Abstract
The L3-secreted Ancylostoma Secreted Protein-2 from the human hookworm Necator americanus (Na-ASP-2) has been selected as a candidate vaccine antigen in anticipation of clinical trials. Its crystal structure revealed that Na-ASP-2 has structural and charge similarities to CC-chemokines, suggesting that it might act as a chemokine mimic when released by the infective larvae during tissue migration. Using the air pouch model of acute inflammation, we found that Na-ASP-2 induced a significant leukocyte influx to the skin pouch, mostly comprised of neutrophils (60%) and monocytes (30%) that was transient and resolved in 24 h. Other hookworm larval proteins did not cause any inflammatory leukocytes to migrate into air pouches. In vitro chemotaxis assays confirmed our results and demonstrated that leukocyte migration was a direct effect of Na-ASP-2 exposure and not caused by other molecules released by host cells in the inflammatory microenvironment or by the expression vector.
Keywords: Na-ASP-2, neutrophils, chemotaxis, vaccine, Necator americanus, air pouch, nematode
Introduction
Human hookworm infection is a leading cause of iron-deficiency anemia and malnutrition with an estimated 600 million cases in the tropical developing world (Bethony et al., 2006). Most hookworms infect a host by penetrating the skin, although some species are orally infective. Third-stage infective larvae (L3) of the canine hookworm, Ancylostoma caninum, and the major human hookworm, Necator americanus, are developmentally arrested and wait in the soil or on grass. They attach to the host upon direct contact and penetrate the skin via hair follicles, crossing the dermis and eventually entering blood or lymphatic capillaries, where they are carried to the pulmonary microcirculation. Once in the lung, they undergo tracheal migration by penetrating into the alveoli to be swept in mucus up the airways and then down into the gut, where they establish as adult parasites. During tissue invasion, infective L3 of parasitic nematodes encounter physicochemical signals that initiate a programmed chain of developmental events resulting in the successful establishment of a parasitic relationship. When L3 of the canine hookworm A. caninum are activated in vitro, they release metalloproteases (Hawdon et al., 1995) and other molecules of unknown function referred to as Ancylostoma Secreted Protein 1 and 2 (Ac-ASP-1, Ac-ASP-2; Hawdon et al., 1996; Hawdon et al., 1999).
Tissue invasion is often associated with granulocyte infiltration. The quantity and quality of inflammatory recruitment following parasitic helminth infection suggest that granulocytes are not simply responding to tissue injury caused by migrating larvae, but they are actively being targeted by molecules secreted by the parasite. Numerous parasite-derived chemotactic factors have been reported to recruit, often selectively, neutrophils or eosinophils (Falcone et al., 2001; Horii et al., 1988; Niwa et al., 1998; Owhashi et al., 1985; Owhashi et al., 1997; Rubio de Kromer et al., 1998; Tanaka et al., 1979; Tanaka and Torisu, 1978). In the few cases in which the mechanism has been studied, it has revealed some degree of interaction between parasite antigens and either β-integrin receptors or the IL-8 pathway (Falcone et al., 2001; Rubio de Kromer et al., 1998).
Hookworm proteins involved in tissue invasion are particularly good candidate antigens for the development of vaccines and drugs (Hotez et al., 2003). On the basis of in vitro data, animal trials and human epidemiological studies (Bethony et al., 2005; Goud et al., 2004; Hotez et al., 2003; Mendez et al., 2005), the L3-secreted N. americanus ASP-2 (Na-ASP-2) was selected as a vaccine antigen to undergo further development (Goud et al., 2005). As mentioned above, ASP-2 is released by the L3 after invading the host and it is likely to play a role in the transition to parasitism (Hotez et al., 2003). Since the function of ASP-2 is currently unknown, structural studies were initiated to clarify the role of Na-ASP-2 as a functional vaccine. Although no protease activity for Na-ASP-2 has been detected so far, our finding that anti-Na-ASP-2 antibodies inhibit larval migration through skin in an in vitro assay (Bethony et al., 2005; Goud et al., 2005) suggests that ASP-2 has an important role in larval host entry and in migration through the tissues before reaching the intestine. Recent crystallography studies have revealed that Na-ASP-2 has structural and charge similarities to CC-chemokines (Asojo et al., 2005), suggesting that this molecule might act as a chemokine mimic when released by the infective larvae during tissue migration.
To study the development of chemotaxis, in vivo animal models have become important. The study of localized inflammation in tissues is often challenging because it is difficult to isolate the immune response towards a particular insult. Thus, there was value in developing a simple inflammatory model that is restricted to a confined location in which population changes can be more readily monitored and regulatory mechanisms identified. The air pouch model in mice represents an ideal location in which to study the development of an inflammatory reaction. The pouches are generated by the injection of sterile air in the skin of mice. Both the stimulus (inflammatory agent) and the response (cellular and humoral) are generated in a sterile environment and can be easily isolated by lavage. In the present study, we were interested to determine, by use of a murine air pouch system, whether Na-ASP-2 had a chemotactic function. We found that Na-ASP-2 caused leukocyte recruitment into the inflammatory air pouch of BALB/c mice, and that the infiltrate was mainly comprised of neutrophils (60%) and monocytes (30%). Other hookworm larval secreted proteins, such as Ac-MTP-1, did not cause any inflammatory infiltrate. In vitro chemotaxis assays using purified neutrophils confirmed that the neutrophil migration was a direct effect of Na-ASP-2 exposure and not caused by other chemokines and/or cytokines that may have been released in vivo in the inflammatory microenvironment of the air pouch.
Materials and Methods
Animals
Female BALB/c were obtained from the National Cancer Institute and used at 6–8 weeks of age. All studies were approved by the Institutional Animal Care and Use Committee at The George Washington University Medical Center.
Recombinant proteins
The rNa-ASP-2 protein was manufactured as previously described (Goud et al., 2005). The recombinant Ancylostoma caninum metalloprotease 1 (Ac-MTP-1) was used as a control. The recombinant protein was provided by Dr. Bin Zhan of the Department of Microbiology, Immunology, and Tropical Medicine at The George Washington University, and produced as described (Zhan et al., 2002). The rationale for selecting Ac-MTP-1 is that this protein is expressed exclusively by the L3 stage of A. caninum and is actively secreted into the culture medium in vitro, supporting a role in tissue invasion. Ac-MTP-1 has significant homology with the N. americanus MTP-1 molecule (Daub et al., 2000), which is not available as a recombinant antigen. Both proteins were suspended in sterile phosphate buffer saline (PBS) for use in in vivo air pouch inoculations. For in vitro chemotaxis assays, proteins were diluted in RPMI supplemented with 1% BSA (Sigma, St. Louis, MO).
Air pouch and leukocyte migration
Air pouches were raised on the dorsum of BALB/c mice as described elsewhere (Edwards et al., 1981). Briefly, on days 0 and 4, mice received a subcutaneous injection of approximately 2 ml sterile air using a 27G needle attached to a 0.2 μ syringe filter. On day 7, mice were injected with 1 ml PBS containing 50–100 μg rNa-ASP-2 or rAc-MTP-1 in the air pouch. The dose of antigen used and timing of sacrifice for each experiment are indicated within each figure legends. After sacrifice, the pouches were flushed with 3 ml PBS to collect their contents. Cells were then filtered through a 70-μ cell strainer (BD Falcon, San Jose, CA), centrifuged and resuspended in 1 ml PBS. Leukocyte numbers were calculated and up to 2.5 × 105 cells were centrifuged onto glass slides by cytospin. Slides were stained with Wright-Giemsa (Camco Chemicals, Florence, KY) to identify leukocyte subpopulations. A total of 300 cells were counted for each slide. Individual leukocyte numbers were combined and expressed as mean ± SEM. Total cell numbers were obtained as the product of total cell number and percentages for each cell type.
Cell purification from peripheral blood
One to two ml of peripheral blood were obtained from the mice by cardiac puncture and treated with ammonium chloride lysis buffer (16 mM NH4Cl, 2 mM KHCO3, and 3 mM EDTA) to lyse red blood cells and enrich for peripheral blood leukocytes (PBLs). In some experiments, once PBLs were obtained, neutrophil purification was performed using a mouse anti-GR-1 biotin MACS separation kit from Miltenyi Biotec (Auburn, CA) according to manufacturer’s instructions.
Boyden Chamber Assays
The apparatus used in all chemotaxis assays was a 48-well Boyden chemotaxis chamber and 5 μm polycarbonate membranes from Neuro Probe, Inc. (Gaithersburg, MD). RANTES and Eotaxin-1 were obtained from PeproTech (Rocky Hill, NJ); FMLP was purchased from Sigma (St. Louis, MO). These chemokines were used as positive controls to attract T cells, eosinophils and neutrophils, respectively. All chemokines were suspended in RPMI + 1% BSA. The upper chamber and rubber gasket were removed and 25 μl of chemoattractants was added to the bottom wells (RANTES 1 ng/ml, Eotaxin-1 270 ng/ml, FMLP 10 μM); the recombinant hookworm proteins were used at varying concentrations (10–250 ng/ml). The 5-μm polycarbonate membrane was then carefully placed onto the bottom chamber and the rubber gasket and upper chamber components were then reattached. Cells were added to the upper wells at a dose of 1 × 104 PBLs or 5 × 103 neutrophils in a total volume of 40 μl. The chamber was incubated at 37 °C, 5% CO2 for 50 min, after which the membrane was removed, the non-migrated cells were scraped off, and the membrane stained with Wright-Giemsa. After staining, the membrane was affixed to slides using Permount and cover slips. All cells in each experimental condition were counted at 40x and identified based on morphology. A chemotactic index was generated for each experimental condition by dividing the number of cells that had migrated through the membrane in response to the chemokine or antigen by the average number of cells that had migrated through the membrane in response to medium alone. Chemotactic indexes were combined and expressed as mean ± SEM, with n = 6 wells per experimental condition.
Pronase protein digestion
For protein digestion, 10 μg of Na-ASP-2 was incubated with 0.1% pronase (Calbiochem, San Diego, CA) overnight in a 37 °C water bath. A separate sample of Na-ASP-2 was incubated overnight at 37 °C without pronase to provide an undigested control. Successful pronase digestion was confirmed by gel electrophoresis followed by Coomassie staining.
Statistics
The statistical significance of differences in cell population numbers was determined by use of the one-way ANOVA test (within a group) or two-way ANOVA test (among groups). After we determined that differences existed among the means, Dunnet’s or Bonferroni post hoc test were used to determine which means differed, respectively. Differences were considered to be statistically significant if the calculated P ≤ 0.05.
Results
Na-ASP-2 causes leukocyte migration into air pouches generated in BALB/c mice
We tested the chemotactic potential of Na-ASP-2 using a well-established in vivo air pouch model of acute inflammation and cellular recruitment. Once the air pouch was established in the dorsum of the mice, 100 μg/ml of Na-ASP-2 was inoculated into the pouches. Total number of cells and leukocyte populations were determined at various time points after Na-ASP-2 inoculation. As shown in Figure 1A, injection of 100 μg of Na-ASP-2 resulted in migration of leukocytes into air pouches that peaked at 6 h post-injection. This inflammatory infiltrate was transient since it greatly, and significantly, decreased after 24 h when compared to the 6 h timepoint (P < 0.001). Injection of sterile PBS as a control did not induce significant cell recruitment (< 105, not shown). When the relative percentage of leukocyte populations was analyzed, we found that neutrophils were the predominant cell type to be recruited into the air pouches following Na-ASP-2 injection, comprising approximately 60% of the leukocytes recovered at any given time. Monocytes/macrophages (Mf) were the second most predominant cell type, representing 30% of the total leukocyte population. Interestingly, very few eosinophils migrated to the air pouch in response to Na-ASP-2 (less than 10%), and only 3% of the cells recovered were lymphocytes (Figure 1B). While the relative proportions were maintained at every time point, the absolute number of cells varied with time, even though neutrophils were always the predominant cell population (Figure 1C). Based on these findings (maximum influx of leukocytes at 6 h post injection), the 6 h time point was selected for all subsequent in vivo studies. Injection of 50 μg induced the same amount of leukocyte recruitment to the pouch (not shown); thus, the lower dose was employed in subsequent experiments.
Figure 1.
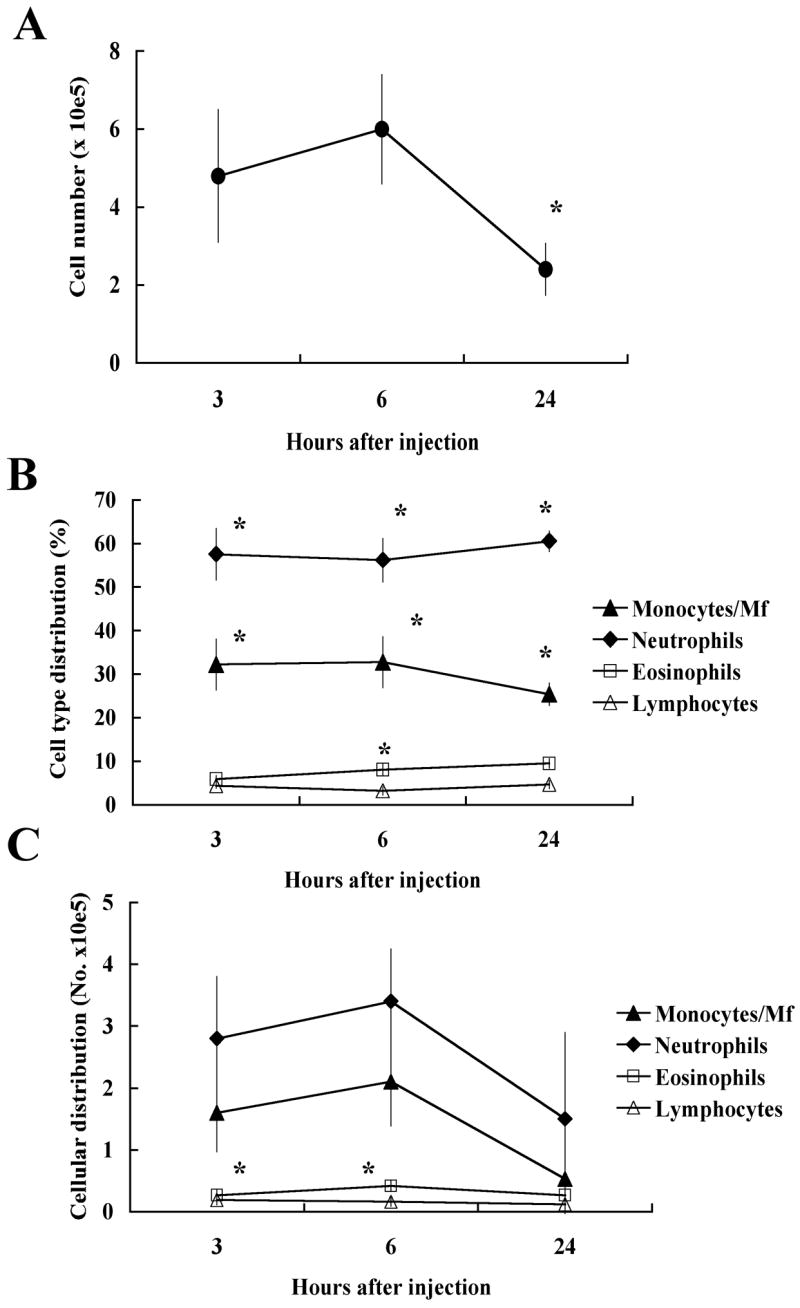
Injection of 100 μg of Na-ASP-2 into the air pouch induces leukocyte migration in BALB/c mice. A. Total number of cells in air pouches at different time points after injection. Data represent the mean ± SEM, n = 8–9. *: Statistically significant when compared with the other time points, P < 0.001. B. Relative cell type distribution (in percentage) of monocytes/macrophages (Mf, ◆), neutrophils (■), eosinophils (□) and lymphocytes (△) at different time points after injection of the antigen. Data represent mean ± SEM, n = 8–9. *: Statistically significant when compared with the other cell populations, P < 0.001. C. Total numbers of monocytes/macrophages (Mf, ◆), neutrophils (■), eosinophils (□) and lymphocytes (△) at different time points after injection of the antigen. Data represent mean ± SEM, n = 8–9. *: Statistically significant when compared with the other cell populations, P < 0.001.
In vivo leukocyte migration occurs in response to Na-ASP-2 but not other larval antigens
To determine whether the recruitment of cells to the air pouch was an effect specific for Na-ASP-2, Ac-MTP-1, a different ancylostomid L3-secreted protein was tested. Figure 2A shows the in vivo recruitment of leukocytes to air pouches of BALB/c mice in response to local injection of 50 μg protein. Sterile PBS was used as a control. Total cell numbers recovered from air pouch exudates collected from mice injected with Na-ASP-2 were 8- and 9-fold higher than those recovered from mice injected with either Ac-MTP-1 or PBS, respectively, confirming that the recruitment of cells into the air pouch occurs following Na-ASP-2 stimulation (P < 0.005). We determined the absolute numbers of the major leukocyte subsets collected from the inflammatory exudates in the air pouches. Figure 2B shows that Na-ASP-2 injection elicited a 4-fold increase in monocyte/Mf numbers (P < 0.0008) and a 20-fold increase in neutrophils numbers (P < 0.0001), if compared to that obtained after Ac-MTP-1 or PBS inoculation. Monocytes/Mf was the predominant cell type recovered from mice inoculated with either PBS or Ac-MTP-1. In contrast, air pouch exudates from Na-ASP-2-injected mice contained a significant higher percentage of neutrophils, followed by monocytes/Mf. Again, recruitment of eosinophils was significantly smaller and lymphocyte recruitment was negligible in all groups analyzed. Taken together, these findings suggest that Na-ASP-2 is responsible for leukocyte recruitment in the in vivo air pouch model, and that this effect is specific for Na-ASP-2 since other larval proteins did not elicit any cell migration.
Figure 2.
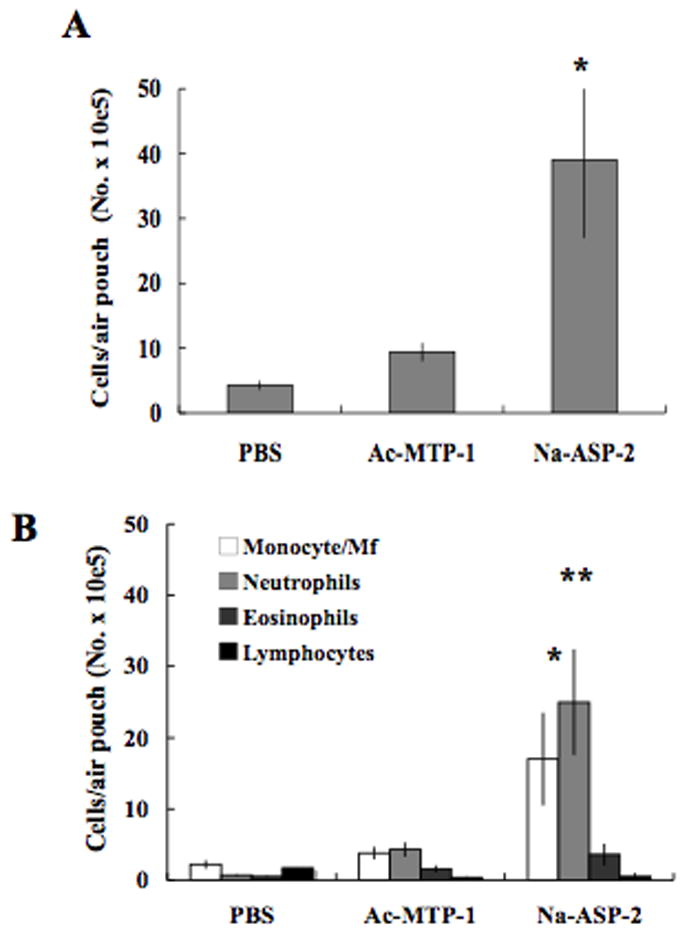
Na-ASP-2 induces neutrophil migration into the dorsal air pouch of BALB/c mice. A. Total number of cells recovered from air pouches 6 h after injection of sterile PBS, 50 μg Ac-MTP-1 or 50 μg Na-ASP-2. Data represent the mean ± SEM, n = 5. B. Total numbers of monocytes/Mf, neutrophils, eosinophils and lymphocytes recovered from air pouches 6 h after injection of sterile PBS, Ac-MTP-1 or Na-ASP-2. Data represent mean ± SEM, n = 5. *, **: Statistically significant when compared to the PBS and Ac-MTP-1 groups, P < 0.0008 and P < 0.0001, respectively.
Na-ASP-2 is directly responsible for chemotaxis of purified neutrophils in vitro
Following our in vivo findings, we wanted to confirm that Na-ASP-2 induced leukocyte migration by itself, and not indirectly via other exogenous factors that might have been present in the in vivo model (i.e. other chemokines released by local cells exposed to the antigen). Thus, we conducted in vitro chemotaxis studies using Boyden chambers, which enabled us to control the chemokine environment present during antigen exposure. We first investigated which leukocyte subset(s) would be induced to migrate in response to Na-ASP-2 by incubating peripheral blood leukocytes (PBLs) with several concentrations of Na-ASP-2. Other well-characterized chemokines such as FMLP, eotaxin-1 and RANTES were used as positive controls to elicit migration of neutrophils, eosinophils and lymphocytes, respectively. As shown in Figure 3A, blood neutrophils were the only subset that demonstrated significant migration in response to Na-ASP-2 stimulation. This response was dose-dependent, with an optimal response at 10 ng/ml. No chemotactic effect was observed if doses lower than 10 ng/ml were tested (not shown), consistent with the typical curves observed when performing chemotaxis assays (decreased chemotaxis at high and low concentrations). To further confirm that the chemotactic effect of Na-ASP-2 on neutrophils was not due to factors released by other leukocytes either in vivo or in vitro, but to the protein itself, chemotaxis assays were conducted using purified neutrophils from peripheral blood. Figure 3B shows that in vitro incubation of purified neutrophils with 10 ng/ml Na-ASP-2 induced cells to migrate in response to the antigen. Finally, we wanted to confirm that the chemotactic effect of Na-ASP-2 was not due to residual carbohydrates that may have been present after expression and purification. Therefore, chemotaxis assays were also conducted using Na-ASP-2 pre-treated with pronase. Pronase is a mixture of endo- and exo-proteinases designed to cleave all peptide bonds in a protein. As shown in Figure 3C, pronase treatment of Na-ASP-2 totally abolished its chemotactic function. Taken together, these findings strongly support the conclusion that the chemotactic effects observed with Na-ASP-2 are due to the protein itself and not to an exogenous contaminant.
Figure 3.
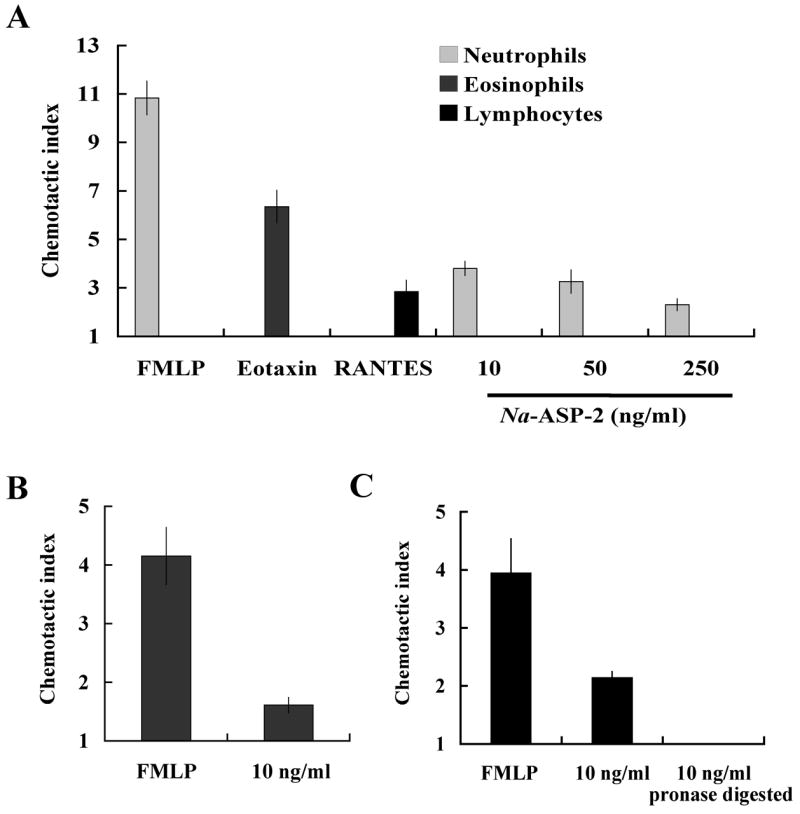
In vitro chemotactic assays confirmed that Na-ASP-2 induces neutrophil chemotaxis. A. Chemotactic indexes for PBLs after 50 min incubation with FMLP 10 μM, eotaxin-1 270 ng/ml or RANTES 1 ng/ml as positive controls, or Na-ASP-2 (10–250 ng/ml). The chemotactic index was determined by dividing the number of cells that migrated through the membrane in response to the chemokine by the average number of cells that migrated in response medium alone. Data represent the mean ± SEM, n = 6. B. Chemotactic index for purified blood neutrophils after 50 min incubation with FMLP (10 μM) or the L3 proteins Na-ASP-2. Data represent mean ± SEM, n = 6. C. Chemotactic index for purified blood neutrophils after 50 min incubation with FMLP (10 μM) or 10 ng/ml Na-ASP-2 incubated overnight with or without 0.1% pronase at 37° C. Data represent mean ± SEM, n = 6.
Discussion
We have shown that murine leukocytes rapidly respond to Na-ASP-2 by the use an in vivo air pouch model of inflammation and an in vitro chemotaxis assay. We have determined that neutrophils, and monocytes to a lesser extent, accumulate in response to Na-ASP-2. This effect was proven to transient, however, since inflammation resolved 24 h after the inoculation of the protein.
Developmental studies carried out with the dog hookworm, A. caninum, indicated that products secreted during larval invasion are likely involved in the transition to parasitism and infectivity (Hawdon et al., 1999). Helminth proteins implicated in the tissue invasion process are particularly good candidate antigens for the development of vaccines and drugs. In particular, tissue-invading hookworm L3 release metalloproteases and ASPs. The function of the A. caninum metalloprotease-1, Ac-MTP-1, has recently been characterized by us (Williamson et al., 2006). We have shown that MTP-1 favors tissue penetration: anti-MTP-1 antiserum inhibited the ability of the recombinant protein to digest collagen by 85% and inhibited larval migration through tissue in vitro by 75% (Williamson et al., 2006). Similarly, rat and dog antiserum against Na-ASP-2 was able to inhibit larval migration through tissue in an in vitro system (Bethony et al., 2005; Goud et al., 2005). Our data suggest that ASP-2 is also central to promote tissue migration in the host. This could also provide the basis by which anti-ASP-2 antibodies are protective against larval challenge infections (Goud et al., 2004; Mendez et al., 2005). Our results also confirm that the mechanisms of action of the two larval proteins are different, since only ASP-2 promoted neutrophil migration. Na-ASP-2 has been selected as a vaccine candidate based on human immuno-epidemiologic studies and laboratory animal vaccine trials (Bethony et al., 2005; Goud et al., 2004; Hotez et al., 2003; Mendez et al., 2005). In order to clarify the role of Na-ASP-2 as a functional vaccine, and in an attempt to unravel its function, structural studies were carried out (Asojo et al., 2005). The crystal structure revealed that this antigen has structural and charge similarities to CC-chemokines, leading to hypothesize that Na-ASP-2 might act as a chemoattractant. Our data confirmed this hypothesis, since Na-ASP-2 treatment induced accumulation of leukocytes both in vivo and in vitro. Our findings also demonstrated this effect was short-lived.
The question remains on what would be the advantage for the parasite to elicit neutrophil accumulation during tissue migration. The literature reveals that both inhibitory and chemoattractant parasite-derived molecules have been described. However, a closer examination of the published data unveiled that tissue-dwelling parasites (usually adults) often release inhibitory factors, possibly in an attempt to reduce the local immune response (Alkarmi and Behbehani, 1989; Culley et al., 2000; Keir et al., 2004; Leid et al., 1987; Lo et al., 1999). Conversely, tissue-migrating larvae usually secrete compounds that promote inflammation (Falcone et al., 2001; Horii et al., 1988; Niwa et al., 1998; Owhashi et al., 1985; Owhashi et al., 1997; Rubio de Kromer et al., 1998; Tanaka et al., 1979; Tanaka and Torisu, 1978). Local inflammation and, in particular, neutrophil-related edema could increase tissue permeability and favor a faster and more successful migration through the host’s tissues. In addition, the specific recruitment of neutrophils could protect migrating parasites from exposure to other cell types that may produce more damage, such as NK cells or eosinophils that cause antibody-dependent cytotoxicity (Attallah et al., 1980; Desakorn et al., 1987).
Our findings presented here suggest that Na-ASP-2 function may have evolved to favor tissue migration by the elicitation of a neutrophil rich inflammatory infiltrate that will favor permeability and edema. A faster migration through the host’s tissues may ultimately result in increased parasite survival and establishment. The existence and role of Na-ASP-2 receptors in the host cells is currently under investigation.
Acknowledgments
We would like to acknowledge Dr. Gaddam Goud and Dr. Bin Zhan for providing rNa-ASP-2 and rAc-MTP-1. This work was supported by the Human Hookworm Vaccine Initiative of the Sabin Vaccine Institute and NIH R01 AI059280 (S.C.). We also thank Dr. Peter Hotez, Dr. Maria Elena Botazzi, and Amy Brown for the critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkarmi T, Behbehani K. Echinococcus multilocularis: inhibition of murine neutrophil and macrophage chemotaxis. Experimental Parasitology. 1989;69:16–22. doi: 10.1016/0014-4894(89)90166-5. [DOI] [PubMed] [Google Scholar]
- Asojo OA, Goud G, Dhar K, Loukas A, Zhan B, Deumic V, Liu S, Borgstahl GE, Hotez PJ. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. Journal of Molecular Biology. 2005;346:801–814. doi: 10.1016/j.jmb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Attallah AM, Lewis FA, Urritia-Shaw A, Folks T, Yeatman TJ. Natural killer cells (NK) and antibody-dependent cell-mediated cytotoxicity (ADCC) components of Schistosoma mansoni infection. Int Archives of Allergy and Applied Immunology. 1980;63:351–354. doi: 10.1159/000232649. [DOI] [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. The Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, Williamson A, Lustigman S, Correa-Oliveira R, Xiao S, Hotez PJ. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. Faseb J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- Culley FJ, Brown A, Conroy DM, Sabroe I, Pritchard DI, Williams TJ. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. The Journal of Immunology. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- Daub J, Loukas A, Pritchard DI, Blaxter M. A survey of genes expressed in adults of the human hookworm, Necator americanus. Parasitology. 2000;120:171–184. doi: 10.1017/s0031182099005375. [DOI] [PubMed] [Google Scholar]
- Desakorn V, Suntharasamai P, Pukrittayakamee S, Migasena S, Bunnag D. Adherence of human eosinophils to infective filariform larvae of Necator americanus in vitro. Southeast Asian Journal of Tropical Medicine and Public Health. 1987;18:66–72. [PubMed] [Google Scholar]
- Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. The Journal of Pathology. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Falcone FH, Rossi AG, Sharkey R, Brown AP, Pritchard DI, Maizels RM. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infection and Immunity. 2001;69:4007–4018. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud GN, Bottazzi ME, Zhan B, Mendez S, Deumic V, Plieskatt J, Liu S, Wang Y, Bueno L, Fujiwara R, Samuel A, Ahn SY, Solanki M, Asojo OA, Wang J, Bethony JM, Loukas A, Roy M, Hotez PJ. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine. 2005;23:4754–4764. doi: 10.1016/j.vaccine.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Goud GN, Zhan B, Ghosh K, Loukas A, Hawdon J, Dobardzic A, Deumic V, Liu S, Dobardzic R, Zook BC, Jin Q, Liu Y, Hoffman L, Chung-Debose S, Patel R, Mendez S, Hotez PJ. Cloning, yeast expression, isolation, and vaccine testing of recombinant Ancylostoma-secreted protein (ASP)-1 and ASP-2 from Ancylostoma ceylanicum. The Journal of Infectious Disease. 2004;189:919–929. doi: 10.1086/381901. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. Journal of Biological Chemistry. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Perregaux MA, Hotez PJ. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Experimental Parasitology. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Molecular and Biochemical Parasitology. 1999;99:149–165. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Horii Y, Owhashi M, Fujita K, Nakanishi H, Ishii A. A comparative study on eosinophil and neutrophil chemotactic activities of various helminth parasites. Parasitology Research. 1988;75:76–78. doi: 10.1007/BF00931196. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Zhan B, Bethony JM, Loukas A, Williamson A, Goud GN, Hawdon JM, Dobardzic A, Dobardzic R, Ghosh K, Bottazzi ME, Mendez S, Zook B, Wang Y, Liu S, Essiet-Gibson I, Chung-Debose S, Xiao S, Knox D, Meagher M, Inan M, Correa-Oliveira R, Vilk P, Shepherd HR, Brandt W, Russell PK. Progress in the development of a recombinant vaccine for human hookworm disease: the Human Hookworm Vaccine Initiative. International Journal for Parasitology. 2003;33:1245–1258. doi: 10.1016/s0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- Keir PA, Brown DM, Clouter-Baker A, Harcus YM, Proudfoot L. Inhibition of neutrophil recruitment by ES of Nippostrongylus brasiliensis. Parasite Immunology. 2004;26:137–139. doi: 10.1111/j.0141-9838.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Leid RW, Grant RF, Suquet CM. Inhibition of neutrophil aggregation by taeniaestatin, a cestode proteinase inhibitor. International Journal for Parasitology. 1987;17:1349–1353. doi: 10.1016/0020-7519(87)90102-0. [DOI] [PubMed] [Google Scholar]
- Lo SK, Rahman A, Xu N, Zhou MY, Nagpala P, Jaffe HA, Malik AB. Neutrophil inhibitory factor abrogates neutrophil adhesion by blockade of CD11a and CD11b beta (2) integrins. Molecular Pharmacology. 1999;56:926–932. doi: 10.1124/mol.56.5.926. [DOI] [PubMed] [Google Scholar]
- Mendez S, Zhan B, Goud G, Ghosh K, Dobardzic A, Wu W, Liu S, Deumic V, Dobardzic R, Liu Y, Bethony J, Hotez PJ. Effect of combining the larval antigens Ancylostoma secreted protein 2 (ASP-2) and metalloprotease 1 (MTP-1) in protecting hamsters against hookworm infection and disease caused by Ancylostoma ceylanicum. Vaccine. 2005;23:3123–3130. doi: 10.1016/j.vaccine.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Niwa A, Asano K, Ito A. Eosinophil chemotactic factors from cysticercoids of Hymenolepis nana. The Journal of Helminthology. 1998;72:273–275. doi: 10.1017/s0022149x00016552. [DOI] [PubMed] [Google Scholar]
- Owhashi M, Horii Y, Ishii A. Isolation of Schistosoma japonicum egg-derived neutrophil stimulating factor: its role on eosinophil chemotactic factor release from neutrophils. International Archives of Allergy and Applied Immunology. 1985;78:415–420. doi: 10.1159/000233924. [DOI] [PubMed] [Google Scholar]
- Owhashi M, Kirai N, Horii Y. Eosinophil chemotactic factor release from neutrophils induced by stimulation with Schistosoma japonicum eggs. Parasitology Research. 1997;83:42–46. doi: 10.1007/s004360050205. [DOI] [PubMed] [Google Scholar]
- Rubio de Kromer MT, Kromer M, Luersen K, Brattig NW. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus. Acta Tropica. 1998;71:45–56. doi: 10.1016/s0001-706x(98)00044-8. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Baba T, Torisu M. Ascaris and eosinophil. II. Isolation and characterization of eosinophil chemotactic factor and neutrophil chemotactic factor of parasite in Ascaris antigen. The Journal of Immunology. 1979;122:302–308. [PubMed] [Google Scholar]
- Tanaka J, Torisu M. Anisakis and eosinophil. I. Detection of a soluble factor selectively chemotactic for eosinophils in the extract from Anisakis larvae. The Journal of Immunology. 1978;120:745–749. [PubMed] [Google Scholar]
- Williamson AL, Lustigman S, Oksov Y, Deumic V, Plieskatt J, Mendez S, Zhan B, Bottazzi ME, Hotez PJ, Loukas A. Ancylostoma caninum MTP-1, an astacin-like metalloprotease secreted by infective hookworm larvae, is involved in tissue migration. Infection and Immunity. 2006;74:961–967. doi: 10.1128/IAI.74.2.961-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan B, Hotez PJ, Wang Y, Hawdon JM. A developmentally regulated metalloprotease secreted by host-stimulated Ancylostoma caninum third-stage infective larvae is a member of the astacin family of proteases. Molecular and Biochemical Parasitology. 2002;120:291–296. doi: 10.1016/s0166-6851(01)00453-4. [DOI] [PubMed] [Google Scholar]


