Abstract
OBJECTIVE
To find out whether the addition of fenofibrate to statin monotherapy produced any synergistic or additive beneficial effects in reducing risk factors, especially plasma fibrinogen, in patients with acute coronary syndrome (ACS) requiring percutaneous coronary interventions.
METHODS
A randomized, non-blinded, prospective study with parallel group design. One hundred two ACS patients who underwent angioplasty were randomly assigned to atorvastatin (20 mg/day, n=25), simvastatin (40 mg/day, n=27), atorvastatin-fenofibrate (10 mg/day-200 mg/day) combination (n=25) or simvastatin-fenofibrate (20 mg/day-200 mg/day) combination (n=25). The serum lipid profile and plasma fibrinogen were recorded before initiation of therapy and after three months of the respective treatments.
RESULTS
All patients already had desirable lipid levels as per the National Cholesterol Education Program – Adult Treatment Panel III guidelines. The addition of fenofibrate to statin monotherapy produced further benefits to the reduction in triglyceride and very low-density lipoprotein levels, and caused an increase in high-density lipoprotein levels. All the treatment groups showed a significant decrease in the plasma fibrinogen levels. Plasma fibrinogen did not correlate with study parameters such as age, body weight, hemo-dynamic characteristics and lipoprotein levels. Statin monotherapy as well as its combination with fenofibrate produced a significant decrease in the fibrinogen levels.
CONCLUSIONS
The addition of fenofibrate to statins seems to be beneficial in patients with ACS. Statins decreased plasma fibrinogen significantly, contrary to results from various reports, and the addition of fenofibrate further enhanced this reduction of the novel risk factor fibrinogen.
Keywords: Acute coronary syndrome, Fenofibrate, Plasma fibrinogen, Statins, Statinfibrate combination
The past century has seen a rapid increase in the global prevalence of coronary artery disease (CAD). Estimates from the Global Burden of Disease Study (1) have predicted that India faces the greatest health burden due to CAD. A number of inflammatory markers have been studied for their ability to predict future cardiovascular events in asymptomatic individuals and patients with established atherosclerotic disease (2). Among the emerging novel cardiac markers, plasma fibrinogen has been identified as an important risk factor for cardiovascular diseases. Many cross-sectional, case control and prospective cohort studies have identified elevated plasma fibrinogen levels as an independent risk factor for CAD, stroke and peripheral vascular disease (3).
Fibrinogen is an acute phase protein that is directly involved in coagulation. The transcription of fibrinogen is stimulated by interleukin-6; its synthesis is suppressed by interleukin-1-beta and tumour necrosis factor-alpha (4,5). Fibrinogen and its metabolites strongly affect hemostasis, hemorheology, platelet aggregation and endothelial function. In fact, fibrinogen’s association with increased mortality is likely due to its ability to promote thromboses, or clots, by causing platelet aggregation in blood vessels. The recognition that fibrinogen is an important factor in the promotion of various disease states has led to the search for specific therapies intended to reduce plasma fibrinogen levels.
The use of statins in the prevention of primary (6) and secondary (7,8) CAD has been demonstrated to significantly reduce cardiovascular events and total mortality. Nevertheless, the majority of patients on statin treatment still experience coronary events (9). A more effective reduction in the incidences of coronary events is needed. This could probably be accomplished by a further reduction in conventional as well as novel risk factors such as plasma fibrinogen, homocysteine and C-reactive protein. Multiple small studies have reported changes in fibrinogen levels with different statins (10,11). While atorvastatin is claimed to increase plasma fibrinogen, simvastatin is reported to either increase plasma fibrinogen or have a neutral effect (12). Although many different pharmacological approaches and strategies for therapeutic modulation of fibrinogen have been tested, the efficacy of different treatments to lower plasma fibrinogen in humans is limited and the mode of action unidentified (13,14). Among the few compounds known to lower circulating fibrinogen levels in humans are certain fibrates.
Fibrates reportedly lower plasma fibrinogen in humans, but the regulatory mechanism of this effect remains to be clarified. Activation of the nuclear hormone receptor peroxisome proliferative activated receptor-alpha (PPARα) mediates the suppression of fibrinogen gene transcription by fibrates in rodents (3). This establishes PPARα as a key regulatory factor in fibrinogen gene expression in rodents and may explain the suppressive effect of fibrates on plasma fibrinogen levels in humans. The fibrinogen molecule is arranged as a dimer, with each monomer composed of three nonidentical polypeptide chains: Aα, Bβ and γ (15). The three fibrinogen chains are encoded by three separate, closely linked genes situated on the same chromosome and located in the sequence γ, Aα, and Bβ, with the last one in opposite transcriptional orientation to the first one (16). Binsack et al (17) reported that in the human hepatoma cell line HepG2, bezafibrate suppressed Aα, Bβ and γ chain messenger RNA levels (18). The genes encoding the three fibrinogen chains are negatively regulated by PPARα and because fibrates act through this receptor, it helps to explain the benefits of fibrate therapy to target a reduction in fibrinogen levels. Maison et al (19) showed that fibrinogen concentration decreased after fibrate therapy, while it increased after statin treatment. Thus, the present study was conducted to investigate the controversial role of statins in modifying fibrinogen levels and to study the benefits of addition of fenofibrate to statin therapy in modifying levels of plasma fibrinogen.
RESEARCH DESIGN AND METHODS
The present study was a controlled, open, clinical trial. It was randomized, nonblind and based on parallel group design. The protocol of the study received ethical clearance from the Institutional Review Board of the Sterling Hospital, Ahmedabad, India. Patients of either sex with acute coronary syndrome (ACS) who had undergone a percutaneous transluminal coronary angioplasty procedure irrespective of the presence of diabetes mellitus were included in the study after obtaining their consent. Patients with second- or third-degree atrioventricular block, renal or hepatic failure, recent cerebrovascular events, valve replacement surgery or balloon mitral valvuloplasty, as well as patients on lipid-lowering therapy other than statins, were excluded from the study. Patients meeting the eligibility criteria were randomly assigned into four treatment groups, consisting of patients given atorvastatin, simvastatin, atorvastatin-fenofibrate combination or simvastatin-fenofibrate combination. Treatment was started after the percutaneous coronary intervention and was continued for three months. Atorvastatin was given in a single dose of 20 mg/day (if alone) and 10 mg/day (in combination with fenofibrate), simvastatin was given in a single dose of 40 mg/day (if alone) and 20 mg/day (in combination with fenofibrate) and fenofibrate was given in a single dose of 200 mg/day (in combination with atorvastatin or simvastatin). The first blood samples were collected before the beginning of treatment and were analyzed for total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), very low-density lipoprotein cholesterol (VLDL-C) and plasma fibrinogen. The second blood samples were collected after three months of treatment and were analyzed for total cholesterol, TG, HDL-C, LDL-C, VLDL-C and plasma fibrinogen, as well as liver function tests that included serum glutamic-pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), total bilirubin and alkaline phosphatase. Total cholesterol, TG, HDL-C, SGPT, SGOT, total bilirubin and alkaline phosphatase were analyzed on an automated VITROS 250 analyzer (Johnson & Johnson, USA) using enzymatic assay methods. LDL-C was analyzed by an enzymatic method. VLDL-C was calculated using Friedewald’s formula (20).
Plasma fibrinogen was assayed by the immunoprecipitation method using the RANDOX kits (Randox Laboratories Ltd, United Kingdom). Many studies measure fibrinogen by the von Clauss method (21). However, data from the Framingham Heart Study (22) suggest that levels determined by the immunoprecipitation test have a stronger association with cardiovascular disease than those obtained by the von Clauss method. The results were analyzed by applying Student’s t test, ANOVA and linear regression to find the degree of correlation between parameters. The value of probability less than 5% (P<0.05) was considered to be statistically significant.
RESULTS
One hundred two patients from the cardiology unit of Sterling Hospital were included in the present study. All of them had angiographically documented CAD and had undergone percutaneous transluminal coronary angioplasty. Twenty-five patients each were enrolled in atorvastatin, atorvastatin-fenofibrate combination and simvastatin-fenofibrate combination treatment groups, and 27 patients were enrolled in the simvastatin treatment group. There were four dropouts each in the atorvastatin-fenofibrate group and the simvastatin monotherapy group, and three dropouts in the simvastatin-fenofibrate group, all due to nontechnical reasons. Therefore, the analysis is based on a total of 91 patients who completed the follow-up successfully. The baseline demographic characteristics such as age, sex, body mass index, smoking, tobacco and alcohol habits, past history of diabetes, hypertension or family history, and other hemodynamic parameters such as hemoglobin, urea and creatinine were recorded (Table 1). These parameters were found to be identical in all the four treatment groups, indicating a symmetrical study design and population.
TABLE 1.
Baseline demographic and hemodynamic characteristics of the patients
| Characteristic | Atorvastatin (n=25) | Atorvastatin + fenofibrate (n=25) | Simvastatin (n=27) | Simvastatin + fenofibrate (n=25) |
|---|---|---|---|---|
| Age, years* | 56.76±9.4 | 56.44±9.95 | 58.44±11.4 | 58.35±11.35 |
| Sex, % | ||||
| Female | 4 | 12 | 7.4 | 20 |
| Male | 96 | 88 | 92.6 | 80 |
| Body mass index, kg/m2* | 25.79±3.5 | 23.62±1.95 | 25.3±3.9 | 25.44±4.25 |
| Smokers, % | 12 | 20 | 14.81 | 8 |
| Tobacco chewers, % | 12 | 12 | 7.4 | 4 |
| Diabetics, % | 24 | 36 | 29.62 | 48 |
| Hypertensives, % | 28 | 28 | 51.85 | 40 |
| Hemoglobin, g/L* | 126.1±16.0 | 121.7±17.5 | 131.6±17.6 | 130.7±16.5 |
| Urea (mmol/L)* | 10.9±6.74 | 9.57±4.26 | 9.61±4.71 | 9.60±4.71 |
| Creatinine (μmol/L)* | 108.73±44.2 | 113.15±97.24 | 97.24±27.4 | 97.24±27.4 |
| Random glucose (mmol/L)* | 7.17±2.16 | 8.07±3.6 | 7.58±3.16 | 7.56±3.11 |
| SBP, mmHg* | 126.54±16.05 | 129.52±24.25 | 128.22±22.47 | 128.84±22.8 |
| DBP, mmHg* | 81.29±8.75 | 78.35±9.00 | 80.55±11.31 | 80.96±11.55 |
| Pulse, beats/min* | 77.09±15.4 | 80.55±10.9 | 75.02±15.46 | 75.29±15.65 |
| LVEF, %* | 46.8±12.35 | 50.94±16.25 | 52.44±12.79 | 52.51±5.76 |
| Number of affected vessels, % | ||||
| One | 68 | 56 | 55.55 | 44 |
| Two | 28 | 28 | 29.62 | 36 |
| Three | 4 | 16 | 14.81 | 20 |
Data expressed as mean ± SD. DBP Diastolic blood pressure; LVEF Left ventricular ejection fraction; SBP Systolic blood pressure
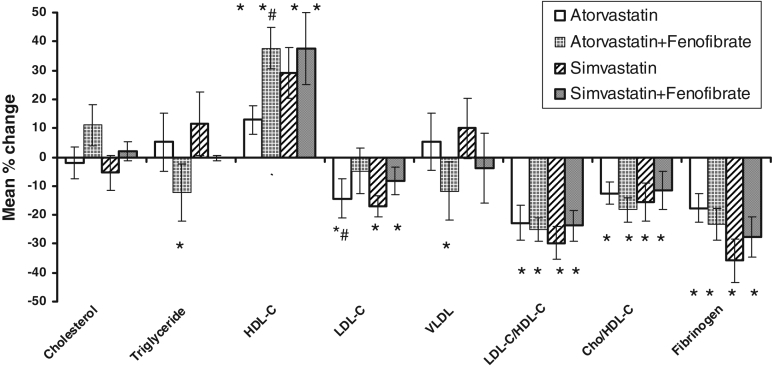
The effects of all the treatments on lipid parameters are given in Table 2. Atorvastatin and simvastatin monotherapy reduced serum LDL-C and, interestingly, increased serum HDL-C significantly (P<0.05). The atorvastatin-fenofibrate combination therapy produced a significant decrease in TG and VLDL-C (P<0.05), and also increased HDL-C significantly compared with atorvastatin alone (P<0.05). Similar to simvastatin monotherapy, simvastatin-fenofibrate combination produced a significant increase in HDL-C and decrease in LDL-C levels (P<0.05). It, however, did not reduce TG significantly. All treatments except atorvastatin-fenofibrate significantly reduced the total cholesterol/HDL-C ratio, and all treatments significantly reduced the LDL-C/HDL-C ratio (P<0.05). ANOVA was applied to find whether there was any difference in the effects among the four groups. The results, however, indicated that the percentage change in lipoprotein levels obtained were not significantly different from each other among the four treatment groups (Figure 1).
TABLE 2.
Effect of treatments on serum lipoprotein levels at baseline and at three months after the start of treatment
| Parameter | Time interval | Atorvastatin* (n=25) | Atorvastatin + fenofibrate† (n=21) | Simvastatin‡ (n=23) | Simvastatin + fenofibrate§ (n=22) |
|---|---|---|---|---|---|
| Total cholesterol, mmol/L | Baseline | 4.07±0.88 | 3.92±1.21 | 3.91±0.81 | 3.87±0.83 |
| Three months | 3.89±1.11 | 4.05±0.66 | 3.81±1.39 | 3.63±0.80 | |
| Triglycerides, mmol/L | Baseline | 1.59±0.84 | 1.71±1.23 | 1.52±0.86 | 1.50±0.87 |
| Three months | 1.47±0.68 | 1.19±0.52¶ | 1.35±1.11 | 1.21±0.52 | |
| HDL-C, mmol/L | Baseline | 0.98±0.24 | 0.91±0.24 | 0.91±0.22 | 0.91±0.22 |
| Three months | 1.08±0.24¶ | 1.22±0.22¶ | 1.13±0.33¶ | 1.13±0.34¶ | |
| LDL-C, mmol/L | Baseline | 2.63±0.87 | 2.37±0.86 | 2.42±0.60 | 2.39±0.63 |
| Three months | 2.14±0.89¶ | 2.25±0.48 | 1.99±0.68¶ | 1.99±0.68¶ | |
| VLDL-C, mmol/L | Baseline | 0.75±0.40 | 0.81±0.58 | 0.71±0.41 | 0.72±0.41 |
| Three months | 0.69±0.39 | 0.56±0.25¶ | 0.63±0.52 | 0.63±0.52 | |
| LDL-C/HDL-C | Baseline | 2.87±1.35 | 2.66±0.82 | 2.89±1.05 | 3.13±1.92 |
| Three months | 2.06±0.95¶ | 1.91±0.50¶ | 1.92±0.81¶ | 1.92±0.79¶ | |
| Total cholesterol/HDL-C | Baseline | 4.33±1.25 | 4.41±1.42 | 4.42±1.29 | 4.37±1.31 |
| Three months | 3.71±1.2¶ | 3.42±0.69 | 3.66±1.77¶ | 3.66±1.78¶ |
Data are expressed as mean ± SD. Degrees of freedom =24,
20,
22,
21;
Significant change from baseline values, Student’s t test, P<0.05. HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; VLDL-C Very low-density lipoprotein cholesterol
Figure 1.
Mean percentage change in lipid levels among treatment groups. *Significant change from baseline values, Student’s t test, P<0.05; #Significant change compared with the respective statin monotherapy or combination, unpaired Student’s t test, P<0.05; ANOVA applied on percentage change in levels, P>0.05. Changes are not significantly different from each other among groups. Cho Cholesterol; HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; VLDL Very low-density lipoprotein cholesterol
The effect of the treatments on plasma fibrinogen are given in Table 3. Atorvastatin and simvastatin were both found to decrease plasma fibrinogen significantly (P<0.05). Combination of statins with fenofibrate also showed a significant decrease in plasma fibrinogen (P<0.05). However, the treatment groups were not significantly different from each other when analyzed by ANOVA. Statins in the present study decreased plasma fibrinogen significantly, while the addition of fenofibrate enhanced this effect, although not significantly.
TABLE 3.
Effect of treatments on plasma fibrinogen levels
| Plasma fibrinogen, g/L | Atorvastatin* (n=25) | Atorvastatin + fenofibrate† (n=21) | Simvastatin‡ (n=23) | Simvastatin + fenofibrate§ (n=22) |
|---|---|---|---|---|
| Initial levels | 4.44±0.26 | 4.29±0.4 | 4.25±0.25 | 4.21±0.27 |
| After 3 months | 3.47±0.18¶ | 3.10±0.28¶ | 3.12±0.14¶ | 3.12±0.15¶ |
Degrees of freedom = 24,
20,
22,
21;
Significant change from baseline values, paired Student’s t test, P<0.05
No correlation was found between fibrinogen levels and various demographic, hemodynamic and biochemical parameters (Table 4). Thus, plasma fibrinogen was found to be an independent risk factor for CAD and was lowered significantly by atorvastatin and simvastatin alone and in combination with fenofibrate.
TABLE 4.
Correlation coefficient between fibrinogen and other study parameters
| Parameter | Correlation coefficient (r) |
|---|---|
| Age | 0.15 |
| Body mass index | −0.02 |
| Hemoglobin | −0.21 |
| Urea | 0.18 |
| Creatinine | −0.21 |
| Blood glucose | −0.14 |
| Total cholesterol | 0.05 |
| Triglycerides | 0.01 |
| HDL-C | −0.10 |
| LDL-C | 0.07 |
| VLDL-C | −0.02 |
| LDL-C/HDL-C | 0.10 |
| Total cholesterol/HDL-C | 0.10 |
HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; VLDL-C Very low-density lipoprotein cholesterol
No significant elevations in the serum bilirubin concentration, transaminases (SGPT and SGOT) and alkaline phosphatase levels were observed in any treatment group (Table 5). Thus, it appeared that all the treatments were safely tolerated in the given doses without any adverse hepatotoxic effects. Two patients in the simvastatin group had complaints of mild muscle pain, but the pain was self-resolving and not severe enough to cause discontinuation of the therapy. The rest of the patients had no complaints of muscle pain, weakness or myalgia. Thus, statin therapy alone or in combination did not lead to undesirable myopathy, as anticipated.
TABLE 5.
Effect of treatments on liver function parameters at the end of three months
| Parameter | Atorvastatin* (n=25) | Atorvastatin + fenofibrate† (n=21) | Simvastatin‡ (n=23) | Simvastatin + fenofibrate§ (n=22) |
|---|---|---|---|---|
| Total bilirubin, μmol/L | 11.97±4.27 | 11.45±3.07 | 12.14±4.79 | 12.14±4.79 |
| SGPT, U/L | 28.16±10.9 | 29.62±7.19 | 31.67±7.99 | 31.48±1.72 |
| SGOT, U/L | 24.46±7.1 | 29.14±6.59 | 27.62±7.28 | 27.55±1.56 |
| Alk phos, U/L | 94.04±23.05 | 69.00±16.48 | 74.51±26.25 | 73.80±5.42 |
Data expressed as mean ± SD. Degrees of freedom = 24,
20,
22,
21. Alk phos Alkaline phosphatase; SGPT serum glutamicpyruvic transaminase; SGOT serum glutamicoxaloacetic transaminase
DISCUSSION
Statins alone as well as in combination with fenofibrate were found to produce beneficial effects. Statins are potent inhibitors of HMG CoA reductase, which decreases LDL-C by upregulating LDL-C receptor activity in the liver and reducing the secretion of apolipoprotein B-containing lipoprotein. The latter is believed to be responsible for the TG-lowering effect of atorvastatin and has profound effects at higher doses (23). Various reports have indicated that atorvastatin lowers LDL-C and increases HDL-C (24–26). The effects of atorvastatin in our study confirm such reports. On the other hand, simvastatin is reported to produce a decrease in LDL-C ranging from 26% to 50% in various doses (27,28). Also, reports from Hunninghake et al (29) showed that simvastatin consistently produces a larger increase in HDL-C than does atorvastatin. Results from our study confirm the above findings because simvastatin in our study also produced a significant decrease in LDL-C and a significant increase in HDL-C compared with atorvastatin.
Fibrates activate PPARα, which ultimately leads to raising of HDL-C and lowering of TG (30,31). Previous studies have suggested increased benefits of a combination of fenofibrate with statins (32,33). Reports are available that show benefits of combining fenofibrate and simvastatin (33,34). A study of the literature also shows that a greater change in HDL-C and TG levels is obtained with a fenofibrate-atorvastatin combination compared with monotherapy (35) with either drug. Our results comply with these reported findings. We observed that combining fenofibrate with statins offered a greater benefit in reducing TG and VLDL-C levels as well as in increasing HDL-C levels. Thus, the combination therapy proved both safe and beneficial in our patients with ACS.
In prospective studies, plasma fibrinogen has been found to be an independent predictor of myocardial infarction in both sexes. It provides information on CAD risk over and beyond that supplied by established risk factors (36). The Framingham study has shown that for both sexes, the risk of cardiovascular diseases was correlated positively to antecedent fibrinogen values higher than 1.3 g/L to 7.0 g/L (37). We found that fibrinogen in our study did not correlate with any of our study parameters including demographic characteristics and lipid levels. Thus, fibrinogen does appear to be an independent risk factor for CAD.
Reports from large-scale trials consistently show statins to have a neutral effect on fibrinogen (38,39). Various studies on plasma fibrinogen indicate that fenofibrate lowers fibrinogen levels but the effects of atorvastatin and simvastatin have been variable and controversial, particularly those of atorvastatin. Wierzbicki et al (40) showed that atorvastatin increases plasma fibrinogen by 22%. Song (41) also found a significant increase in fibrinogen with atorvastatin treatment whereas simvastatin was reported to have a neutral effect. Various reports indicated that fenofibrate decreased plasma fibrinogen significantly whereas atorvastatin produced an increase in fibrinogen levels (42–44). Otto et al (45) showed that plasma fibrinogen and other hemorheological parameters were unchanged during atorvastatin treatment compared with during simvastatin treatment. Athyros et al (46) reported that plasma fibrinogen was unaffected by atorvastatin and was significantly reduced by fenofibrate and a combination of both. Ceska et al (47) showed that fenofibrate is a potent hypolipidemic drug with only rare side effects and that it reduces fibrinogen significantly. In our study, however, contrary to all above reports for statins, we observed that atorvastatin as well as simvastatin produced a significant decrease in plasma fibrinogen levels. However, our results comply with some recent findings by Kadikoylu et al (48) that showed a decrease in plasma fibrinogen by atorvastatin and simvastatin. Another study by Leibovitz et al (49) proved that atorvastatin reduces fibrinogen levels in patients with severe hypercholesterolemia; fibrinogen levels dropped by almost 18% in this study. Tekin et al (50) have also shown a reduction in plasma fibrinogen levels by atorvastatin in hyperlipidemic patients with angiographically proven CAD. Our findings with atorvastatin are consistent with these recent data. The combination of statins with fenofibrate also produced a further, although not significant, decrease in fibrinogen. These results are in agreement with the reported claims of fenofibrate to decrease plasma fibrinogen.
Previous studies have indicated an increased risk of myopathy with a statinfibrate combination (32,51). However, no cessation of therapy was required in any patient due to anticipated undesirable muscle-related complications of the therapy in our study. Liver function tests of all patients were also normal at the end of three months of treatments, indicating no adverse hepatotoxic effects. These findings suggest that it may be safe to use fenofibrate and statins in combination, contrary to reported contraindications for the combined use of these two classes of drugs. However, we have not looked into the long-term toxicity associated with the use of these drugs in combination. Moreover, the strategy appears to be distinctly beneficial in lowering the risk factor plasma fibrinogen. Thus, the therapy offers a new method to treat patients with ACS.
CONCLUSIONS
The addition of fenofibrate to statins seems to be beneficial in patients with ACS. Statins decreased plasma fibrinogen significantly, contrary to results from various reports. Also, in combination with fenofibrate, statins enhanced reduction of the novel risk factor fibrinogen.
REFERENCES
- 1.Murray JL, Lopez AL. Alternative projections of mortality and disability by cause 1990–2020. Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Tsimihodimos V, Kostoula A, Kakafika A, et al. Effect of fenofibrate on serum inflammatory markers in patients with high triglyceride values. J Cardiovasc Pharmacol Ther. 2004;9:27–33. doi: 10.1177/107424840400900i105. [DOI] [PubMed] [Google Scholar]
- 3.Kockx M, Gervois PP, Poulain P, et al. Fibrates suppress fibrinogen gene expression in rodents via activation of the peroxisome proliferator-activated receptor-alpha. Blood. 1999;93:2991–8. [PubMed] [Google Scholar]
- 4.Green F, Humphries S. Control of plasma fibrinogen levels. Baillieres Clin Haematol. 1989;2:945–59. doi: 10.1016/s0950-3536(89)80053-8. [DOI] [PubMed] [Google Scholar]
- 5.Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: The central role of interleukin-6. Eur Heart J. 2002;21:1574–83. doi: 10.1053/euhj.1999.2207. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd J, Cobbe SM, Ford I. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 7.Sacks FM, Pfeffer MA, Moye LA. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 8.The Scandinavian Simvastatin Survival Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 9.Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Athyros VV, Kontopoulos AG. Effect of atorvastatin versus simvastatin on lipid profile and plasma fibrinogen in patients with hypercholesterolemia. A pilot, randomized, double-blind, dose-titrating study. Clin Drug Investig. 1998;16:219–27. doi: 10.2165/00044011-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–4. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 11.Rosenson RS, Tangney CC. Anti-atherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;279:1643–50. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 12.Broncel M, Marczyk I, Chojnowska-Jezierska J, Michalska M, Sikora J, Kostka B. The comparison of simvastatin and atorvastatin effects on hemostatic parameters in patients with hyperlipidemia type II. Pol Merkur Lekarski. 2005;18:380–4. [PubMed] [Google Scholar]
- 13.Handley DA, Hughes TE. Pharmacological approaches and strategies for therapeutic modulation of fibrinogen. Thromb Res. 1997;87:1–5. doi: 10.1016/s0049-3848(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 14.Ernst E, Resch KL. Therapeutic interventions to lower plasma fibrinogen concentration. Eur Heart J. 1995;16:47–51. doi: 10.1093/eurheartj/16.suppl_a.47. [DOI] [PubMed] [Google Scholar]
- 15.Fuller GM. Fibrinogen: A multifunctional acute phase protein. In: Mackiewicz A, Kushner I, Baumann H, editors. Acute Phase Proteins: Molecular Biology, Biochemistry and Clinical Applications. New York: Doubleday; 1993. p. 169. [Google Scholar]
- 16.Kant JA, Fornace AJ, Saxe D, Simon MI, McBride OW, Crabtree GR. Evolution and organization of the fibrinogen locus on chromosome 4: Gene duplication accompanied by transposition and inversion. Proc Natl Acad Sci USA. 1985;82:2344–50. doi: 10.1073/pnas.82.8.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binsack R, Stegmeier K, Dorge L, Volkl A. Bezafibrate down-regulates fibrinogen biosynthesis in human hepatoma HepG2 cells. Eur J Clin Invest. 1998;28:151–63. doi: 10.1046/j.1365-2362.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 18.Roy SN, Mukhopadhyay G, Redman CM. Regulation of fibrinogen assembly. Transfection of Hep G2 cells with B beta cDNA specifically enhances synthesis of the three component chains of fibrinogen. J Biol Chem. 1990;265:6389–93. [PubMed] [Google Scholar]
- 19.Maison P, Mennon L, Sapinho D, et al. for the DESIR Study Group. A pharmacoepidemiological assessment of the effect of statins and fibrates on fibrinogen concentration. Atherosclerosis. 2002;160:155–60. doi: 10.1016/s0021-9150(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Clauss A. [Rapid physiological coagulation method in determination of fibrinogen. ] Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 22.Stec JJ, Silbershatz H, Tofler GH, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation. 2000;102:1634–8. doi: 10.1161/01.cir.102.14.1634. [DOI] [PubMed] [Google Scholar]
- 23.Burnett JR, Wilcox LT, Telford DE, et al. Inhibition of HMG CoA reductase by atorvastatin decreases both VLDL and LDL, apolipoprotein B production in miniature pigs. Arterioscler Thromb Vasc Biol. 1997;17:2589–600. doi: 10.1161/01.atv.17.11.2589. [DOI] [PubMed] [Google Scholar]
- 24.Neil HA, Fowler G, Patel H, Eminton Z, Maton S. An assessment of the efficacy of atorvastatin in achieving LDL cholesterol target levels in patients with coronary heart disease: A general practice study. Int J Clin Pract. 1999;53:422–6. [PubMed] [Google Scholar]
- 25.Marais AD, Firth JC, Bateman M, Jones J, Mountney J. Atorvastatin is a powerful and safe agent for lowering plasma cholesterol concentration in heterozygous familial hypercholesterolemia. Atherosclerosis. 1994;109:316–20. [Google Scholar]
- 26.Frost R, Otto C, Geiss C, Schwandt P, Parhofer KG. Effects of atorvastatin versus fenofibrate on lipoprotein profiles, low density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia. Am J Cardiol. 2001;87:44–8. doi: 10.1016/s0002-9149(00)01270-4. [DOI] [PubMed] [Google Scholar]
- 27.Jones P, Kafonek S, Laurova I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin and fluvastatin in patients with hypercholesterolemia. (the CURVES Study) Am J Cardiol. 1998;81:582–7. doi: 10.1016/s0002-9149(97)00965-x. (Erratum in 1998;82:128) [DOI] [PubMed] [Google Scholar]
- 28.Crouse JR, III, Frolich J, Ose L, Mercuri M, Tobert JA. Effects of high doses of simvastatin and atorvastatin on high-density lipoprotein cholesterol and apolipoprotein A-1. Am J Cardiol. 1999;83:1476–7. doi: 10.1016/s0002-9149(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 29.Hunninghake DB, Ballantyne CM, Maccubbin DL, Shah AK, Gumbiner B, Mitchel YB. Comparative effects of simvastatin and atorvastatin in hypercholesterolemic patients with characteristics of metabolic syndrome. Clin Ther. 2003;25:1670–86. doi: 10.1016/s0149-2918(03)80162-5. [DOI] [PubMed] [Google Scholar]
- 30.Staels B, Dallongvile J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–93. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 31.Watts GF, Dimmit SB. Fibrates, dyslipoproteinemia and cardiovascular disease. Curr Opin Lipid. 1999;10:561–74. doi: 10.1097/00041433-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Athyros VG, Papageorgiou AA, Hatziknonstandinou HA, et al. Safety and efficacy of long term statin-fibrate combination in patients with refractory familial combined hyperlipidemia. Am J Cardiol. 1997;80:608–13. doi: 10.1016/s0002-9149(97)00430-x. [DOI] [PubMed] [Google Scholar]
- 33.Ellen RL, McPherson R. Long term efficacy and safety of fenofibrate and statin in treatment of combined hyperlipidemia. Am J Cardiol. 1998;81:60B–65B. doi: 10.1016/s0002-9149(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 34.Vega GL, Ma PT, Cater NB, et al. Effects of adding fenofibrate (200 mg/day) in patients with combined hyperlipidemia and metabolic syndrome. Am J Cardiol. 2003;91:956–60. doi: 10.1016/s0002-9149(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 35.Keating GM, Ormod D. Micronised fenofibrate: An updated review of its clinical efficacy in the management of dyslipidaemia. Drugs. 2002;62:1909–44. doi: 10.2165/00003495-200262130-00013. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich J, Assmann G. Fibrinogen and cardiovascular risk. J Cardiovasc Risk. 1995;2:197–205. [PubMed] [Google Scholar]
- 37.Kannel WB, Neaton JD, Wentworth D, et al. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT trial. Multiple Risk Factor Intervention Trial. Am Heart J. 1987;112:825–36. doi: 10.1016/0002-8703(86)90481-3. [DOI] [PubMed] [Google Scholar]
- 38.Rosenson RS, Koenig W. Utility of inflammatory markers in the management of coronary artery disease. Am J Cardiol. 2003;92:10i–8i. doi: 10.1016/s0002-9149(03)00504-6. [DOI] [PubMed] [Google Scholar]
- 39.Lowe G, Rumley A, Norrie J. Blood rheology, cardiovascular risk factors and cardiovascular disease: The west of Scotland Coronary Prevention Study. Thromb Haemost. 2000;84:553–8. (Erratum in 2001;85:946) [PubMed] [Google Scholar]
- 40.Wierzbicki AS, Lumb PJ, Semra YK. Effect of atorvastatin on plasma fibrinogen. Lancet. 1998;351:569–70. doi: 10.1016/s0140-6736(05)78556-1. [DOI] [PubMed] [Google Scholar]
- 41.Song JC, White CM. Do HMG CoA reductase inhibitors affect fibrinogen? Ann Pharmacother. 2001;35:236–41. doi: 10.1345/aph.10211. [DOI] [PubMed] [Google Scholar]
- 42.Bairaktari ET, Tzallas CS, Tsimihodimos VK, Liberopoulos EN, Miltiadous GA, Elisaf MS. Comparison of the efficacy of atorvastatin and micronized fenofibrate in the treatment of mixed hyperlipidemia. J Cardiovasc Risk. 1999;6:113–6. doi: 10.1177/204748739900600208. [DOI] [PubMed] [Google Scholar]
- 43.Frost R, Otto C, Geiss C, Schwandt P, Parhofer KG. Effects of atorvastatin versus fenofibrate on lipoprotein profiles, low density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia. Am J Cardiol. 2001;87:44–8. doi: 10.1016/s0002-9149(00)01270-4. [DOI] [PubMed] [Google Scholar]
- 44.Serna G, Cadarso C. Fenofibrate decreases plasma fibrinogen, improves lipid profile, and reduces uricemia. Clin Pharmacol Ther. 1999;66:166–72. doi: 10.1053/cp.1999.v66.99709. [DOI] [PubMed] [Google Scholar]
- 45.Otto C, Geiss HC, Donner MG, Parhofer KG, Schwandt P. Influence of atorvastatin versus simvastatin on fibrinogen and other hemorheological parameters in patients with severe hypercholesterolemia treated with regular low-density lipoprotein immunoadsorption apheresis. Ther Apher. 2000;4:244–8. doi: 10.1046/j.1526-0968.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 47.Ceska R, Sobra J, Kvasnicka J, Prochazkova R, Kvasilova M, Haas T. The effect of micronized fenofibrate on lipid parameters and fibrinogen in heterozygous familial hypercholesterolemia and familial combined hyperlipidemia. Cas Lek Cesk. 1996;135:413–6. [PubMed] [Google Scholar]
- 48.Kadikoylu G, Yukselen V, Yavasoglu I, Bolaman Z. Hemostatic effects of atorvastatin versus simvastatin. Ann Pharmacother. 2003;37:478–84. doi: 10.1345/aph.1C189. [DOI] [PubMed] [Google Scholar]
- 49.Leibovitz E, Hazanov N, Frieman A, Elly I, Gavish D. Atorvastatin reduces fibrinogen levels in patients with severe hypercholesterolemia: Additional evidence to support the anti-inflammatory effects of statins. Isr Med Assoc J. 2004;6:456–9. [PubMed] [Google Scholar]
- 50.Tekin A, Tekin G, Guzelsoy D, et al. Effects of atorvastatin (10 mg) on hemostatic and inflammatory parameters in hyperlipidemic patients with angiographically proven coronary artery disease. Am J Cardiol. 2004;94:206–9. doi: 10.1016/j.amjcard.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 51.Tikkanen MJ. Statins: Within-group comparisons, statin escape and combination therapy. Curr Opin Lipidol. 1996;7:385–8. doi: 10.1097/00041433-199612000-00008. [DOI] [PubMed] [Google Scholar]