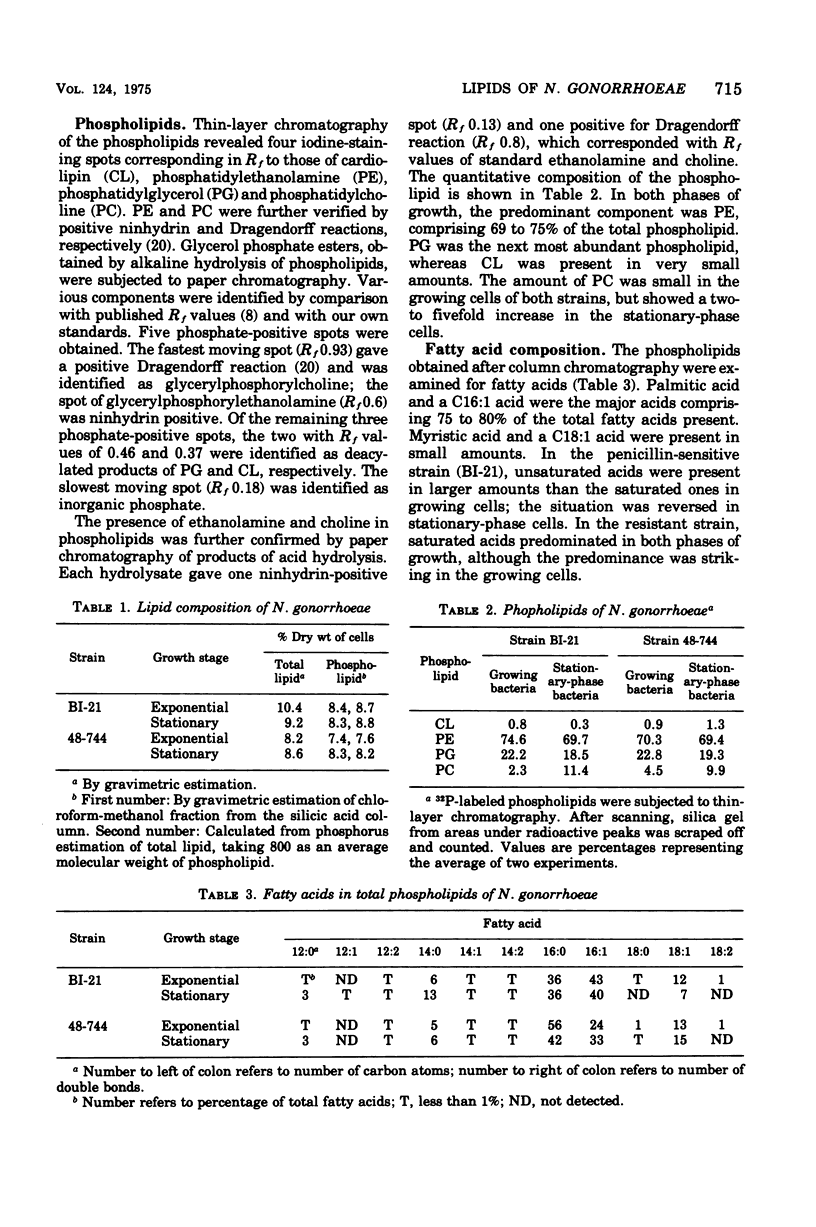
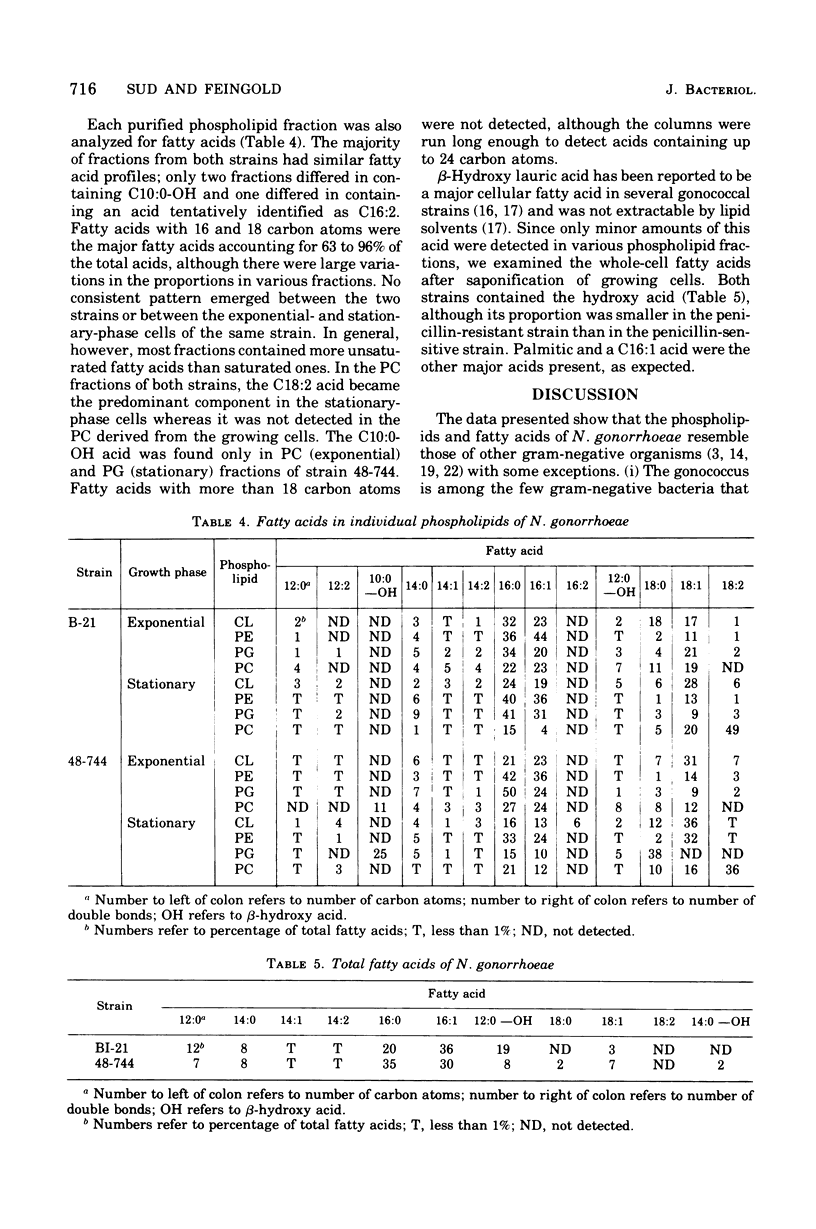
Abstract
The phospholipids and fatty acids of two strains of Neisseria gonorrhoeae of different penicillin susceptibilities were examined. The phospholipids, which comprise about 8% of the dry weight of the cells, consisted of phosphatidylethanolamine (70%) and phosphatidylglycerol (20%); small amounts of phosphatidylcholine and traces of cardiolipin were also present. Growing and stationary-phase cells were similar in content and composition of phospholipids except for phosphatidylcholine, which increased two- to fivefold in the stationary-phase cells. The fatty acids of the phospholipids were characterized by two major acids, palmitic and a C16:1, with myristic and a C18:1 acid present in smaller amounts. The fatty acids present in purified phospholipid fractions varied considerably in relative proportions from fraction to fraction. No significant difference in the composition of phospholipids from the two strains was evident. Large amounts of beta-hydroxy lauric acid were detected only after saponification of the organisms. Differences in the lipid composition between the gonococcus and other gram-negative bacteria are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A. Structural investigations on a cell-wall lipopolysaccharide from Neisseria sicca. Can J Biochem. 1971 Feb;49(2):243–250. doi: 10.1139/o71-035. [DOI] [PubMed] [Google Scholar]
- Anderes E. A., Sandine W. E., Elliker P. R. Lipids of antibiotic-sensitive and -resistant strains of Pseudomonas aeruginosa. Can J Microbiol. 1971 Nov;17(11):1357–1365. doi: 10.1139/m71-217. [DOI] [PubMed] [Google Scholar]
- Chang C. Y., Molar R. E., Tsang J. C. Lipid content of antibiotic-resistant and -sensitive strains of Serratia marcescens. Appl Microbiol. 1972 Dec;24(6):972–976. doi: 10.1128/am.24.6.972-976.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J. K., O'Leary W. M. Correlation of bacteria lipid composition with antibiotic resistance. J Bacteriol. 1970 Mar;101(3):892–900. doi: 10.1128/jb.101.3.892-900.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR J. W., INSULL W., Jr, ROSEN P., STOFFEL W., AHRENS E. H., Jr The analysis of fatty acid mixtures by gas-liquid chromatography; construction and operation of an ionization chamber instrument. Nutr Rev. 1959 Aug;17(8 Suppl):1–30. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hugo W. B., Stretton R. J. The role of cellular lipid in the resistance of gram-positive bacteria to penicillins. J Gen Microbiol. 1966 Jan;42(1):133–138. doi: 10.1099/00221287-42-1-133. [DOI] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Hollis D. G., Moss C. W., Weaver R. E., Thomas M. L. Cellular fatty acids of nonpathogenic Neisseria. Can J Microbiol. 1971 Dec;17(12):1491–1502. doi: 10.1139/m71-239. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kellogg D. S., Jr, Farshy D. C., Lambert M. A., Thayer J. D. Cellular fatty acids of pathogenic Neisseria. J Bacteriol. 1970 Oct;104(1):63–68. doi: 10.1128/jb.104.1.63-68.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lewis V. J. Characterization of clostridia by gas chromatography. I. Differentiation of species by cellular fatty acids. Appl Microbiol. 1967 Mar;15(2):390–397. doi: 10.1128/am.15.2.390-397.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Stokinger H. E., Ackerman H., Carpenter C. M. Studies on the gonococcus: I. Constituents of the cell. J Bacteriol. 1944 Feb;47(2):129–139. doi: 10.1128/jb.47.2.129-139.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Mechanism of polymyxin B resistance in Proteus mirabilis. J Bacteriol. 1970 Oct;104(1):289–294. doi: 10.1128/jb.104.1.289-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARLOV A. R., KENNEDY E. P. THE BETA-GALACTOSIDE PERMEASE SYSTEM AND THE METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1965 Jan;240:49–53. [PubMed] [Google Scholar]


