Abstract
The aim of this study was to describe the microbiologic agents and pathologic processes in fatal bovine respiratory disease (BRD) of feedlot cattle and to investigate associations between agents and pathologic processes. Ninety feedlot calves diagnosed at necropsy with BRD and 9 control calves without BRD were examined, using immunohistochemical (IHC) staining and histopathologic studies. Mannheimia haemolytica (MH) (peracute, acute, and subacute cases) and Mycoplasma bovis (MB) (subacute, bronchiolar, and chronic cases) were the most common agents identified in fatal BRD cases. Significant associations (P < 0.10) were detected between microbiologic agents and between agents and pathologic processes. When IHC staining was used, 25/26 (96%) of animals that were positive for bovine viral diarrhea virus (BVDV) were also positive for MH; 12/15 (80 %) of animals that were positive for Histophilus somni (HS) were also positive for MB; and all of the animals that were positive for HS were negative for MH and BVDV. This quantitative pathological study demonstrates that several etiologic agents and pathologic processes are involved in fatal BRD of feedlot cattle.
Résumé
Observations microbiologiques et histopathologiques concernant des cas mortels de maladie respiratoire bovine chez des bovins gardés en parcs d’engraissement dans l’ouest du Canada. Le but de cette étude était de décrire les agents microbiologiques et les processus pathologiques impliqués dans la maladie respiratoire bovine (MRB) mortelle chez des bovins en parcs d’engraissement et de rechercher les associations entre agents et processus pathologiques. Quatre-vingt-dix veaux de parcs d’engraissement diagnostiqués positifs à la MRB à la nécropsie et 9 veaux témoins exempts de MRB ont été examinés par coloration immunohistochimique (IHC) et études histopathologiques. Mannheimia haemolytica (MH) (cas suraigus, aigus et subaigus) et Mycoplasma bovis (MB) (cas suraigus, aigus et subaigus) étaient les agents les plus communs identifiés dans les cas mortels de MRB. Des associations significatives (P < 0,10) ont été détectées entre les agents microbiologiques et entre les agents et les processus pathologiques. À la coloration IHC, 25/26 (96 %) des animaux positifs au virus de la diarrhée virale bovine (VDVB) l’étaient également à MH; 12/15 (80 %) des animaux positifs à Histophilus somni (HS) l’étaient également à MB et tous les animaux positifs à HS étaient négatifs à MH et au VDVB. Cette étude pathologique quantitative démontre que plusieurs agents étiologiques et processus pathologiques sont impliqués dans la MRB mortelle des bovins gardés en parcs d’engraissement.
(Traduit par Docteur André Blouin)
Introduction
The prevention and control of undifferentiated fever (UF)/bovine respiratory disease (BRD) in Canadian feedlots remains an important component of optimizing the welfare of cattle and the costs of beef production. Current prevention and control strategies for UF/BRD are directed towards the common etiologic agents involved in UF/BRD of feedlot calves (1–4). A variety of diagnostic tools, including bacterial and viral culture and serologic testing, have been used over the years to identify the etiologic agents that are involved in UF/BRD of feedlot calves (5–10). Recent diagnostic developments, such as immunohistochemical (IHC) staining, allow for a more standardized and perhaps more sensitive diagnostic work-up of samples (11). Previous studies have been conducted using IHC staining to determine the etiologic agents involved in feedlot mortality (12–15). However, general extrapolation of the results from these studies has been limited because of the sampling methods used, the populations studied, the lesions sampled, and the targeted use of IHC staining to confirm the presence of specific etiologic agents in lesions identified by histopathologic examination.
In western Canada, approximately 10% to 30% of auction market-derived calves are treated for UF/BRD. The mortality rate in animals treated for UF/BRD commonly ranges from 5% to 10% and the overall population mortality rate of auction market-derived calves is 2% to 4% (1,2,4,6,16). Postmortem examination findings collected at Feedlot Health Management Services Ltd. (FHMS) indicate that infectious respiratory disease in Alberta feedlots is comprised of both acute fatal respiratory disease and prolonged intractable respiratory disease cases.
Improving our understanding of the etiologic agents involved in the pathogenesis of UF/BRD is critical for developing appropriate preventive and treatment strategies. There are few current cross-sectional studies that investigate the etiologic agents and pathologic processes of the various chronologic stages of fatal UF/BRD. However, determination of the various etiologic agents and pathologic processes involved in fatal UF/BRD could be accomplished by selecting naturally occurring cases throughout the disease process continuum and systematically examining them by using a standardized combination of IHC staining and histopathologic examination. The descriptive information gathered, using this type of approach from a recent study, would be valuable for identifying any changes that occur over time in the spectrum of causative agents and pathologic processes involved in fatal UF/BRD of feedlot calves.
The aim of this study was to determine the microbiologic agents and pathologic processes involved in fatal UF/BRD of feedlot cattle and to investigate associations between agents as well as between agents and pathologic processes.
Materials and methods
Experimental design
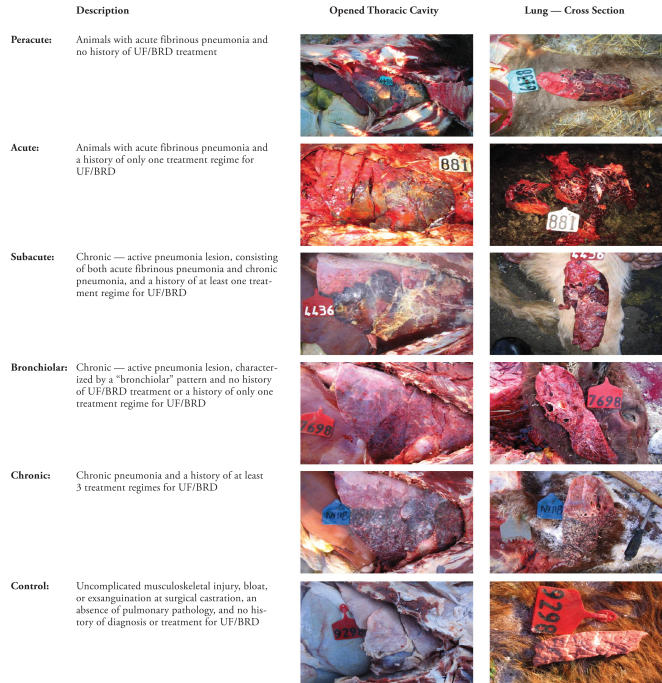
The study was designed so that standardized gross and microscopic postmortem examinations, in addition to laboratory testing, were performed on targeted fall-placed feedlot calves dying in the first 60 d of feedlot occupancy. A diagnosis of UF/BRD was made when animals showed evidence of depression, as characterized by lack of response to stimulation, reluctance to move, and/or abnormal posture/carriage of the head; a lack of abnormal clinical signs referable to body systems other than the respiratory system; and a rectal temperature > 40.5°C. The definition of UF/BRD cases; the prophylactic antimicrobial, viral vaccination, and bacterial vaccination programs; and the treatment protocols were standardized for all feedlots from which study animals were enrolled. Upon arrival at the feedlot, animals that were candidates for enrollment in the study were moved through a hydraulic chute for a group of procedures known collectively as processing. In this study, processing included applying unique, individual animal identification and administering a modified-live infectious bovine rhinotracheitis virus (IBRV) and a type I and II bovine viral diarrhea virus (BVDV) vaccine, a multivalent clostridial bacterin/toxoid, a Mannheimia haemolytica (MH)/Pasteurella multocida (PM) bacterin-toxoid, a Histophilus somni (HS) bacterin, prophylactic parenteral long-acting oxytetracycline, an anabolic growth implant, and a topical external and internal parasite control product. Animals diagnosed with BRD by a FHMS veterinarian were enrolled in the study and classified into 1 of 5 BRD study groups, based on the duration of the illness and the severity and distribution of lung lesions: peracute, acute, subacute, bronchiolar, and chronic infectious BRD (Figure 1). Animals that died of causes, such as uncomplicated musculoskeletal injury, bloat, or exsanguination at surgical castration, in which there was no evidence of pulmonary lesions and no diagnosis or treatment history for UF/BRD were enrolled in the study and classified as the control group. The study animals were from different feedlots that utilized a standardized set of methods and procedures for disease prevention, control, and treatment. In addition, the study feedlots used a computerized individual animal data recording system [Feedlot Health Animal Record Management (FHARM), FHMS, Okotoks, Alberta] to record all of an animal’s events from feedlot arrival to exit. Up to 25 animals were enrolled in each BRD study group and up to 25 animals were enrolled in the control study group.
Figure 1.
Definition of bovine respiratory disease (BRD) and control study groups used to examine the microbiological and histopathological findings in cases of fatal BRD of feedlot cattle in western Canada. The definition of the study groups are accompanied by a digital image of the open thoracic cavity and cross section of the lung of animals in the different groups.
UF — undifferentiated fever
Pneumonia was diagnosed when there was evidence for lung consolidation +/− atelectasis
Fibrinous peumonia was diagnosed when there was evidence for lung consolidation, edema, atelectasis, and fibrinous exudate, with or without associated fibrinous pleuritis
Lesions indicative of chronic pneumonia included pulmonary abscessation, adhesions, and/or shrinkage (presence of fibrous tissue)
Lesions indicative of acute pneumonia included pulmonary edema, swelling, or both
Bronchiolar pattern was diagnosed based on the presence of prominent and thickened bronchioles with or without exudate or debris in the lumen, which was detected on gross examination of the cross section of the lung
In order to maximize study resources and ensure that only appropriate target animals were enrolled in the study, a 2-stage enrollment process was implemented. Enrollment of appropriate animals in the study was done by the attending FHMS veterinarian, who collected the required study samples. The attending veterinarian made sure that animals met all the selection criteria. However, prior to final enrollment, the FHMS research team reviewed the digital images and treatment histories of each candidate animal to determine final inclusion in the study.
In animals that were enrolled in the study, the amount of diseased lung was recorded by using digital images, and pathologic processes were characterized by using gross postmortem findings to determine the most predominant lesion. In addition, 3 fixed lung samples (1 each from cranioventral, midlateral, and caudodorsal areas of the lung, hereafter referred to as lung sampling areas) and an ear skin biopsy were collected from each animal. Samples were fixed in formalin and submitted for IHC staining and histopathologic examination at Prairie Diagnostic Services (PDS), Saskatoon, Saskatchewan. All lung tissues and ear skin biopsies were removed from formalin and processed into paraffin blocks within 3 wk of sample collection.
Immunohistochemical staining was performed on all lung samples by following a standardized approach to identifying the microbiological agents present in the lung of each animal. The microbiological agents tested for by IHC staining included IBRV, BVDV, bovine respiratory syncytial virus (BRSV), parainfluenza-3 virus (PI3V), MH, HS, and Mycoplasma bovis (MB). Unfortunately, IHC staining for other agents, such as PM and Actinomyces pyogenes, could not be conducted because appropriate antibodies could not be identified for detecting these agents in formalin-fixed tissues, or for differentiating these microbiologic agents from organisms with similar antigenicity. A standardized IHC scoring system was used by a single technician to characterize staining in lung samples (Table 1). In addition, ear skin biopsies were tested for BVDV by IHC staining to identify animals that were persistently infected with BVDV. Lesions identified during histological examinations of lung samples were characterized by a single pathologist, using a standarized scoring system (Table 2). The IHC staining and histopathologic assessments for each sample were performed independently and all laboratory personnel were blinded as to the study group status of each sample.
Table 1.
Definition of the immunohistochemical staining scoring system used in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada
| Immunohistochemical staining score | Definition |
|---|---|
| Mannheimia haemolytica (MH) and Histophilus somni (HS) | |
| 0 | Negative |
| 1+ | Positive staining in foci in some but not all fields on the section (4× objective) |
| 2+ | Positive staining in all fields on the section (4× objective) but < 50% of each field is comprised of tissue that is positively stained for antigen |
| 3+ | Positive staining in all fields on the section (4× objective) and > 50% of each field is comprised of tissue that is positively stained for antigen |
| Mycoplasma bovis (MB) | |
| 0 | Negative |
| 1+ | Fewer than 10 lesions/section and < 50% of the area of fields is comprised of tissue that is positively stained for antigen (small and medium sized foci) |
| 2+ | More than 10 lesions/section but < 50% of the area of the fields are comprised of tissue that is positively stained for antigen (small and medium sized foci) |
| 3+ | More than 10 lesions/section and > 50% of the area of most fields is comprised of tissue that is positively stained for antigen (large sized foci) |
| Parainfluenza-3 virus (PI3V) and Bovine respiratory syncytial virus (BRSV) | |
| 0 | Negative |
| 1+ | Positive staining of an occasional bronchus or alveolus only, not all fields (4× objective) have positive airways or alveoli |
| 2+ | Some positive staining in bronchi or alveoli in all fields (4× objective) |
| 3+ | Majority of bronchi (and some alveoli) in all fields have positive staining |
| Bovine viral diarrhea virus (BVDV) | |
| 0 | Negative |
| 1+ | Occasional vessel has positive staining of tunica media (less than 1 vessel/field at 10× objective) |
| 2+ | Most fields have positive staining of 1 or more vessels (10× objective) |
| 3+ | All fields have positive staining of 1 or more vessels |
| 4+ | Positive staining in epithelial cells, as well as in vessels, in all fields of the section (PI/mucosal disease suspect) |
| Infectious bovine rhinotracheitis virus (IBRV) | |
| 0 | Negative |
| 1+ | Occasional foci of positive staining of epithelial cells (fewer than 1 focus/field at 10× objective) |
| 2+ | Most fields have positive staining of 1 or more foci of epithelial cells (10× objective) |
| 3+ | All fields have positive staining of 1 or more foci of epithelial cells (10× objective) |
| 4+ | Positive staining in epithelial cells that comprise more than 50% of the tissues present in the section |
1. Positive control samples for MH, HS, MB, and BVDV had an immunohistochemical staining score of 2+ for each pathogen
2. Positive control samples used for quality assurance had an immunohistochemical staining score of 3+ for PI3V, BRSV, and IBRV
3. All immunohistochemical staining scores were determined by a single technician
Table 2.
Definition of histopathologic scoring system used in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada
| Pathologic process | Definition |
|---|---|
| Suppurative bronchopneumonia | Suppurative bronchopneumonia/neutrophil infiltrations |
| Chronic suppurative pneumonia | Chronic suppurative pneumonia/abscesses |
| Bronchiectasis | Bronchiectasis/eosinophilic necrotic exudate |
| Fibrinonecrotizing pneumonia | Fibrinonecrotizing pneumonia/alveolar necrosis & fibrin; oat cells |
| Fibrinous pleuritis | Fibrinous pleuritis or interlobular inflammation/fibrin; edema; thrombotic lymphangitis |
| Hemorrhage | Hemorrhage/alveolar, perivascular, or septal |
| Bronchiolar necrosis | Bronchiolar necrosis/necrosis with or without syncytial cells |
| Bronchiolitis obliterans | Bronchiolitis obliterans |
| Acute/subacute interstitial pneumonia | Acute or subacute interstitial pneumonia/hypercellularity of alveolar septa; congestion; edema/hyaline membranes |
| Proliferative interstitial pneumonia | Proliferative interstitial pneumonia/type 2 pneumocytes; fibrosis |
| Other lesions | Bronchi-associated lymphoid tissue hyperplasia; vasculitis/granulomatous pneumonia; fungi; parasites |
1. The scoring system for each pathologic process was 0 — none, 1 — focal or multifocal lesion, 2 — single locally extensive lesion, 3 — widespread, extensive, or multiple locally extensive lesions, or 4 — diffuse lesion (throughout section)
2. All histopathologic examinations were performed by a single pathologist
The results of the gross postmortem, IHC staining, and histopathologic examinations were entered into an electronic spreadsheet (Microsoft Office Excel 2003; Microsoft Corporation, Redmond, Washington, USA) and verified.
Statistical analysis
Data were analyzed by using a software program (SAS for Windows, Release 9.1; SAS Institute, Cary, North Carolina, USA). Descriptive statistics, including cross-tabulations, were used to evaluate simple relationships between variables and the effect of using different levels of IHC staining and histopathologic scores to categorize animals as positive or negative for each agent or process. Spearman rank-sum correlations were used to evaluate the associations between IHC staining and histopathologic scores (17). Chi-square tests were used to evaluate the simple associations between categorical variables (18). Linear regression techniques were used to evaluate the complex associations between study groups and laboratory scores, using study group as the independent variable and the sums within animals for IHC staining and/or histopathologic scores as the dependent variables (19). A critical alpha of 0.10 was used in statistical comparison.
Results
Ninety-nine animals from 17 feedlots met the enrollment criteria. Ninety animals were enrolled in the BRD study groups (13 peracute, 24 acute, 25 subacute, 10 bronchiolar, and 18 chronic) and 9 animals were enrolled in the control group.
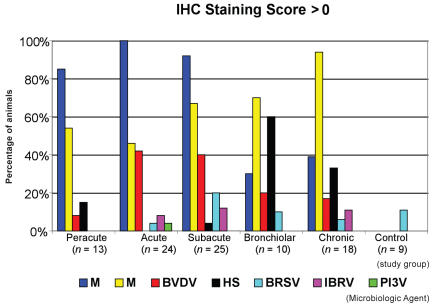
Immunohistochemical staining results from the different lung sampling areas revealed that MH and MB were the most commonly identified microbiologic agents at the animal level (IHC score > 0). Mannheimia haemolytica was more commonly found in the peracute, acute, and subacute study groups, and MB was more commonly found in the subacute, bronchiolar, and chronic study groups. Overall, both BVDV and HS were found less commonly than MH and MB. The highest frequencies of animal-level BVDV and HS detection occurred in the acute and subacute study groups and the bronchiolar and chronic study groups, respectively. On average, the other microbiologic agents in this study were identified in fewer than 10% of each study group. Regardless of the cut-off value used to define agent-positive animals, 2 or more microbiologic agents were detected in 40% to 60% of study animals (Table 3). The percentage of agent-positive animals in each study group, using an IHC staining score cut-off of > 0, is summarized in Figure 2. Utilization of different cut-off values for defining animal-level agent-positive status changed the absolute estimates for each agent, but it had minimal effects on the relative estimates between agents. Only 1 animal was identified as positive on IHC staining for PI3V; however, this animal, along with 2 other animals, was persistently infected with BVDV. In the control group, 1 animal was identified as positive for BRSV on IHC staining.
Table 3.
Within-animal distribution of the number of microbiologic agents detected by using an immunohistochemical staining score cut-off value of > 0 in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada
| Number of microbiologic agents detected
|
|||||
|---|---|---|---|---|---|
| Immunohistochemical staining cut-off values | 0 | 1 | 2 | 3 | 4 |
| > 0 | 9 | 24 | 47 | 16 | 3 |
1. Refer to Table 1 for definition of immunohistochemical staining scoring system used
Figure 2.
Percentage of pathogen-positive animals in each study group based on an immunohistochemical staining score cut-off of > 0 in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada.
MH — Mannheimia haemolytica
MB — Mycoplasma bovis
BVDV — bovine viral diarrhea virus
HS — Histophilus somni
BRSV — bovine respiratory syncytial virus
IBRV — infectious bovine rhinotracheitis virus
PI3V — parainfluenza-3 virus
The distributions of IHC staining scores for all microbiologic agents were very similar between the cranioventral and midlateral lung sampling areas, except for MB, which demonstrated higher IHC staining scores in the cranioventral sampling area than in the midlateral sampling area. In the caudodorsal sampling area, the distributions of IHC staining scores for all of the viral agents were very similar to the distributions of IHC staining scores in cranioventral and midlateral sampling areas. The distributions of IHC staining scores for all of the bacterial agents were markedly lower in the caudodorsal sampling area than in the cranioventral and midlateral sampling areas.
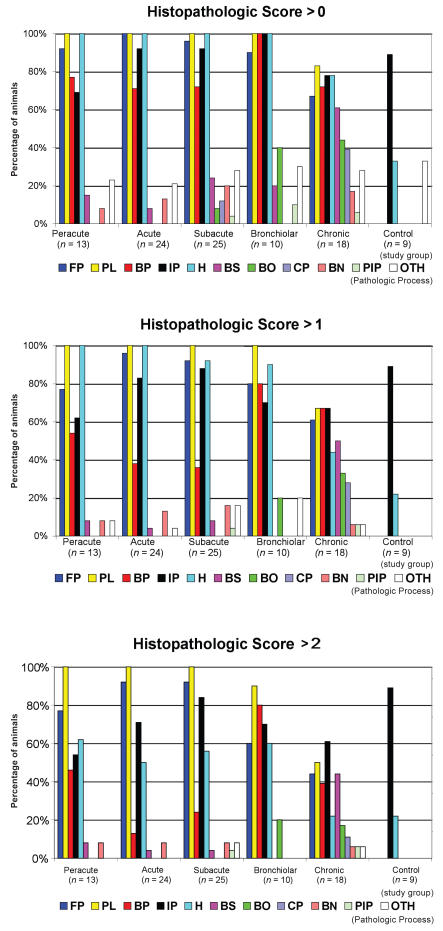
Results of histopathologic scoring revealed that fibrinous pleuritis, fibrinonecrotizing pneumonia, acute/subacute interstitial pneumonia, suppurative bronchopneumonia, and hemorrhage were the most commonly described pathologic processes. The occurrence of these 5 processes was remarkably similar across the peracute, acute, subacute, and bronchiolar study groups at the animal level. Histopathologic scoring data are summarized in Table 4 and Figure 3.
Table 4.
Within-animal distribution of the number of pathologic processes detected by using various histopathologic score cut-off values in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada
| Number of pathological processes detected
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Histopathologic cut-off values | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| > 0 | 0 | 6 | 1 | 6 | 11 | 40 | 21 | 9 | 5 |
| > 1 | 0 | 8 | 2 | 15 | 34 | 30 | 7 | 3 | 0 |
| > 2 | 0 | 8 | 13 | 36 | 30 | 10 | 1 | 1 | 0 |
1. Refer to Table 2 for definition of the histopathologic scoring system used
Figure 3.
Percentage of pathologic process-positive animals in each study group based on histopathologic score cut-offs of > 0, > 1, or > 2 in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada.
FP — fibrinonecrotizing pneumonia
PL — fibrinous pleuritis
BP — suppurative bronchopneumonia
IP — acute/subacute interstitial pneumonia
HM — hemorrhage
BS — bronchiectasis
BO — bronchiolitis obliterans
CP — chronic suppurative pneumonia
BN — bronchiolar necrosis
PIP — proliferative interstitial pneumonia
OTH — other lesions
Regardless of the cut-off values used for the histopathologic scores to define pathologic process-positive animals, 4 or more categories of processes were detected in 53% to 87% of the study animals (Table 5). Regarding histopathologic scores from each lung sampling area, the distributions of scores for suppurative bronchopneumonia, fibrinonecrotizing pneumonia, fibrinous pleuritis, and hemorrhage were highest in the cranioventral sampling area, slightly lower in the midlateral sampling area, and markedly lower in the caudodorsal sampling area. Conversely, distribution of scores for acute/subacute interstitial pneumonia was lowest in the cranioventral sampling area, higher in the midlateral sampling area, and highest in the caudodorsal sampling area.
Table 5.
Frequency of pathologic process detection in pathogen-positive animals for each microbiologic agent based on histopathologic score cut-offs of > 0, > 1, or > 2 in a study of the microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada
| Microbiologic agent
|
|||||||
|---|---|---|---|---|---|---|---|
| Pathologic process | IBRV (n = 7) | BVDV (n = 26) | PI3V (n = 1) | BRSV (n = 9) | MB (n = 59) | MH (n = 68) | HS (n = 15) |
| Histopathologic score > 0 | |||||||
| Suppurative bronchopneumonia | 57% | 69% | 0% | 60% | 78% | 68% | 100% |
| Chronic suppurative pneumonia | 14% | 8% | 0% | 0% | 17% | 4% | 27% |
| Bronchiectasis | 29% | 19% | 100% | 10% | 37% | 18% | 33% |
| Fibrinonecrotizing pneumonia | 100% | 96% | 100% | 80% | 86% | 99% | 87% |
| Fibrinous pleuritis | 100% | 100% | 100% | 90% | 97% | 100% | 93% |
| Hemorrhage | 100% | 96% | 100% | 80% | 93% | 99% | 93% |
| Bronchiolar necrosis | 29% | 23% | 0% | 10% | 17% | 15% | 7% |
| Bronchiolitis obliterans | 0% | 8% | 0% | 20% | 19% | 6% | 33% |
| Acute/subacute interstitial pneumonia | 57% | 88% | 100% | 90% | 86% | 88% | 80% |
| Proliferative interstitial pneumonia | 0% | 4% | 0% | 0% | 3% | 1% | 7% |
| Other lesions | 14% | 27% | 100% | 20% | 25% | 26% | 27% |
| Histopathologic score > 1 | |||||||
| Suppurative bronchopneumonia | 29% | 23% | 0% | 50% | 53% | 35% | 100% |
| Chronic suppurative pneumonia | 14% | 0% | 0% | 0% | 8% | 0% | 20% |
| Bronchiectasis | 14% | 12% | 100% | 0% | 22% | 10% | 13% |
| Fibrinonecrotizing pneumonia | 100% | 88% | 100% | 80% | 80% | 94% | 67% |
| Fibrinous pleuritis | 100% | 92% | 100% | 90% | 92% | 97% | 93% |
| Hemorrhage | 100% | 88% | 100% | 80% | 81% | 91% | 87% |
| Bronchiolar necrosis | 14% | 23% | 0% | 10% | 12% | 13% | 0% |
| Bronchiolitis obliterans | 0% | 0% | 0% | 10% | 12% | 0% | 27% |
| Acute/subacute interstitial pneumonia | 57% | 85% | 100% | 60% | 81% | 81% | 67% |
| Proliferative interstitial pneumonia | 0% | 4% | 0% | 0% | 2% | 1% | 0% |
| Other lesions | 14% | 12% | 100% | 10% | 8% | 10% | 7% |
| Histopathologic score > 2 | |||||||
| Suppurative bronchopneumonia | 14% | 19% | 0% | 40% | 37% | 19% | 93% |
| Chronic suppurative pneumonia | 0% | 0% | 0% | 0% | 3% | 0% | 13% |
| Bronchiectasis | 14% | 12% | 100% | 0% | 19% | 7% | 13% |
| Fibrinonecrotizing pneumonia | 100% | 81% | 100% | 80% | 69% | 90% | 47% |
| Fibrinous pleuritis | 100% | 92% | 100% | 90% | 85% | 97% | 73% |
| Hemorrhage | 43% | 58% | 100% | 50% | 46% | 49% | 60% |
| Bronchiolar necrosis | 0% | 15% | 0% | 0% | 7% | 9% | 0% |
| Bronchiolitis obliterans | 0% | 0% | 0% | 10% | 7% | 0% | 13% |
| Acute/subacute interstitial pneumonia | 57% | 85% | 100% | 50% | 78% | 72% | 67% |
| Proliferative interstitial pneumonia | 0% | 4% | 0% | 0% | 2% | 1% | 0% |
| Other lesions | 0% | 4% | 0% | 0% | 3% | 3% | 0% |
There were several significant (P < 0.05) associations detected between microbiologic agents and between agents and pathologic processes. When the lowest (least conservative) cut-off values for the IHC staining data were used, 25/26 (96%) animals that were identified as positive on IHC staining for BVDV were also positive for MH. Twelve of 15 (80%) animals that were positive for HS were also positive for MB. Conversely, none of the 15 samples that were positive for HS were positive for either MH or BVDV. There was no significant (P ≥ 0.10) association between MH and MB, as detected by IHC staining.
Significant relationships were also detected between IHC staining scores for microbiologic agents and histopathologic scores. In all 3 lung sampling areas, significant (P < 0.05) positive correlations (r= 0.27 to 0.55) existed between IHC staining for MH and the occurrence of fibrinonecrotizing pneumonia and fibrinous pleuritis. In the cranioventral and midlateral sampling areas, there were positive correlations between IHC staining for MH and hemorrhage (r = 0.35). In addition, there were significant (P < 0.05) negative correlations ( r= −0.20 to −0.35) between IHC staining for MH and the occurrence of chronic suppurative pneumonia, bronchiectasis, bronchiolitis obliterans, and acute/subacute interstitial pneumonia.
With respect to relationships between MB and pathologic processes, significant (P < 0.05) positive correlation ( r= 0.45) existed between IHC staining for MB and the occurrence of bronchiectasis in the cranioventral sampling area, but the correlation was weaker in the midlateral sampling area (r = 0.26) and nondetectable in the caudodorsal sampling area. There were significant (P < 0.05) positive correlations (r = 0.20 to 0.34) between IHC staining for MB and the occurrence of suppurative bronchopneumonia, chronic suppurative pneumonia, fibrinonecrotizing pneumonia, fibrinous pleuritis, and proliferative interstitial pneumonia that were inconsistently observed throughout the various lung sampling areas. There was a significant (P < 0.05) negative correlation ( r = −0.30) between IHC staining for MB and the occurrence of acute/subacute interstitial pneumonia, but this correlation was detected only in the cranioventral sampling area.
With respect to the relationships between HS and pathologic processes, there were significant (P < 0.05) positive correlations (r = 0.22 to 0.34) between IHC staining for HS and the occurrence of suppurative bronchopneumonia and chronic suppurative bronchopneumonia that were consistently identified whenever these processes were detected. In addition, there were significant (P < 0.05) positive correlations between IHC staining for HS and the occurrence of bronchiolitis obliterans (r = 0.20) in the cranioventral sampling area and fibrinous pleuritis (r = 0.32) in the caudodorsal sampling area. There was a significant negative correlation (r = −0.20) between IHC staining for HS and the occurrence of fibrinonecrotizing pneumonia, but this correlation was detected only in the cranioventral sampling area.
With respect to the relationships between BVDV and pathologic processes, there were significant (P < 0.05) positive correlations between IHC staining for BVDV and the occurrence of fibrinous pleuritis (r= 0.22) and bronchiolar necrosis ( r= 0.33) in the midlateral sampling area and bronchiectasis (r = 0.23) in the caudodorsal sampling area. There were no significant (P ≥ 0.10) positive correlations between IHC staining for any of the microbiological agents and acute/subacute interstitial pneumonia. The frequency of pathologic process detection in animals staining IHC positive for each microbiologic agent is summarized in Table 5.
Discussion
To the authors’ knowledge, this is the first standardized study to intensively investigate the microbiologic agents and pathologic processes associated with both acute and chronic pulmonary lesions observed in fatal BRD cases from the same feedlot cohorts. It commonly identified multiple microbiological agents and pathological processes in both acute and chronic cases of fatal BRD. Perhaps, synergistic relationships exist between the different microbiological agents and between the different pathological processes.
The results of this study clearly showed that MH, along with its pathological processes, was the predominant microbiological agent found in acute cases of fatal BRD, while MB, along with its processes, was the predominant microbiological agent found in chronic cases of fatal BRD. However, MB and MH, were also found to a lesser extent in acute and chronic cases of fatal BRD, respectively, and relative to MB and MH, it was less common to find other microbiological agents. These results may appear to contradict previously reported IHC staining-based data generated from other feedlot studies (12–15) that MB and BVDV were the most commonly identified microbiologic agents in fatal BRD cases. In the current study, MB and BVDV were not identified as the primary microbiologic agents and no significant (P ≥ 0.10) association was detected between MB and BVDV. However, the differences observed in pathogen detection levels between the current study and previous studies are likely due to 2 reasons: the 1st reason is the difference in the target BRD study groups; in the current study, all stages of uncomplicated UF/BRD were evaluated rather than putting an emphasis on animals with chronic disease and/or animals with multi-systemic disease. The 2nd reason is the standardization of potential confounding variables, which include disease prevention and treatment programs, animal age, and the stage of the feeding period at the time of death. In a recent study, BVDV did not appear to preferentially predispose to MB pneumonia and was more frequently associated with fibrinosuppurative bronchopneumonia than with MB pneumonia (20), which is supportive of the findings of the current study.
Interestingly, over 80% of the cattle in the control group had a histopathologic score for interstitial pneumonia of > 2. The exact reason for this finding is unknown. Perhaps, it is because the presence of congestion, which is not a specific finding for pneumonia, was one of the criteria for the diagnosis of interstitial pneumonia.
Several key factors that could have influenced the study findings were identified in the development of the project. These included selection of a target population, standardization of feedlot preventive and treatment strategies, definition of the target feedlot occupation period interval, specification of the target postmortem findings, development of standardized procedures for study enrollment, determination of appropriate laboratory procedures, and the use of standardized laboratory evaluation and scoring systems. Each of these factors was carefully considered and the final determination of the experimental design was based on a balance between scientific objectives, available resources, and study costs.
Fall-placed calves arriving at feedlots in western Canada were identified as the target population of interest for this study, because, historically, this population typically experiences the highest levels of UF/BRD-related mortality (1,2). In addition, the seasonal distribution of the calving period in western Canada results in a relatively narrow age span for the majority of calves entering feedlots in the fall. Moreover, these calves usually have minimal previous exposure to UF/BRD prior to feedlot arrival. In theory, variations in preventive and treatment programs used in this study could significantly impact the variation observed in the etiologic agents and pathologic processes. So, the preventive and treatment programs used in this study were standardized across all study candidates. The collection of standardized, real-time, electronic data for all feedlot events was deemed mandatory to facilitate accurate data collection and verification of study eligibility. Therefore, candidate animals were only selected from feedlots that used FHARM. In most instances, UF/BRD-related mortality is the most common cause of mortality in feedlot calves during the feeding period. Epidemiologic data from FHMS indicate that 70% of BRD mortality occurs in the first 60 d of feedlot occupation (1,2). Other causes of death, including the various manifestations of histophilosis and arthritis, are also commonly observed in feedlot calves (1–3,4,6,16). However, in order to have a sufficient experimental power to adequately describe the microbiologic agents and pathologic processes involved in fatal UF/BRD and to remove as much of the “background noise” as possible from the data, the resources of the study were focused only on uncomplicated BRD occurring in the first 60 d of the feeding period.
The original intent of the study was to include only 4 UF/BRD study groups (peracute, acute, subacute, and chronic). However, animals with gross postmortem findings characteristic of bronchiolar BRD did not fit easily into the original study groups because of an inability to effectively classify the gross postmortem findings as acute or chronic. As a result, a 5th UF/BRD study group was included for animals exhibiting uncomplicated bronchiolar BRD on gross postmortem examination.
The conventional methods typically used by diagnostic laboratories to report IHC staining results (positive/negative) tend to lack detail on intensity/distribution of staining and histopathologic findings (descriptive text) tend to lack standardization, consistent detail on severity/distribution, and appropriateness for statistical analysis. As a result, the PDS IHC staining and pathology teams developed the categorization and scoring systems that were used in the study. The scoring systems provided the opportunity for more detailed description of the study findings and exploration of the associations between microbiologic agents and pathologic processes involved in fatal UF/BRD.
Many statistical comparisons and univariate associations were made in this study, which creates a potential for Type II error that should be recognized. However, because of the descriptive nature of the study, the results and conclusions are less likely to be affected by this consideration.
Interagent associations warranting further investigation were identified. Moreover, if IHC staining for other agents, such as Pasteurella multocida and Actinomyces pyogenes, become available, the resulting data may improve interpretation of the current study results. Finally, the descriptive nature of the study results provide a standardized, cross-sectional benchmark of feedlot BRD lesions throughout the disease process that can be used to assess comparative changes over time.
Acknowledgments
We thank the management and staff of Prairie Diagnostic Service, Saskatoon, Saskatchewan, with specific reference to Dr. Ted Clark, Dr. Debbie Haines, Mrs. Melissa Koehnlein, Dr. Brendan O’Connor, and Dr. Jim Orr, for their assistance and expertise in conducting the laboratory components of this study. In addition, we thank the members of the support team at Feedlot Health Management Services Ltd., Okotoks, Alberta, for preparing, processing, and cutting all tissues collected for this study. CVJ
Footnotes
Authors’ contributions
Drs. Abutarbush and Booker wrote and edited the manuscript. Drs. Morley and Booker analyzed the data. Drs. Booker, Schunicht, Guichon, Jim, Wildman, Pittman, Perrett, Fenton, and Janzen helped in designing and conducting the experiment as well as collecting the samples.
This research was funded by the Beef Cattle Research Council, a Division of the Canadian Cattlemen’s Association, and the Alberta Beef Producers.
References
- 1.Schunicht OC, Guichon PT, Booker CW, et al. A comparison of prophylactic efficacy of tilmicosin and a new formulation of oxytetracycline in feedlot calves. Can Vet J. 2002;43:355–362. [PMC free article] [PubMed] [Google Scholar]
- 2.Schunicht OC, Booker CW, Jim GK, Guichon PT, Wildman BK, Hill BW. Comparison of a multivalent viral vaccine program versus a univalent viral vaccine program on animal health, feedlot performance, and carcass characteristics of feedlot calves. Can Vet J. 2003;44:43–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Schunicht OC, Booker CW, Guichon PT, et al. An evaluation of the relative efficacy of a new formulation of oxytetracycline for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 2002;43:940–945. [PMC free article] [PubMed] [Google Scholar]
- 4.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:179–184. [PMC free article] [PubMed] [Google Scholar]
- 5.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine. 9. Philadelphia: WB Saunders; 2000. pp. 829–846. [Google Scholar]
- 6.Booker CW, Guichon PT, Jim GK, Schunicht OC, Harland RJ, Morley PS. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:40–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Harris WH, Janzen ED. The Haemophilus somnus complex (Hemophilosis): A review. Can Vet J. 1989;30:816–822. [PMC free article] [PubMed] [Google Scholar]
- 8.Martin SW, Bateman KG, Shewan PE, Rosendal S, Bohac JG, Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res. 1990;54:337–342. [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor A, Martin SW, Nagy E, Menzies P, Harland R. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in three Ontario feedlots. Can Vet J. 2001;65:137–142. [PMC free article] [PubMed] [Google Scholar]
- 10.Rosendal S, Martin SW. The association between serological evidence of Mycoplasma infection and respiratory disease in feedlot calves. Can J Vet Res. 1986;50:179–183. [PMC free article] [PubMed] [Google Scholar]
- 11.Haines DM, Chelack BJ. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed, paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991;3:101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- 12.Adegboye DS, Halbur PG, Nutsch RG, Kadlec RG, Rosenbusch RF. Mycoplasma bovis-associated pneumonia and arthritis complicated with pyogranulomatous tenosynovitis in calves. J Am Vet Med Assoc. 1996;209:647–649. [PubMed] [Google Scholar]
- 13.Haines DM, Moline KM, Sargent RA, Campbell JR, Myers DJ, Doig PA. Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can Vet J. 2004;45:231–234. [PMC free article] [PubMed] [Google Scholar]
- 14.Haines DM, Martin KM, Clark EG, Jim GK, Janzen ED. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can Vet J. 2001;42:857–860. [PMC free article] [PubMed] [Google Scholar]
- 15.Shahriar FM, Clark EG, Janzen ED, West K, Wobeser G. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can Vet J. 2002;43:863–868. [PMC free article] [PubMed] [Google Scholar]
- 16.Booker CW, Jim GK, Guichon PT, Schunicht OC, Thorlakson BE, Lockwood PW. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1997;38:555–560. [PMC free article] [PubMed] [Google Scholar]
- 17.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames, Iowa: Iowa State Univ Pr; 1987. pp. 175–193. [Google Scholar]
- 18.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3. Hoboken, New Jersey: Wiley-Interscience; 2003. [Google Scholar]
- 19.Paulson DS. Applied Statistical Designs for the Researcher. New York: Dekker; 2003. [Google Scholar]
- 20.Gagea MI, Bateman KG, Shanahan RA, et al. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest. 2006;18(1):29–40. doi: 10.1177/104063870601800105. [DOI] [PubMed] [Google Scholar]