missing in 22%. Psychiatric comorbidities were identified in 12% (n = 3,760) of patients using inpatient secondary diagnosis codes, 32% of patients (n = 10,252) using prior outpatient diagnoses, and 31% of patients (n = 9,876) using prior mental health clinic visits. Rates of identifying individual psychiatric conditions were also higher using outpatient codes than inpatient codes for each of the 5 conditions (Fig. 1).
Table 1.
Characteristics of Study Patients with Congestive Heart Failure (CHF) and Pneumonia
| CHF (n = 15,146) | Pneumonia (n = 16,927) | |
|---|---|---|
| Mean age, in years ± SD | 70.7 ± 11.5 | 70.3 ± 12.7 |
| Gender: male (%) | 16,622 (98.1) | 18,776 (97.4) |
| Race (%) | ||
| White | 8,804 (58.1) | 10,691 (63.2) |
| Black | 2,930 (19.4) | 2,207 (13.0) |
| Hispanic | 202 (1.3) | 190 (1.1) |
| Missing | 3,215 (21.2) | 3,820 (22.6) |
| Admission from skilled care facility (%) | 350 (2.3) | 1,128 (6.6) |
| Service-connected disability (%) | 5,038 (33.3) | 6,111 (36.1) |
| Comorbid medical conditions (%) | ||
| Hypertension | 8,062 (50.4) | 7,759 (43.6) |
| Chronic obstructive lung disease | 5,675 (34.5) | 7,963 (44.8) |
| Diabetes | 7,242 (45.3) | 4,824 (27.2) |
| Renal failure | 1,555 (9.7) | 1,142 (6.4) |
| Obesity | 1,059 (6.6) | 446 (2.5) |
| Liver disease | 475 (2.9) | 643 (3.6) |
| Neurological disease | 403 (2.5) | 1,309 (7.4) |
| Metastatic disease | 149 (0.9) | 582 (3.3) |
| Coagulapathy | 351 (2.2) | 483 (2.7) |
| Weight loss | 223 (1.4) | 633 (3.6) |
| Fluid disorder | 2,242 (14.0) | 4,306 (24.2) |
| Anemia | 113 (0.7) | 106 (0.6) |
| Mean laboratory severity score (SD) | 31.2 (10.9) | 29.2 (11.3) |
| Mean length of stay ± SD (days) | 6.4 ± 9.8 | 7.8 ± 11.8 |
| Psychiatric comorbidity, N, (%) | ||
| Inpatient secondary diagnosis codes | 1,435 (9.0) | 2,443 (13.7) |
| Depression | 776 (4.9) | 1,251 (7.1) |
| Anxiety | 229 (1.4) | 345 (2.0) |
| PTSD | 232 (1.5) | 403 (2.3) |
| Bipolar | 87 (0.5) | 246 (1.4) |
| Psychosis | 212 (1.3) | 463 (2.6) |
| Prior outpatient diagnosis codes | 4,939 (30.9) | 5,807 (32.7) |
| Depression | 3,263 (20.4) | 3,342 (18.8) |
| Anxiety | 1,867 (11.7) | 2,297 (13.0) |
| PTSD | 971 (6.1) | 628 (6.5) |
| Bipolar | 278 (1.7) | 455 (2.6) |
| Psychosis | 367 (2.3) | 628 (3.5) |
| Prior mental health clinic visits | 4,398 (27.5) | 5,974 (33.6) |
Figure 1.
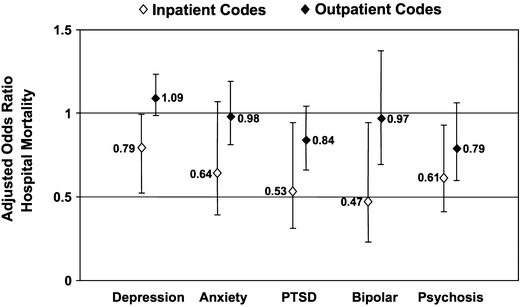
Adjusted odds of death of patients for individual psychiatric conditions as identified by inpatient or prior outpatient diagnosis codes.
Individuals with psychiatric comorbidity tended to be younger, have lower laboratory severity scores, and have lower predicted probabilities of death for all 3 methods (Table 2). Agreement between the 3 methods was highest for outpatient diagnosis and mental health visits; the kappa statistics of 0.51 (P < .001) for pneumonia and 0.50 for CHF (P < .001) indicated moderate agreement.15 Of the 10,252 patients identified by outpatient diagnosis codes, 66% (n = 6,690) had 1 or more mental health visits, whereas of the 9,876 patients with mental health visits, 32% (n = 3,186) did not have an outpatient diagnosis code for 1 of the 5 major psychiatric comorbidities that were studied. In contrast, agreement was only fair for inpatient and outpatient diagnosis codes (κ = 0.31 [P < .001] for pneumonia and 0.24 [P < .001] for CHF) and for inpatient diagnosis codes and mental health visits (κ = 0.20 [P < .001] for pneumonia and 0.25 [P < .001] for CHF).
Table 2.
Differences in Markers of Severity of Illness in Patients with and without Psychiatric Comorbidity, as Identified by the Three Different Methods
| Method of Identifying Psychiatric Comorbidity | Inpatient Diagnosis Codes | Outpatient Diagnosis Codes | Mental Health Visits | |||
|---|---|---|---|---|---|---|
| Yes, n = 1,435 | No, n = 14,566 | Yes, n = 4,939 | No, n = 11,062 | Yes, n = 4,398 | No, n = 11,603 | |
| Congestive heart failure* | ||||||
| Mean Age (SD) | 66.0 (12.1) | 71.1 (11.1) | 68.1 (12.0) | 72.3 (12.7) | 66.0 (11.9) | 72.7 (10.4) |
| Mean laboratory severity score (SD) | 29.1 (10.9) | 31.4 (10.9) | 30.6 (11.0) | 31.5 (10.8) | 30.2 (11.0) | 31.6 (10.8) |
| Mean predicted risk of death (%) (SD) | 3.3 (4.3) | 4.9 (6.5) | 4.3 (6.4) | 4.8 (6.3) | 5.4 (6.2) | 7.0 (6.9) |
| Pneumonia* | ||||||
| Mean age (SD) | 65.3 (12.7) | 71.3 (12.0) | 67.4 (13.0) | 72.1 (11.4) | 65.3 (13.0 | 73.0 (11.1) |
| Mean laboratory severity score (SD) | 26.3 (10.5) | 29.7 (11.3) | 28.1 (11.2) | 29.7 (11.3) | 28.0 (11.3) | 29.9 (11.3) |
| Mean predicted risk of death (%) (SD) | 6.5 (7.1) | 9.5 (10.5) | 7.7 (9.4) | 9.8 (10.5) | 7.4 (9.4) | 9.8 (10.5) |
*Differences mean ages, laboratory scores, and predicted risks of death for patients with and without psychiatric comorbidity were significant (P < .001) for each of the methods of identifying psychiatric comorbidity for both pneumonia and CHF
Overall, 6.9% of patients died during the admission; mortality was higher for pneumonia than for CHF (9.2% versus 4.3%; P < .001). Unadjusted mortality rates were lower (P < .05) for individuals with psychiatric comorbidities for each of the 3 methods, although magnitudes of the differences tended to be highest for inpatient diagnosis and mental health visits (Table 3). In GEE analyses controlling for demographic and clinical confounders, the adjusted odds of death remained lower for patients with psychiatric comorbidity, as identified by mental health visits for both pneumonia and CHF, and as identified by inpatient secondary diagnosis codes for pneumonia (Table 3). The adjusted odds of death were similar for patients with psychiatric comorbidity, as identified by prior outpatient codes. These results were generally similar in analyses that examined individual psychiatric comorbidities (Fig. 2) identified by inpatient or outpatient diagnosis codes.
Table 3.
Unadjusted Mortality in Patients with and without Psychiatric Comorbidity and Adjusted Odds of Death, for the Three Identification Methods
| Psychiatric Comorbidity Present Number of Deaths/(%) | Psychiatric Comorbidity Absent/Number of Deaths (%) | P Value | Adjusted Odds* of Death (95% CI) [P value] | |
|---|---|---|---|---|
| Congestive heart failure | ||||
| Inpatient diagnosis codes | 36 (2.5) | 672 (4.6) | <.001 | 0.75 (0.52–1.08) [.13] |
| Outpatient diagnosis codes | 192 (3.9) | 516 (4.7) | .027 | 0.93 (0.77–1.12) [.45] |
| Mental health clinic visits | 124 (2.8) | 584 (5.0) | <.001 | 0.70 (0.57–0.86) [<.001] |
| Pneumonia | ||||
| Inpatient diagnosis codes | 109 (4.5) | 1,506 (9.8) | <.001 | 0.63 (0.52–0.77) [=.001] |
| Outpatient diagnosis codes | 455 (7.8) | 1,160 (9.7) | <.001 | 1.04 (0.93–1.16) [.46] |
| Mental health clinic visits | 411 (6.9) | 1,204 (10.2) | <.001 | 0.85 (0.75–0.96) [.01] |
*Adjusted odds of death in patients with psychiatric comorbidity, relative to patients without psychiatric comorbidity, as determined by generalized estimating equations, adjusting for demographic and clinical confounders, and accounting for clustering of patients within hospitals.
To gain further insight into the differences in mortality between outpatient diagnosis codes and mental health clinic visits, we assessed the odds of death in patients who were identified as having psychiatric comorbidities by both methods, by 1 of the methods, but not the other, and by neither method (Table 4). These analyses found that the lowest risks of death for both pneumonia and CHF were observed in patients seen in mental health clinics but whose mental health visits was not associated with diagnosis codes for depression, PTSD, anxiety, psychosis, or bipolar disorder. In addition, among patients with 1 of these 5 diagnoses, adjusted odds of death were lower in patients seen in mental health clinics than in patients not seen in mental health clinics.
Table 4.
Adjusted Odds of Death for Subgroups Defined According to Prior Outpatient Diagnosis Codes and Mental Health Visits
| N | Adjusted Odds of Death* | 95% CI | P Value | |
|---|---|---|---|---|
| Congestive heart failure | ||||
| Subgroup | ||||
| Outpatient diagnosis† and >1 mental health visits | 3,025 | 0.76 | 0.60–0.97 | .03 |
| Outpatient diagnosis and no mental health visits | 1,914 | 1.04 | 0.80–1.35 | .77 |
| No outpatient diagnosis and >1 mental health visits | 1,373 | 0.59 | 0.39–0.89 | .01 |
| No outpatient diagnosis and no mental health visits | 9,689 | 1.00 (Referent group) | – | – |
| Pneumonia | ||||
| Subgroup | ||||
| Outpatient diagnosis* and >1 mental health visits | 3,975 | 0.91 | 0.80–1.05 | .21 |
| Outpatient diagnosis and no mental health visits | 1,832 | 1.17 | 1.00–1.36 | .05 |
| No outpatient diagnosis and >1 mental health visits | 1,999 | 0.81 | 0.66–0.99 | .04 |
| No outpatient diagnosis and no mental health visits | 9,976 | 1.00 (Referent group) | – | – |
*Adjusted odds of death relative to patients without outpatient diagnosis codes and mental health visits, as determined by generalized estimating equations, adjusting for demographic and clinical confounders and accounting for clustering of patients within hospitals
†Diagnosis of depression, anxiety, psychosis, PTSD, or bipolar disorder on 1 or more outpatient encounters
DISCUSSION
The current study applied 3 previously used methodological approaches for identifying psychiatric comorbidity and examined the impact of these different methods on hospital mortality for 2 of the most common causes of hospitalization—CHF and pneumonia. We emphasize the following 3 findings. First, rates of psychiatric comorbidity varied according to the method used to identify such comorbidity. Rates were relatively similar using prior outpatient diagnoses or mental health clinic visits but were substantially lower using inpatient secondary diagnosis codes. Second, the agreement between the different approaches was only fair to moderate; agreement was highest between prior outpatient diagnosis codes and mental health visits. Third and perhaps most important, the risk of hospital death varied depending on the method of identifying psychiatric comorbidity. Thus, the method of identifying psychiatric comorbidity substantially affected the prognostic impact. These findings suggest that different diagnostic approaches likely identify individuals with different types of psychiatric comorbidity and different illness constructs.
In interpreting our findings, several explanations are possible. First, the findings of lower mortality for patients with secondary diagnoses of psychiatric comorbidity may indicate that these codes are a marker for lower unmeasured severity. Because the number of secondary diagnoses in discharge abstracts is limited, medical record coding may be more likely to include a psychiatric comorbidity in patients with less complicated hospital courses. This explanation may also underlie the lower mortality in some studies for patients with secondary diagnoses of hypertension and diabetes.16,17 Indeed, in the current analysis, patients with psychiatric comorbidity from inpatient codes had the lowest predicted mortality (Table 2)—supporting the hypothesis that secondary inpatient codes may serve as a marker for lower severity. Moreover, some prior studies have also demonstrated that patients with psychiatric comorbidity identified by secondary inpatient codes had lower mean predicted risk of mortality.7,9
Second, it is possible that the lower mortality in patients with psychiatric secondary diagnoses may be due to greater vigilance by providers or greater likelihood to admit lower severity patients with psychiatric comorbidities. Third, the lower mortality may reflect lower utilization of invasive diagnostic or therapeutic modalities. For example, 2 prior studies of acute myocardial infarction (AMI) found that patients with psychiatric secondary diagnoses were less likely to undergo coronary revascularization,9,20 which may be associated with worse hospital outcomes but better long-term outcomes.18 In contrast, a study of general medical and surgical inpatients4 found that patients with an inpatient diagnosis code of schizophrenia (0.2% of the sample) had a 2- to 2.5-fold higher risk of several adverse events (e.g., postoperative sepsis, iatrogenic infections).
Other studies of patients with ischemic heart disease provide further evidence of the potential impact of different diagnostic methods. In contrast to the findings noted above9,20 of lower use of coronary revascularization in patients with inpatient psychiatric codes, Jones and Carney19 examined privately insured patients with AMI in a single state and found that patients with psychiatric diagnoses captured before or during hospitalization or within 30 days after discharge had similar rates of coronary revascularization. However, in an analysis of VA patients with AMI during 1994–1995, Petersen et al.20 found that patients identified by prior psychiatric admissions, secondary inpatient codes, or mental health provider visits had lower rates of coronary angiography and revascularization but had similar 30-day mortality and were equally likely to receive indicated medications. Nonetheless, the latter finding contrasts with the study by Druss et al.,9 who found that psychiatric patients identified by inpatient secondary diagnosis inpatient codes were less likely to receive indicated medications after AMI. A final analysis by Young and Foster8 of patients with acute coronary syndromes found that in-hospital mortality was 21% lower in patients with psychiatric secondary diagnosis codes in among patients 65 years and older but was higher for patients with schizophrenia and substance abuse diagnoses who were less than 65.
Whereas the inconsistent findings across prior studies may reflect differences in patient characteristics (e.g., age, type of health insurance), it is also likely that the inconsistencies may reflect the different methods used to identify psychiatric comorbidity. Consistent with our findings, rates of identifying psychiatric comorbidity varied widely in these earlier studies depending on the methods employed, varying from roughly 5% using inpatient codes7–9 to 40% using outpatient codes.19 Thus, our findings build on prior studies and represent the first analysis to directly compare the differences in rates of identification and in prognostic impact of psychiatric comorbidity in the same patient population.
Several limitations should be discussed. First, mental illnesses often go unrecognized21 and inpatient and outpatient diagnoses from claims data may underestimate the prevalence of such conditions. Second, the sensitivity and specificity of ICD-9-CM codes in administrative databases may vary across individual diagnosis.22 Nevertheless, numerous prior studies have used administrative data to measure quality23,24 including several VA studies.25–27 Moreover, in a comparison of statistical models based on administrative data and clinical data from medical records, Krakauer et al.28 found that administrative data are satisfactory for characterizing variations in hospital mortality rates, whereas Ash et al.29 concluded that prediction models based on claims data can be accurate. Additionally, our inclusion of a laboratory-based measure addresses some of the limitations of administrative data regarding unmeasured severity of illness.30,31
Third, it is important to acknowledge that methods we used to identify psychiatric comorbidity may identify constructs of psychiatric disease that vary with respect to disease severity or spectrum (e.g., acute, chronic, newly diagnosed illness). Such constructs may, in turn, have different associations with hospital mortality either directly or indirectly through their influence on health care delivery. Differences in findings across prior studies may, in fact, reflect the differences in disease constructs identified by these studies.
Fourth, our use of mental health visits may identify a heterogeneous group of patients and may not capture patients with ongoing illness. These concerns may also be true for diagnosis codes captured on encounter data.
Despite these limitations, this study has several implications for research and practice. First, the study highlights the limitations of using secondary psychiatric diagnoses from inpatient codes to identify patients. Second, the study highlights that assessing the care delivered to patients with psychiatric illnesses using claims data may require the triangulation of multiple methodological approaches, given that different approaches may identify different disease constructs. Lastly, the findings are reassuring that patients with psychiatric illnesses do not appear to be at increased risk of adverse hospital outcomes for 2 common causes of hospitalization within the VA system. However, given the robust resources and novel clinical programs for managing mental health problems32 and the VA’s integrated electronic medical record, our findings should be replicated in other settings.
In conclusion, as the recognition of psychiatric illnesses improves through better screening approaches and public awareness, the monitoring of outcomes in such populations will become increasingly important. This is particularly true for the increasing number of veterans and nonveterans being diagnosed with serious mental illnesses, such as PTSD. The current findings suggest that additional research in determining sensitive and specific methods of identifying such patients, using existing data sources, is warranted.
Acknowledgments
This research was conducted and completed by only those authors listed on the title page. No other authors contributed in a significant manner to this work.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development (HSR&D) Service through the Center for Research in the Implementation of Innovative Strategies in Practice (CRIISP) (HFP 04-149). Dr. Abrams is a fellow associate supported by additional VA funding through the Office of Academic Affairs. This work was presented at the Annual Society of General Internal Medicine conference in Toronto, Canada on April 27, 2007.
Conflict of Interest The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest in regards to this study.
References
- 1.Kessler RC, Chiu TC, Demler O, et al. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. [DOI] [PMC free article] [PubMed]
- 2.Kisely S, Smith M, Lawrence D, et al. Mortality in individuals who have had psychiatric treatment: population-based study in Nova Scotia. Br J Psychiatry. 2005;187:552–8. [DOI] [PubMed]
- 3.Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21:1133–7. [DOI] [PMC free article] [PubMed]
- 4.Daumit GL, Pronovost PJ, Anthony CB, et al. Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Arch Gen Psychiatry. 2006;63:267–72. [DOI] [PubMed]
- 5.Jaffe AS, Krumholz HM, Catellier DJ, et al. Prediction of medical morbidity and mortality after acute myocardial infarction in patients at increased psychosocial risk in the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study. Am Heart J. 2006;152:126–35. [DOI] [PubMed]
- 6.Lesperance F, Frasure-Smith N, Juneau M, et al. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160:1354–60. [DOI] [PubMed]
- 7.Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58:565–72. [DOI] [PubMed]
- 8.Young JK, Foster DA. Cardiovascular procedures in patients with mental disorders. JAMA. 2000;283:3198. author reply 3198–9. [DOI] [PubMed]
- 9.Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506–11. [DOI] [PubMed]
- 10.Lawrence DM, Holman CD, Jablensky AV, et al. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980–1998. Br J Psychiatry. 2003;182:31–6. [DOI] [PubMed]
- 11.Maynard C, Lowy E, Rumsfeld J, et al. The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System. Arch Intern Med. 2006;166:1410–6. [DOI] [PubMed]
- 12.Elixhauser A, Steiner C, Harris D, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed]
- 13.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–36. [DOI] [PubMed]
- 14.Iezzoni LI. The risks of risk adjustment. JAMA. 1997;278:1600–7. [DOI] [PubMed]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [DOI] [PubMed]
- 16.Iezzoni LI, Foley SM, Daley J, Hughes J, Fishe, et al. Comorbidities, complications, and coding bias. Does the number of diagnosis codes matter in predicting in-hospital mortality? JAMA. 1992;267:2197–203. [DOI] [PubMed]
- 17.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. The role of length of stay and comorbidities. JAMA. 1988;260:2240–6. [DOI] [PubMed]
- 18.Ottervanger JP, Armstrong P, Barnathan ES, et al. Association of revascularisation with low mortality in non-ST elevation acute coronary syndrome, a report from GUSTO IV-ACS. Eur Heart J. 2004;25:1494–501. [DOI] [PubMed]
- 19.Jones LE, Carney CP. Mental disorders and revascularization procedures in a commercially insured sample. Psychosom Med. 2005;67:568–76. [DOI] [PubMed]
- 20.Petersen LA, Normand SL, Druss BG, et al. Process of care and outcome after acute myocardial infarction for patients with mental illness in the VA health care system: are there disparities? Health Serv Res. 2003;38:41–63. [DOI] [PMC free article] [PubMed]
- 21.Glasser M, Stearns JA. Unrecognized mental illness in primary care. Another day and another duty in the life of a primary care physician. Arch Fam Med. 1994;3:862–4. [DOI] [PubMed]
- 22.Hsia DC, Krushat WM, Fagan AB, et al. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med. 1988;318(6):352–5. [DOI] [PubMed]
- 23.Hannan EL, Vaughan-Sarrazin MS, Doran D, et al. Provider profiling and quality improvement efforts in CABG surgery: the effect on short-term mortality among Medicare beneficiaries. Med Care. 2003;41(10):1164–72. [DOI] [PubMed]
- 24.Vaughan-Sarrazin MS, Hannan EL, Gormley CJ, et al. Mortality in Medicare beneficiaries following coronary artery bypass graft surgery in states with and without certificate of need regulation. JAMA. 2002;288(15):1859–66. [DOI] [PubMed]
- 25.Department of Veterans Affairs, Office of Policy and Planning. Program Evaluation of Cardiac Care Programs in the Veterans Health Administration. Part 1: Acute Myocardial Infarction (AMI) and Percutaneous Coronary Interventions (PCI) Cohort Analyses. Contract Number V101 (93) 1444. 4-11-2003. Washington DC: Department of Veterans Affairs. http://www.va.gov/opp/organizations/progeval.htm (Report).
- 26.Department of Veterans Affairs, Veterans Health Services Research Administration. A Report on the Quality of Surgical Care in the Department of Veterans Affairs: The Phase III Report. R&D no. IL 10-87-9. 4-21-1991. Washington, DC: Administrator of Veterans Affairs; 1991(findings also found in Stremple JH, Bross DS, Davis CL, McDonald DO. Comparison of postoperative mortality and morbidity in VA and nonfederal hospitals. J Surg Res. 1994;56:405–16.) (Report). [DOI] [PubMed]
- 27.Department of Veterans Affairs, Veterans Health Services Research Administration. A Report on the Quality of Surgical Care in the Department of Veterans Affairs: The Phase II Report. R&D no. IL 10-87-8. 4-12-1989. Washington, DC: Administrator of Veterans Affairs; 1989(findings also found in Stremple JH, Bross DS, Davis CL, McDonald DO. Comparison of postoperative mortality in VA and private hospitals. Ann Surg. 1993;217:272–85.) (Report). [DOI] [PMC free article] [PubMed]
- 28.Krakauer H, Bailey RC, Skellan KJ, et al. Evaluation of the HCFA model for the analysis of mortality following hospitalization. Health Serv Res. 1992;27:317–35. [PMC free article] [PubMed]
- 29.Ash AS, Posner MA, Speckman J, et al. Using claims data to examine mortality trends following hospitalization for heart attack in Medicare. Health Serv Res. 2003;38(5):1253–62. [DOI] [PMC free article] [PubMed]
- 30.Pine M, Norusis M, Jones B, et al. Predictions of hospital mortality rates: a comparison of data sources. Ann Intern Med. 1997;126:347–54. [DOI] [PubMed]
- 31.Pine M, Jordan HS, Elixhauser A, et al. Enhancement of claims data to improve risk adjustment of hospital mortality. JAMA. 2007;297:71–6. [DOI] [PubMed]
- 32.Felker BL, Chaney E, Rubenstein LV, et al. Developing effective collaboration between primary care and mental health providers. Primary care companion. J Clin Psychiatry. 2006;8(1):12–16. [DOI] [PMC free article] [PubMed]


