Abstract
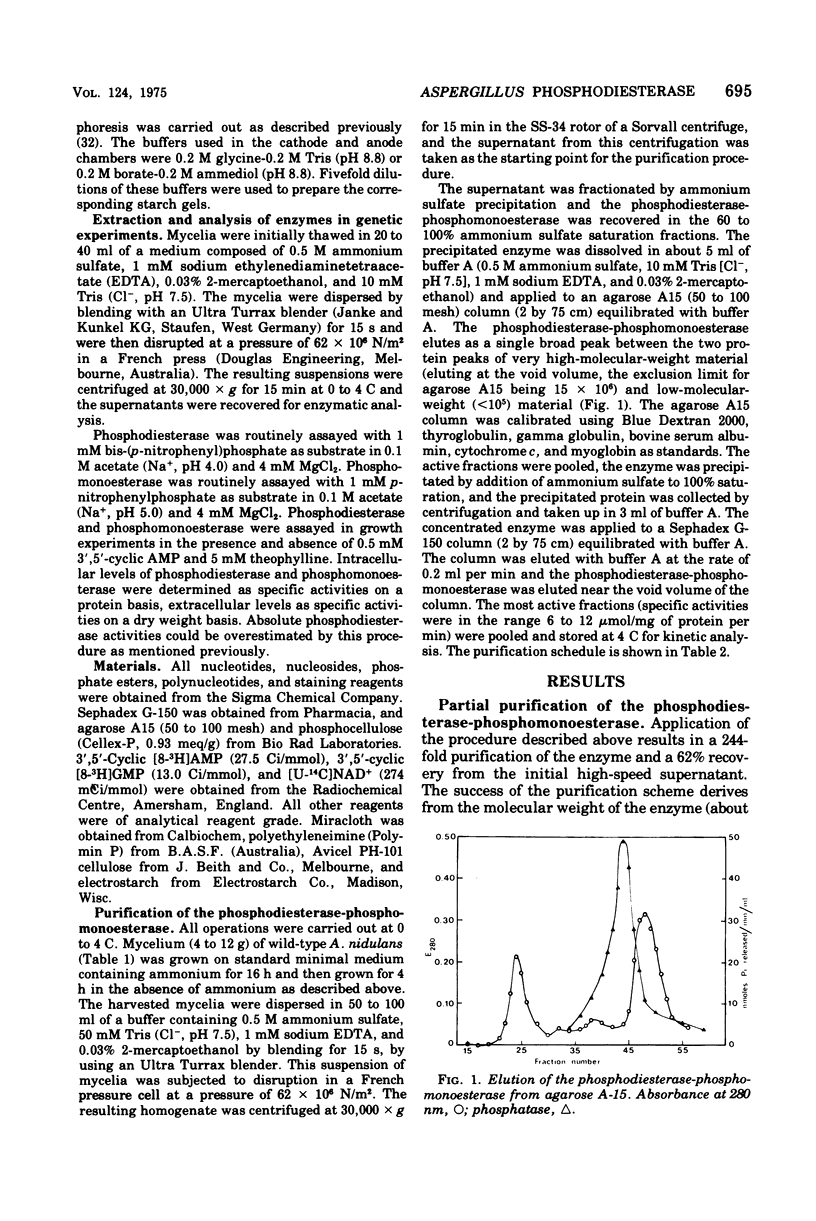
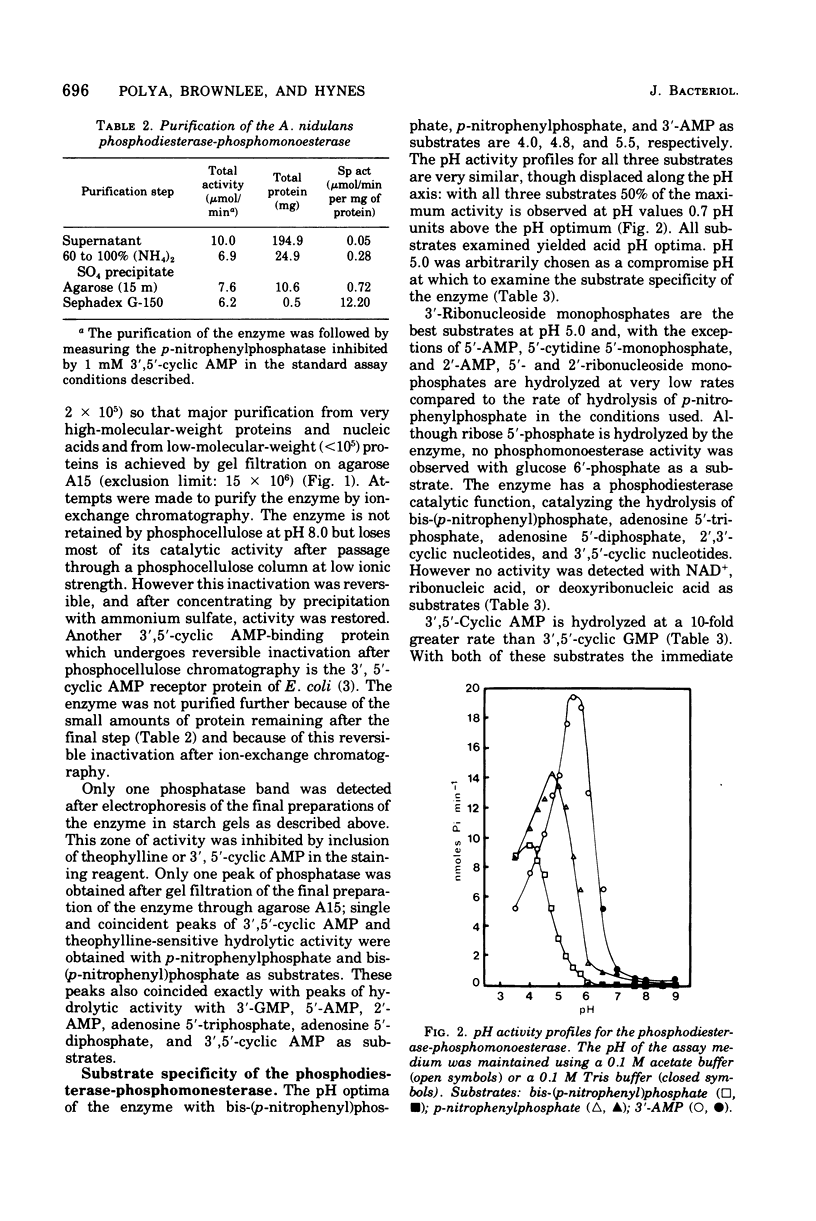
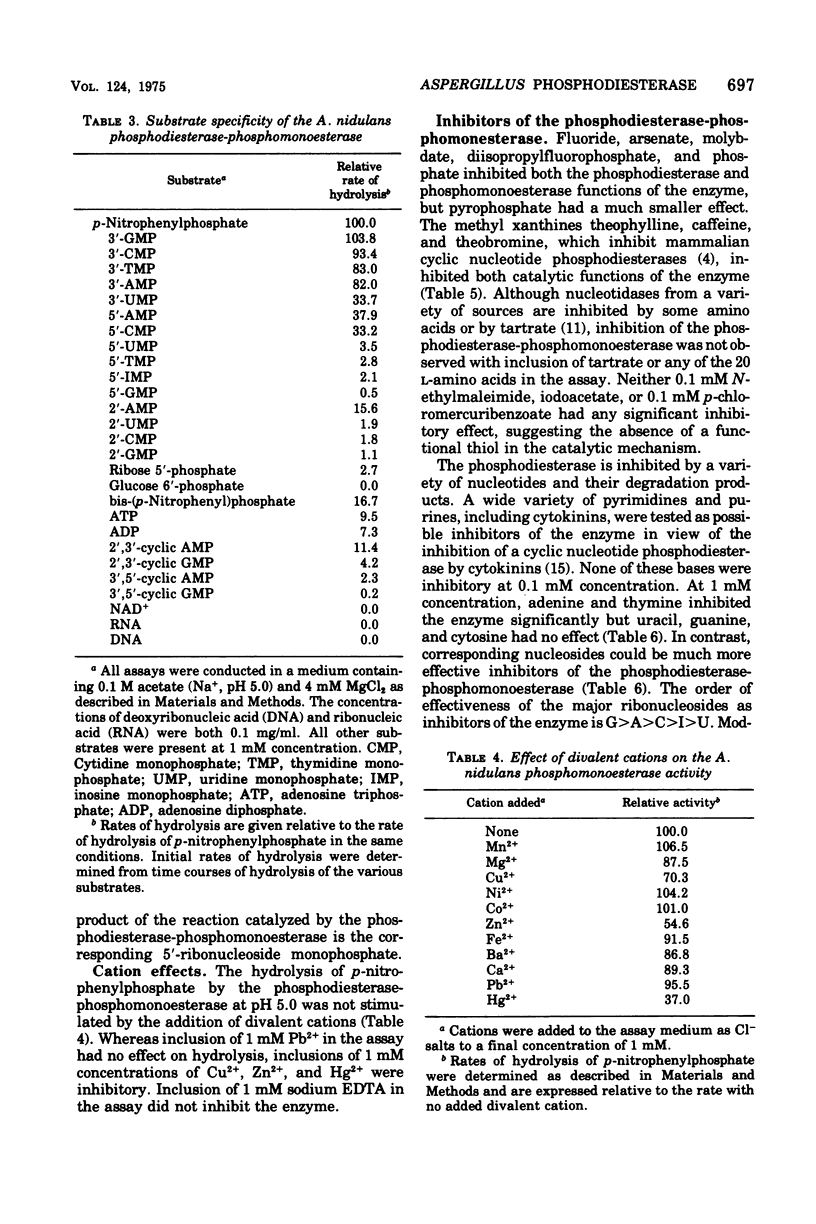
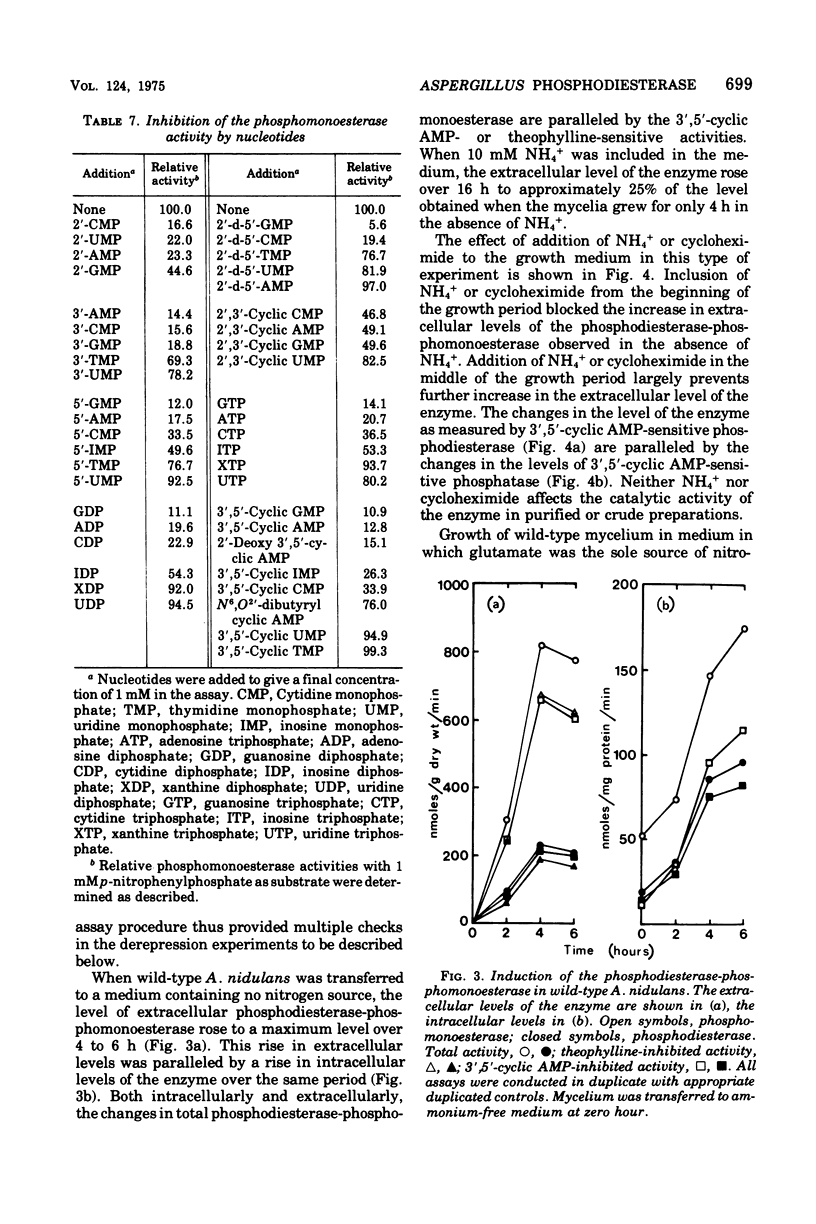
A cyclic nucleotide-binding phosphohydrolase that possesses both a phosphomonoesterase and a phosphodiesterase catalytic function has been partially purified from Aspergillus nidulans. The enzyme hydrolyzes both p-nitrophenylphosphate and bis-(p-nitrophenyl)-phosphate. o'-Nucleoside monophosphates are the best physiological phosphomonesterase substrates but 5'- and 2'-nucleoside monophosphates are also hydrolyzed. The enzyme catalyzes the hydrolysis of adenosine 5'-triphosphate, adenosine 5'-diphosphate, and 2',3'- and 3'5'-cyclic nucleotides, but not of ribonucleic acid, deoxyribonucleic acid, or nicotinamide adenine dinucleotide. The enzyme has acid pH optima and is not activated by divalent cations. Nucleosides and nucleotides inhibit the enzyme. Cyclic nucleotides are competitive inhibitors of the phosphodiesterase-phosphomonoesterase. The enzyme can occur extracellularly. The phosphodiesterase-phosphomonoesterase is present at high levels in nitrogen-starved mycelium, and it is strongly repressed during growth in media containing ammonium or glutamine and weakly repressed during growth in glutamate-containing medium. Experiments with various area mutants show that this regulatory gene is involved in the control of the enzyme. No evidence for regulation of the enzyme by carbon or phosphorus starvation has been found.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Pastan I. The cyclic AMP receptor of Escherichia coli: immunological studies in extracts of Escherichia coli and other organisms. Biochim Biophys Acta. 1973 Oct 5;320(3):577–587. doi: 10.1016/0304-4165(73)90137-2. [DOI] [PubMed] [Google Scholar]
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Arst H. N., Jr, Cove D. J. Nitrogen metabolite repression in Aspergillus nidulans. Mol Gen Genet. 1973 Nov 2;126(2):111–141. doi: 10.1007/BF00330988. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, MacDonald D. W. A mutant of Asperigillus nidulans lacking NADP-linked glutamate dehydrogenase. Mol Gen Genet. 1973 May 9;122(3):261–265. doi: 10.1007/BF00278601. [DOI] [PubMed] [Google Scholar]
- Bideon G. M. Purification and characterization of a cyclic nucleotide-regulated 5'-nucleotidase from potatoe. Biochim Biophys Acta. 1975 Apr 19;384(2):443–457. doi: 10.1016/0005-2744(75)90045-5. [DOI] [PubMed] [Google Scholar]
- Brownlee S. T., Heath E. C. An extracellular 5'-nucleotidase with both monoesterase and diesterase activity from Micrococcus sodonensis. Arch Biochem Biophys. 1975 Jan;166(1):1–7. doi: 10.1016/0003-9861(75)90357-4. [DOI] [PubMed] [Google Scholar]
- Cohen B. L. Regulation of intracellular and extracellular neutral and alkaline proteases in Aspergillus nidulans. J Gen Microbiol. 1973 Dec;79(2):311–320. doi: 10.1099/00221287-79-2-311. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- DORN G. GENETIC ANALYSIS OF THE PHOSPHATASES IN ASPERGILLUS NIDULANS. Genet Res. 1965 Feb;6:13–26. doi: 10.1017/s0016672300003943. [DOI] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Activation of membrane-bound adenylate cyclase by glucagon in Neurospora crassa. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2870–2873. doi: 10.1073/pnas.69.10.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. 3. Modulation by glucagon and insulin. J Biol Chem. 1973 Jul 10;248(13):4517–4520. [PubMed] [Google Scholar]
- Hamagishi Y., Yoshida H. Phosphodiesterase-phosphomonoesterases from Fusarium moniliforme. V. Mode of action on various nucleotides. J Biochem. 1974 Jul;76(1):81–89. doi: 10.1093/oxfordjournals.jbchem.a130563. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Faulkner R. D., Hawrelak S. D. Competitive inhibition of beef heart cyclic AMP phosphodiesterase by cytokinins and related compounds. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4670–4674. doi: 10.1073/pnas.71.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J. Alterations in the control of glutamate uptake in mutants of Aspergillus nidulans. Biochem Biophys Res Commun. 1973 Sep 18;54(2):685–689. doi: 10.1016/0006-291x(73)91477-0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Effects of ammonium, L-glutamate, and L-glutamine on nitrogen catabolism in Aspergillus nidulans. J Bacteriol. 1974 Dec;120(3):1116–1123. doi: 10.1128/jb.120.3.1116-1123.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J. Induction and repression of amidase enzymes in Aspergillus nidulans. J Bacteriol. 1970 Aug;103(2):482–487. doi: 10.1128/jb.103.2.482-487.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J. Mutants with altered glucose repression of amidase enzymes in Aspergillus nidulans. J Bacteriol. 1972 Sep;111(3):717–722. doi: 10.1128/jb.111.3.717-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J., Pateman J. A. The genetic analysis of regulation of amidase synthesis in Aspergillus nidulans. I. Mutants able to utilize acrylamide. Mol Gen Genet. 1970;108(2):97–106. doi: 10.1007/BF02430516. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. The effect of lack of a carbon source on nitrate-reductase activity in Aspergillus nidulans. J Gen Microbiol. 1973 Nov;79(1):155–157. doi: 10.1099/00221287-79-1-155. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. The effects of carbon source on glutamate dehydrogenase activities in Aspergillus nidulans. J Gen Microbiol. 1974 Mar;81(1):165–170. doi: 10.1099/00221287-81-1-165. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn J. R., Pateman J. A. NAD and NADP l-glutamate dehydrogenase activity and ammonium regulation in Aspergillus nidulans. J Gen Microbiol. 1973 Sep;78(1):39–46. doi: 10.1099/00221287-78-1-39. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman J. F., Gleason M. K., Ahlgren S. K., Metzenberg R. L. Regulation of phosphate metabolism in Neurospora crassa. Characterization of regulatory mutants. Genetics. 1973 Sep;75(1):61–73. doi: 10.1093/genetics/75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londesborough J. C. Soluble and membrane-bound cyclic AMP diesterase activity with a low Michaelis constant in baker's yeast. FEBS Lett. 1975 Feb 1;50(2):283–287. doi: 10.1016/0014-5793(75)80509-6. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Regulation of gene transcription in Escherichia coli by cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:11–16. [PubMed] [Google Scholar]
- Pateman J. A., Rever B. M., Cove D. J. Genetic and biochemical studies of nitrate reduction in Aspergillus nidulans. Biochem J. 1967 Jul;104(1):103–111. doi: 10.1042/bj1040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M. Regulation of a plant 5'(3')-ribonucleotide phosphohydrolase by cyclic nucleotides and pyrimidine, purine, and cytokinin ribosides. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1299–1303. doi: 10.1073/pnas.71.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Schlanderer G., Dellweg H. Cyclid AMP and catabolite repression in yeasts, In Schizosaccharomyces pombe glucose lowers both intracellular adenosine 3':5'-monophosphate levels and the activity of catabolite-sensitive enzymes. Eur J Biochem. 1974 Nov 1;49(1):305–316. doi: 10.1111/j.1432-1033.1974.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Solomon B. Cyclic 3',5'-AMP phosphodiesterase of Neurospora crassa. Biochem Biophys Res Commun. 1973 Aug 6;53(3):1024–1030. doi: 10.1016/0006-291x(73)90194-0. [DOI] [PubMed] [Google Scholar]
- Speziali G. A., Van Wijk R. Cyclic 3',5'-AMP phosphodiesterase of Saccharomyces carlsbergensis. Inhibition by adenosine 5'-triphosphate, inorganic pyrophosphate and inorganic polyphosphate. Biochim Biophys Acta. 1971 Jun 16;235(3):466–472. doi: 10.1016/0005-2744(71)90288-9. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sakai K., Morishita Y., Yamamura H., Nishizuka Y. Functional similarity of yeast and mammalian adenosine 3',5'-monophosphate-dependent protein kinases. Biochem Biophys Res Commun. 1974 Jul 24;59(2):646–652. doi: 10.1016/s0006-291x(74)80028-8. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Flawiá M. M., Torres H. N. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):990–996. doi: 10.1016/s0006-291x(74)80241-x. [DOI] [PubMed] [Google Scholar]
- Uno I., Yamaguchi M., Ishikawa T. The effect of light on fruiting body formation and adenosine 3':5'-cyclic monophosphate metabolism in Coprinus macrorhizus. Proc Natl Acad Sci U S A. 1974 Feb;71(2):479–483. doi: 10.1073/pnas.71.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Suzuki I. Cyclic AMP and citric acid accumulation by Aspergillus niger. Biochem Biophys Res Commun. 1973 Jan 23;50(2):237–244. doi: 10.1016/0006-291x(73)90831-0. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Suzuki I. Promotion of conidia aggregation in Aspergillus niger by cyclic AMP and 5'-GMP. Biochem Biophys Res Commun. 1973 Dec 10;55(3):824–830. doi: 10.1016/0006-291x(73)91218-7. [DOI] [PubMed] [Google Scholar]


