Abstract
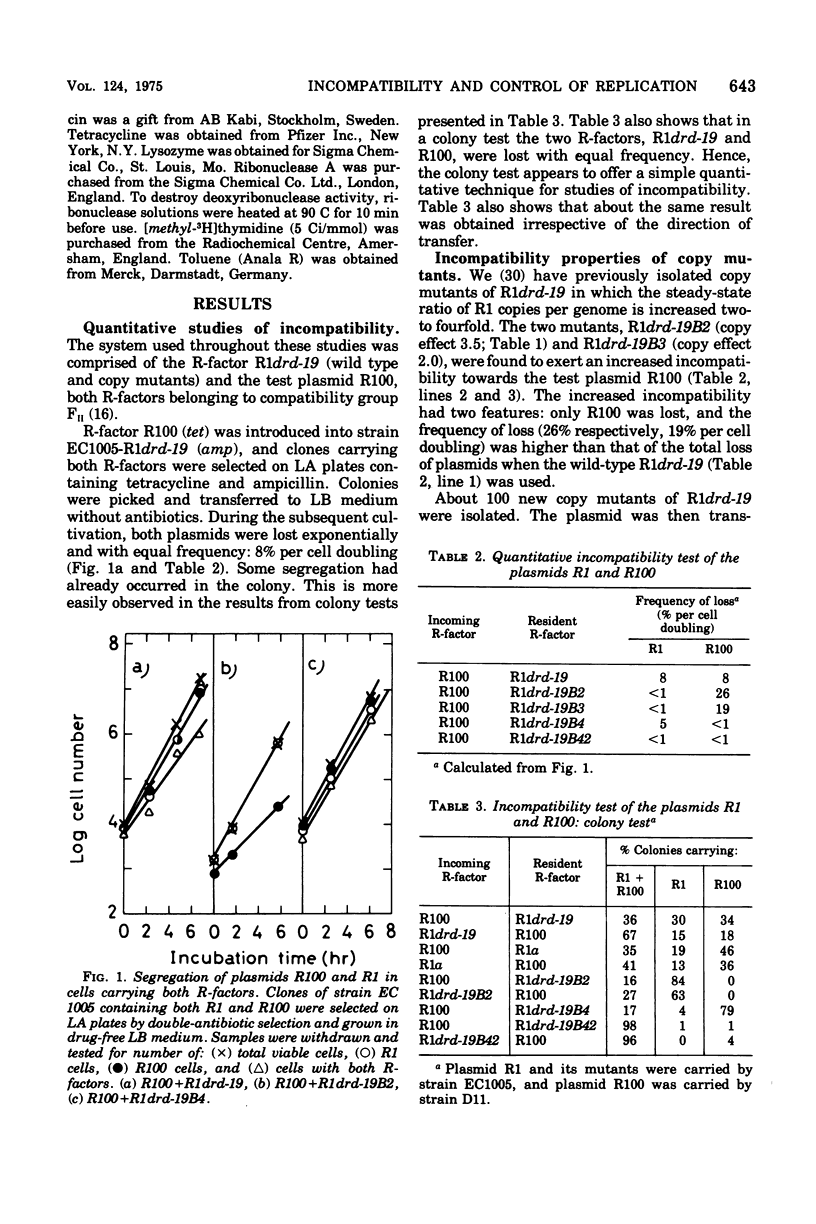
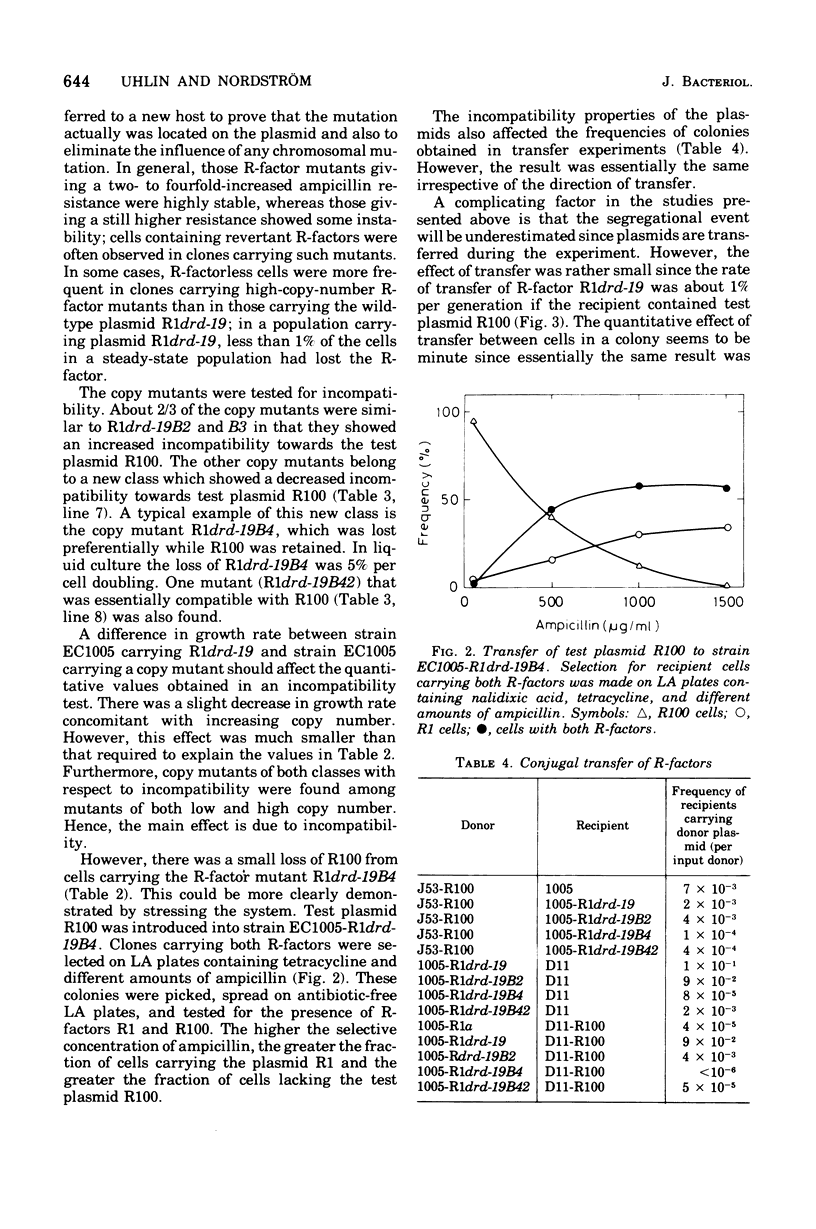
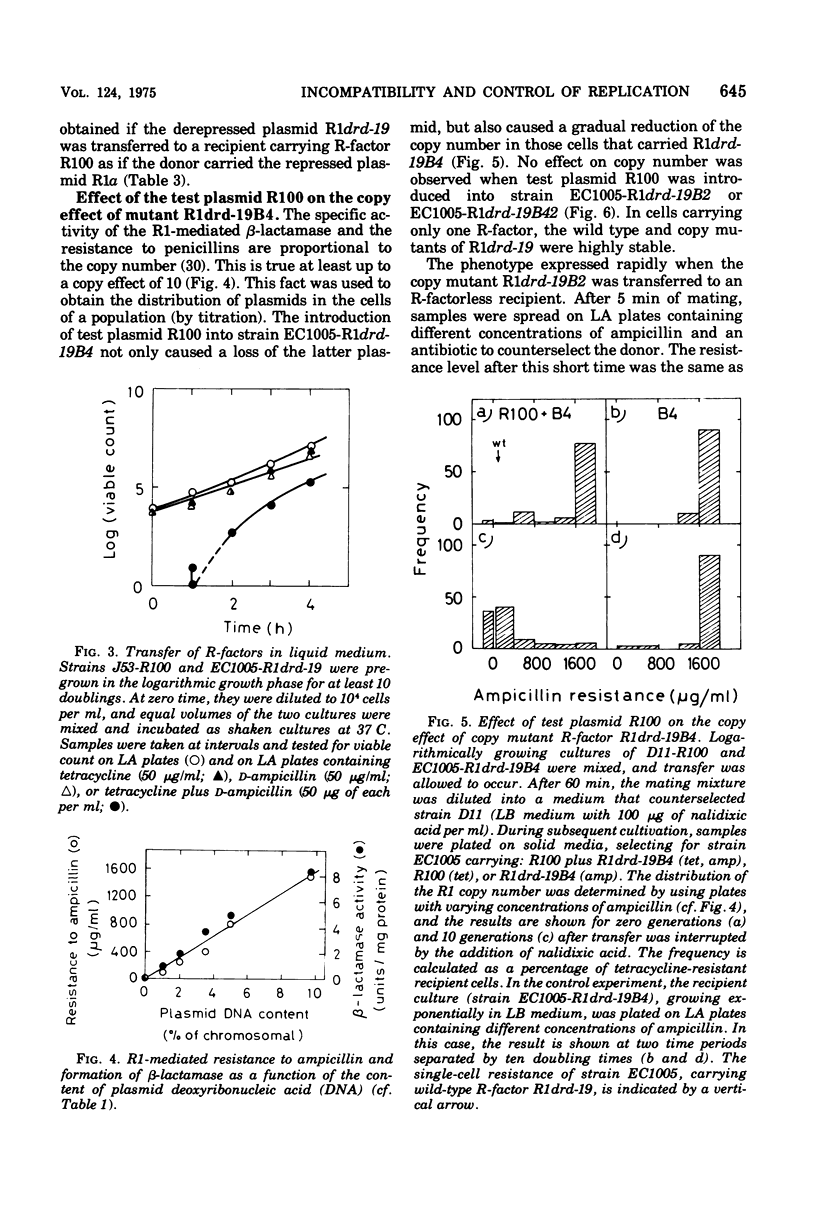
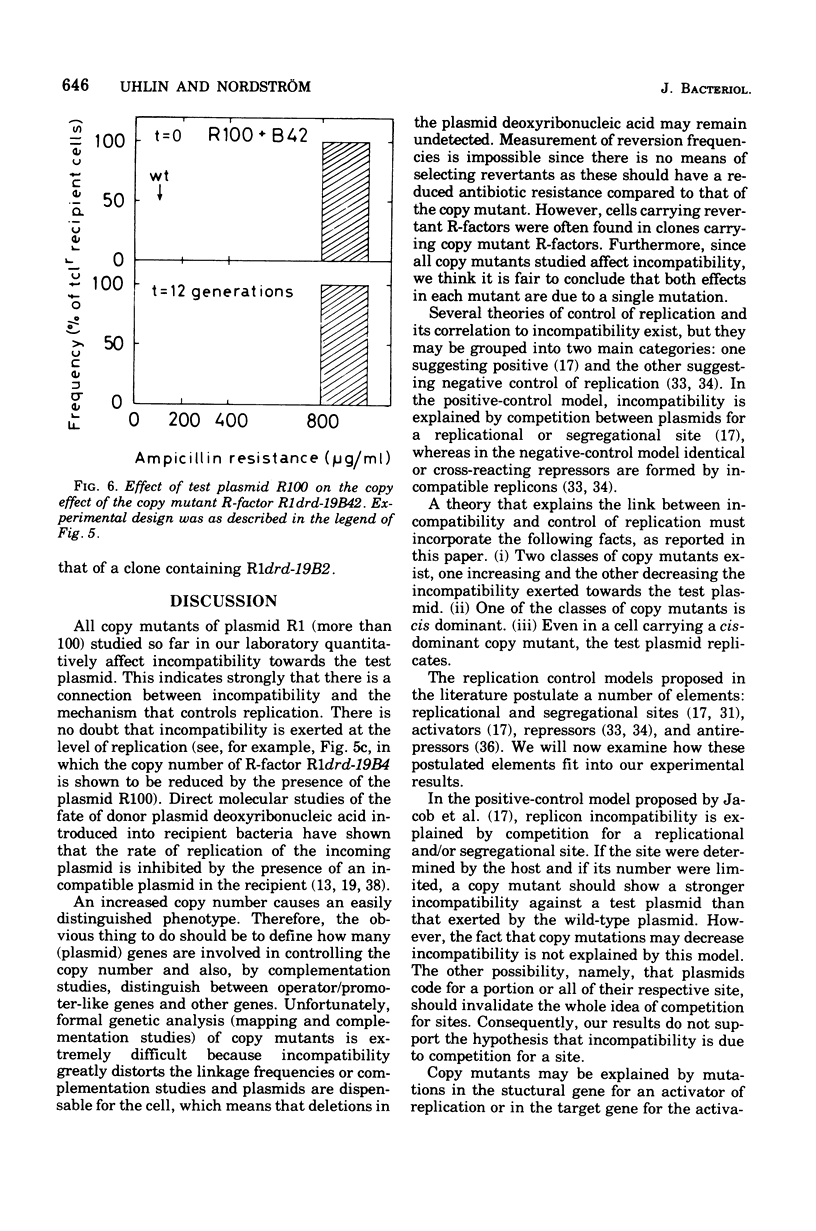
Plasmid incompatibility was studied in Escherichia coli K-12. By double-antibiotic selection, clones were constructed that carried the two R-factors R1 and R100, both belonging to the compatibility group FII. After release of the selection pressure, each of the two plasmids was lost at the same rate (8% per generation). Mutants of R-factor R1 showing an increased number of copies per chromosome (copy mutants) were tested for their incompatibility towards R-factor R100. The results indicate that plasmid incompatibility is quantitative and not just a qualitative property. All copy mutants studied affected incompatibility, and there were two classes of mutants: one increasing and one decreasing the incompatiblity exerted towards the test plasmid R100. Evidence is presented that incompatibility is related to the mechanisms that control replication. The implications of such a relation on proposed models for control of replication are discussed. The data do not support the hypothesis that plasmid incompatibility is due to competition for a replicational or segregational site.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E. Genes of phage lambda essential for lambda dv plasmids. Virology. 1974 Nov;62(1):224–233. doi: 10.1016/0042-6822(74)90317-1. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOWES R. C., ROWLEY D. Some observations on linkage effects in genetic recombination in Escherichia coli K-12. J Gen Microbiol. 1954 Oct;11(2):250–260. doi: 10.1099/00221287-11-2-250. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Pritchard R. H. Relationship between chromosome replication and F'lac episome replication in Escherichia coli. J Mol Biol. 1973 Jun 25;78(1):143–155. doi: 10.1016/0022-2836(73)90434-8. [DOI] [PubMed] [Google Scholar]
- DeVries J. K., Maas W. K. Description of an incompatibility mutant of Escherichia coli. J Bacteriol. 1973 Jul;115(1):213–220. doi: 10.1128/jb.115.1.213-220.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERRINGTON F. P., POWELL E. O., THOMPSON N. GROWTH CHARACTERISITICS OF SOME GRAM-NEGATIVE BACTERIA. J Gen Microbiol. 1965 Apr;39:109–123. doi: 10.1099/00221287-39-1-109. [DOI] [PubMed] [Google Scholar]
- Engberg B., Nordström K. Replication of R-factor R1 in Scherichia coli K-12 at different growth rates. J Bacteriol. 1975 Jul;123(1):179–186. doi: 10.1128/jb.123.1.179-186.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K. Random replication of the stringent plasmid R1 in Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):443–448. doi: 10.1128/jb.123.2.443-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. doi: 10.1099/00221287-81-1-171. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Falkow S. Studies on superinfection immunity among transmissible plasmids in Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):689–701. doi: 10.1016/0022-2836(73)90057-0. [DOI] [PubMed] [Google Scholar]
- Lindström E. B., Nordström K. Automated method for determination of penicillins, cephalosporins, and penicillinases. Antimicrob Agents Chemother. 1972 Feb;1(2):100–106. doi: 10.1128/aac.1.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K., Goldschmidt A. D. A mutant of Escherichia coli permitting replication of two F factors. Proc Natl Acad Sci U S A. 1969 Mar;62(3):873–880. doi: 10.1073/pnas.62.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Weatherly G. G., Curtiss R., 3rd R6K plasmid replication: influence of chromosomal genotype in minicell-producing strains of Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1387–1400. doi: 10.1128/jb.120.3.1387-1400.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL E. O., ERRINGTON F. P. Generation times of individual bacteria: some corroborative measurements. J Gen Microbiol. 1963 May;31:315–327. doi: 10.1099/00221287-31-2-315. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Chandler M. G., Collins J. Independence of F replication and chromosome replication in Escherichia coli. Mol Gen Genet. 1975;138(2):143–155. doi: 10.1007/BF02428118. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F., Ungers G. The negative control mechanism for E. coli DNA replication. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1410–1417. doi: 10.1073/pnas.63.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Hiraga S. F deoxyribonucleic acid superinfected into phenocopies of donor strains. J Bacteriol. 1975 Mar;121(3):1007–1013. doi: 10.1128/jb.121.3.1007-1013.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Maaloe O. Autorepressor model for control of DNA replication. Nat New Biol. 1973 Jan 31;241(109):133–135. doi: 10.1038/newbio241133a0. [DOI] [PubMed] [Google Scholar]
- Timmis K., Winkler U. Gene dosage studies with pleiotropic mutants of Serratia marcescens superactive in the synthesis of marcescin A and certain other exocellular proteins. Mol Gen Genet. 1973 Aug 17;124(3):207–217. doi: 10.1007/BF00293092. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Novick R. P. Studies on plasmid replication. IV. Complementation of replication-defective mutants by an incompatibility-deficient plasmid. Mol Gen Genet. 1974;135(2):149–161. doi: 10.1007/BF00264782. [DOI] [PubMed] [Google Scholar]



