In companion animals, a number of forms of cancer arise within the oral or nasal cavities, the brain, or other facial, aural, or rostral tissues. Cure or long-term control can be achieved in a good proportion of such cancers with early diagnosis and the appropriate selection and implementation of treatment modality. While a combination of therapies is often required to enhance outcome, full-course external beam radiation treatment has proven to be a valuable component in the treatment of several of these malignancies. For instance, adenocarcinomas, lymphomas, mast cell tumors, melanomas, meningiomas, plasmacytomas, soft tissue sarcomas, squamous cell carcinomas, and acanthomatous ameloblastomas have shown benefit from the use of radiation. A full-course radiation protocol generally involves 3 to 4 wk of daily treatments.
Whatever tissues or organs lie in the path of the treatment beams will be affected as the radiation passes through them and deposits dose. Certain regions of the skin will of necessity receive dose when an external source of radiation is used. Treatment of tumors of the head typically also directs radiation through, and hence causes side effects in, tissues associated with the oropharyngeal passage. Affected tissues include those that lie within the treatment fields designed for a given patient, such as mucous membranes and salivary glands. One of the goals influencing the planning of treatment is to reduce, as much as possible, the exposure of normal, disease-free tissues to the radiation that is being directed at the intended target. Accomplishment of this goal is facilitated through three-dimensional (3-D) treatment planning based primarily on computed tomographic images of the patient, along with accurate daily positioning of the patient. However, with existing technologies it is not yet possible to achieve a treatment plan that will altogether eliminate exposure of normal tissues.
The side effects that can be expected in these exposed tissues are discussed in the following paragraphs, as well as some of the management approaches. Acute effects begin during the course of treatment and usually resolve, with proper care, within the 2 to 4 wk after treatment has concluded.
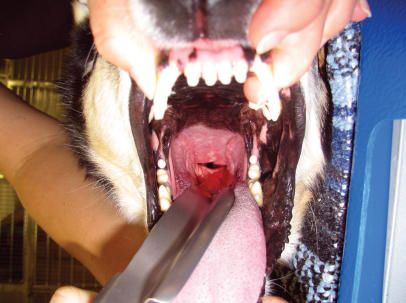
Mucous membranes are particularly prone to radiation injury because of their high cell turnover rate, the ease with which tissue trauma can occur during normal oral function, and the diversity of potentially infectious microorganisms inhabiting their surfaces (1–3). As described in the Veterinary Radiation Therapy and Oncology Group Acute Morbidity Scoring Scheme (4), the typical sequence of effects begins with injection without apparent inflammation (Figure 1). Then, mucositis initiates its appearance in isolated patches on various mucosal surfaces. This can begin as early as the 2nd wk of radiation therapy, with the worst symptoms occurring during the final week (1). In the most severe cases, effects progress to confluent fibrinous mucositis that sometimes can include areas of ulceration, hemorrhage, or necrosis (Figure 1). Even when this form of mucositis develops, healing is typically complete within the 2 wk following completion of therapy.
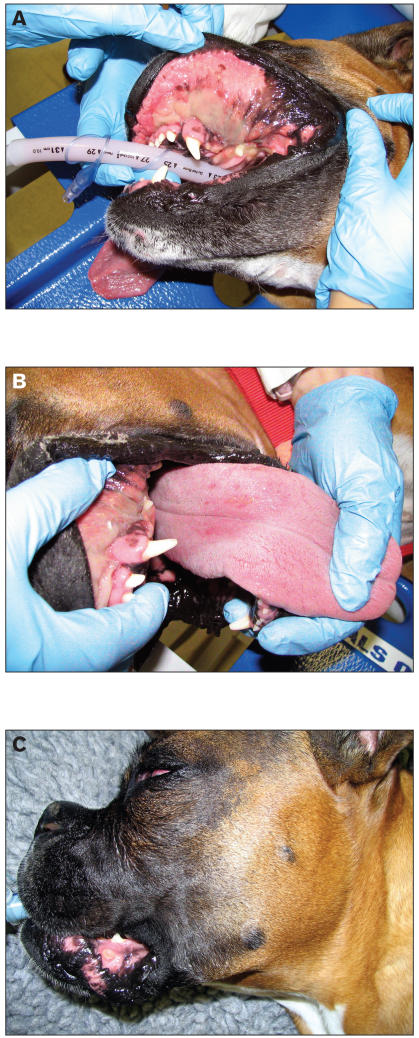
Figure 1.
Patient receiving radiation treatment for an undifferentiated carcinoma of the nasal cavity, with slow-release cisplatin as a radiation sensitizer. A: The patient has received 3900 cGy of a prescribed 5400 cGy, or 13 of 18 fractions. Note the confluent fibrinous mucositis along the buccal surfaces. B: The patient after 14 of 18 fractions. The tongue is not being subjected to full dose, and therefore shows little more than injection on its dorsal surface. C: At the completion of full-course treatment, ulceration is evident on the lower lip.
Human cancer patients who receive radiation treatment in the oropharyngeal region typically require progressively stronger interventions to alleviate and control pain as the mucous membranes suffer damage. Dysphagia and odynophagia (painful swallowing) are extremely common complaints (3). Since it is known that dogs and cats have neural pathways related to pain that are similar to those in humans, it is highly probable that our patients experience pain in a similar manner (5). Therefore the daily assessment of patients includes noting changes in behavior and physiological signs that are indicative of discomfort. Management involves a range of interventions such as changes in diet, local anesthetics, and analgesics. While responding to observed signs of discomfort is critical, preemptive strategies are also a significant component in a compassionate approach to pain prevention and management (5). If the patient’s usual diet consists of hard kibble, the practice at the Veterinary Teaching Hospital of the Western College of Veterinary Medicine (WCVM) is to soften the kibble with the addition of water or low-salt broth, or introduce canned food, prior to the visible onset of mucositis. In addition, a topical anesthetic slurry consisting of diphenhydramine, viscous lidocaine, and Maalox, is applied daily throughout the course of treatment (Figure 2). This slurry is administered after the daily radiation dose while the patient is still under general anesthesia. If the dog will permit irrigation of its oral mucosa while it is awake, the mixture will be applied 2 or 3 additional times per day. At the WCVM, oral L-glutamine is also given 3 times per day. L-glutamine may reduce the severity of oral mucositis (6), and most patients will readily accept it. Dental nerve blocks (7) are used later in the course of treatment, once the patient has begun to show signs of oral discomfort (Figure 3). As well, a number of systemic analgesics are prescribed in various combinations and levels of dosing, including nonsteroidal anti-inflammatory drugs, opioids, and other pain medications. On occasion, the complementary therapy of acupuncture is also incorporated into a patient’s care plan. Anecdotally, the families of patients have reported that acupuncture seems to relieve pain as well as stimulate appetite.
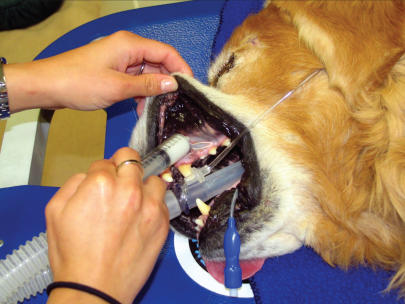
Figure 2.
Administration of topical anesthetic to the mucous membranes of the oral cavity.
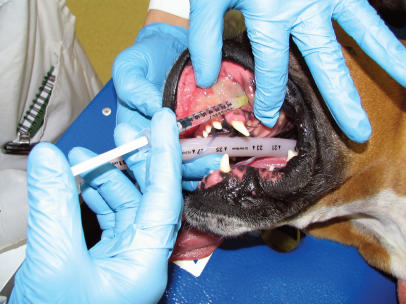
Figure 3.
Injection of local anesthetic through the infraorbital foramen.
Appetite suppression is generally an undesired effect for patients during cancer treatment. When a patient is losing interest in its food, especially palatable options such as fish or chicken can be offered as encouragement; sometimes appetite stimulants are given. Inadequately controlled pain can nevertheless reduce the patient’s ability to consume appropriate amounts of water or other nutrients needed for repair of normal tissue and other functions. Should this occur, the administration of fluid therapy (1) or temporary placement of a feeding tube could be indicated (1,8). Another outcome could be that the patient might require a break in the prescribed schedule of radiation treatment to permit tissues to partially heal before treatment is resumed. This decision in patient management cannot be made lightly, as its associated effect is a reduction in the probability of long-term tumor control (9). It is incumbent upon the entire team overseeing the care of the patient to monitor the dog or cat closely, to communicate effectively regarding observed changes in behavior, and to adjust care accordingly.
In addition to odynophagia, another reason that a patient could begin to lose interest in its usual diet is a treatment-induced alteration or loss of taste. This frequently occurs with human patients undergoing therapy involving the oropharyngeal region (10), and it is assumed that it also occurs in animals. Food that is served at room temperature can be more aromatic than cold food and hence more appealing. Radiation damage to the taste buds is believed to be primarily causative, although a reduction in saliva production also affects the perception of taste (10).
During the treatment planning process, the dose to the salivary glands will be limited as much as the tumor location and the treatment machine’s capabilities will allow. However, it is often impossible to avoid the glands altogether, and when this is the case, their function can be affected and xerostomia can result. Initially, xerostomia manifests itself as mild oral dryness with slightly thickened saliva and has an indiscernable effect on feeding behavior. However, beginning in the 3rd week, it can progress to moderate dryness with the saliva having considerable viscosity (Figure 4). To increase patient comfort, it is essential that food be moist and fresh water be readily available. Xerostomia is an effect that can improve soon after treatment is completed, as affected tissues recover, or as other non-irradiated glands compensate. However, xerostomia can also persist as a chronic condition that will necessitate a life-long diet of well-moistened food plus other interventions depending on its severity. Slices of cold cucumber and apple have been shown in human studies to stimulate residual salivary function and sooth the mucous membranes (11), and could benefit some canine patients. In addition to relieving pain, acupuncture has also demonstrated merit as a salivary stimulant (11). More traditional approaches include the prescription of a medication designed to alleviate xerostomia (12). At the WCVM, use of an oral moisturizer (Moi-Stir; PendoPharm, division of Pharmascience, Mont-Royal, Quebec) is recommended, although other medications are being considered in an effort to find a palatable yet effective tool. Human studies have shown that certain formulations are more helpful than others in improving patient comfort (11–12), and some offer the added benefit of strengthening, or at least not damaging, the enamel of patients’ teeth (13).
Figure 4.
Patient receiving treatment for an incompletely excised soft tissue sarcoma of the right aspect of the mandible. The patient has received 3510 cGy of a prescribed 5400 cGy, or 13 of 20 fractions. Note the ropey, extremely viscous saliva.
The latter point is significant in that teeth have a greater susceptibility to dental caries, due in large part to decreased salivary function (10,14). As well, the risk of complications following dental surgery is increased in irradiated tissues. For this reason, patients who are scheduled to receive radiation therapy of the oral region should first have a thorough dental exam (12,14). When possible, prophylaxis along with needed extractions should be performed. Adequate healing should be allowed before radiation is initiated. After the completion of full-course therapy, the animal’s caregivers should be encouraged to continue providing good oral hygiene, or else be trained in how to begin this practice. Regular dental exams will constitute an even more important component in the ongoing care received at the clinic of the referring veterinarian.
It should be noted that, in general, side effects tend to have an earlier onset and greater overall severity when radiation is given soon after surgical procedures, such as dental extractions or the actual tumor excision. If the tissues are not fully healed before the first dose of radiation is delivered, the additional assault upon cells in the process if repair and replacement will have an effect. This must be weighed against the fact that time spent waiting to initiate treatment equals time allotted for remaining tumor cells to increase in numbers. As well, side effects tend to appear earlier and be more severe when radiation is administered concurrently with chemotherapy. Drugs used to systemically treat cancer often also serve to potentiate the effects of radiation, and can themselves cause side effects. Mucositis in particular is often worse in patients undergoing concurrent therapies. Also, the larger the portion of the oropharyngeal passage that is irradiated, the more severe the effects on the involved tissues. When the targeted tumor lies in the brain, little more than the caudal aspect of the mouth will be affected (Figure 5). When the lesion is within the oral or nasal cavity, substantially more oropharyngeal tissue is of course involved. However, even when side effects are temporarily traumatic, with appropriate management, these effects will resolve. The impact of treatment can be difficult for a few weeks, but it should be possible for most patients and their families to return to a good quality of life shared together after the completion of a full-course radiation protocol.
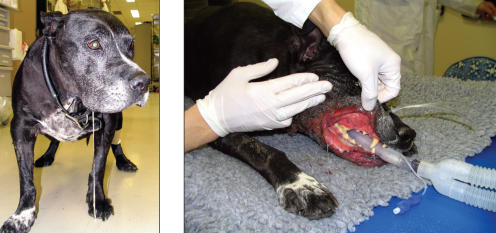
Figure 5.
Patient receiving treatment for lesion of the right dorsolateral brainstem, probable meningioma. The patient has received 2750 cGy of a prescribed 5500 cGy, or 11 of 22 fractions. Note the mucositis at the caudal aspect of the mouth.
References
- 1.Gillette EL, LaRue SM, Gillette SM. Normal tissue tolerance and management of radiation injury. Semin Vet Med Surg (Small Anim) 1995;10:209–213. [PubMed] [Google Scholar]
- 2.Sonis ST, Peterson DE, McGuire DB, editors. Mucosal injury in cancer patients: New strategies for research and treatment. J Natl Cancer Inst Monogr. 2001;29:1–54. [Google Scholar]
- 3.Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–83. doi: 10.1016/S1470-2045(06)70580-0. [DOI] [PubMed] [Google Scholar]
- 4.LaDue T, Klein MK. Toxicity criteria of the Veterinary Radiation Therapy Oncology Group. Vet Radiol Ultrasound. 2001;42:475–476. doi: 10.1111/j.1740-8261.2001.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 5.Hellyer P, Rodan I, Brunt J, Downing R, Hagedorn JE, Robertson SA. AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc. 2007;43:235–248. doi: 10.5326/0430235. [DOI] [PubMed] [Google Scholar]
- 6.Lana SE, Hansen RA, Kloer L, LaRue SM, Bachand AM, Ogilvie GK. The effects of oral glutamine supplementation on plasma glutamine concentrations in dogs experiencing radiation-concentrations and PGE2 induced mucositis. Int J Appl Res Vet Med. 2003;4:1–7. [Google Scholar]
- 7.Reuss-Lamky H. Administering dental nerve blocks. J Am Anim Hosp Assoc. 2007;43:298–305. doi: 10.5326/0430298. [DOI] [PubMed] [Google Scholar]
- 8.Wortinger A. Care and use of feeding tubes in dogs and cats. J Am Anim Hosp Assoc. 2006;42:401–406. doi: 10.5326/0420401. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5:23–31. [PubMed] [Google Scholar]
- 10.Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 11.Nieuw Amerongen AV, Veerman ECI. Current therapies for xerostomia and salivary gland hypofunction associated with cancer therapies. Support Care Cancer. 2003;11:226–231. doi: 10.1007/s00520-002-0409-5. [DOI] [PubMed] [Google Scholar]
- 12.Vissink A, Burlage FR, Spijkervet FKL, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–225. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 13.Kielbassa AM, Parichereh Shohadai S, Schulte-Montag J. Effect of saliva substitutes on mineral content of demineralized and sound dental enamel. Support Care Cancer. 2000;9:40–47. doi: 10.1007/s005200000148. [DOI] [PubMed] [Google Scholar]
- 14.Hancock PJ, Epstein JB, Sadler GR. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc. 2003;29:585–590. [PubMed] [Google Scholar]