Abstract
Silent myocardial ischemia (SMI) is increasingly being recognized as part of the spectrum of ischemic heart disease. The spectrum of SMI ranges from asymptomatic coronary artery disease to critical illness necessitating intensive care. Although many diagnostic tools have been used to identify low- and high-risk subgroups, their use is limited by modest sensitivities and specificities. The present review identifies current concepts in the management of SMI in various clinical settings, as well as emerging technologies that may simplify the diagnosis and treatment of this condition.
Keywords: Continuous monitoring, Coronary artery disease, Silent myocardial ischemia
Silent myocardial ischemia (SMI) was recognized as early as the beginning of the 20th century (1). It is defined as objective evidence of myocardial ischemia in the absence of chest discomfort or other anginal equivalents. This objective evidence includes ST-segment shifts (usually depression), reversible regional wall motion abnormalities, and perfusion defects on scintigraphic studies (2). In fact, 70% to 80% of transient ischemic episodes are not associated with anginal chest pain or any other symptoms (silent ischemia) (3).
SMI is an important public health issue, and its early detection may prevent many episodes of sudden cardiac death annually (4). According to the World Health Organization, one of the cornerstone features in the diagnosis of acute coronary syndrome (ACS) is the presence of chest pain; however, not all patients with myocardial ischemia present with chest pain. The extent to which this phenomenon occurs is largely unknown (5). Episodes of asymptomatic ischemia occur in approximately 25% to 50% of patients with coronary artery disease (CAD) and may outnumber symptomatic episodes by a ratio of more than 20:1 (6).
OBJECTIVES
With increasing recognition of the frequency and prevalence of SMI, several questions arise about the need for detection, the prognostic implications and the approach to therapy, such as
the usefulness of early detection of SMI and risk stratification,
the need for actively diagnosing SMI and appropriate management strategies, and
the need to identify newer technologies for continuous monitoring.
MECHANISMS OF SMI
Silent ischemia results from variable combinations of decreased sensitivity to painful stimuli and coronary microvascular dysfunction (7). The association between diabetes and both silent ischemia and ‘painless infarctions’ has been attributed to autonomic neuropathy (4,8). Patients with silent ischemia have been shown to have a higher threshold for other forms of pain, such as that resulting from an electric shock, limb ischemia, cutaneous application of heat or balloon inflation in the coronary artery (9). Hypertensive patients who demonstrated a high incidence of silent ischemia were shown to have higher pain thresholds for and lower reactions to tooth pulp stimulation than normotensive subjects (10).
Another area of investigation suggests that silent ischemia may be due to cerebral cortical dysfunction, rather than peripheral nerve dysfunction. Frontal cortical activation appears to be necessary to experience cardiac pain, and some evidence indicates that in patients with silent ischemia, afferent pain impulses from the heart are subject to abnormal neural processing (11).
A study (12) using positron emission tomography scanning of the brains of nondiabetic patients with exercise-induced ischemia may explain why some episodes of ischemia are asymptomatic. Patients with asymptomatic ischemia had significantly less cortical activation than those who experienced angina. In addition, extracardiac factors may exert a critical influence on the central processing of afferent stimuli. Amplifying and abating influences may include analgesic effects arising from concurrent exercise or vagal stimulation, emotional status and personality characteristics (13). Mental stress can trigger ischemia in 40% to 70% of patients with CAD and has been reported to be a frequent trigger for the development of asymptomatic ischemia, acute myocardial infarction and sudden cardiac death (14,15). In fact, the development of ischemia in response to mental stress in the laboratory is independently associated with higher rates of fatal and nonfatal cardiac events, and predicts events over and above exercise-induced ischemia (16). Increased levels of beta-endorphin, an endogenous opiate, have been noted in patients with asymptomatic myocardial ischemia during exercise (17). The expression of peripheral benzodiazepine receptors is higher in patients with SMI than in symptomatic patients (18). Significantly elevated levels of anti-inflammatory cytokines (interleukin-4 and -10, transforming growth factor-beta), together with a decrease in leukocyte adhesion molecule expression (CD11b), may help identify some of the mechanisms of silent ischemia (19). SMI is also recognized as a common manifestation of percutaneous coronary intervention (PCI), possibly due to induction of coronary artery spasm (20).
CLASSIFICATION OF SMI
Cohn (4) divided SMI into three categories:
Type I: This is the least common form and occurs in completely asymptomatic patients with CAD (which may be severe) in the absence of anginal symptoms.
Type II: This type occurs in patients with documented previous myocardial infarction.
Type III: This is the most common form and occurs in patients with the usual forms of chronic stable angina, unstable angina and vasospastic angina.
POPULATIONS AT RISK
Although SMI has been recognized as a distinct clinical entity, considerable controversy still surrounds the application of appropriate screening guidelines in the asymptomatic, high-risk population (21).
In patients with CAD
The prevalence of SMI in asymptomatic patients with moderate coronary atherosclerosis is high (18%) (22). Holter monitoring studies (23,24) have shown that almost all patients with chronic angina have silent ischemic episodes four times as often as symptomatic episodes, and that these anginal episodes represent only the tip of the iceberg compared with the overall prevalence of ischemic episodes in patients with angina. In addition, two-thirds of patients with unstable angina may have silent ischemic episodes (25,26). The probability of an acute cardiac event is significantly high in asymptomatic patients who have episodes of ST-segment changes documented by Holter monitoring, especially if the total ischemic time is more than 1 h over the 24 h monitoring period for recurrent angina during the 30-day follow-up in patients with unstable angina (27,28).
Silent ischemia is more frequent in the convalescent phase of myocardial infarction than in the acute phase (29,30). It is not uncommon for patients with ACS presenting without chest pain to be misdiagnosed and undertreated. This patient population is less likely to receive effective therapy for ACS, and more likely to experience greater in-hospital morbidity and mortality (13% versus 4.3%, respectively; P<0.0001) than patients with typical symptoms (31).
In the intensive care unit
Critically ill patients admitted to the intensive care unit (ICU) for noncardiac causes are at risk of acute myocardial ischemia. It has been shown that transient myocardial ischemia and advanced age are important predictors of cardiac events (32). Silent ischemia is strongly associated with troponin elevation in critically ill ICU patients, and predicts both early and late mortality (33). Although the occurrence of myocardial injury in ICU patients (defined by elevated levels of cardiac troponin I) was unexpectedly high in one study, it was often unrecognized clinically (34).
In surgery
There is a relatively high incidence of perioperative SMI in the geriatric population undergoing elective surgery. In two different studies (35,36), approximately 6% to 18% of patients who underwent coronary artery bypass graft surgery had episodes of SMI detected by Holter monitoring. The diagnosis may be confounded by multiple factors, including postoperative chest discomfort, universal serum enzyme elevations and nonspecific electrocardiogram (ECG) changes. No single test is reliable, but a combination of many diagnostic tests, which are often expensive and time consuming, may increase the sensitivity and specificity of diagnosis. SMI occurs in as many as 60.8% of patients undergoing surgery for peripheral vascular disease and in 67.5% of carotid endarterectomy patients having real-time ECG monitoring during the pre-, intra- and postoperative periods (37).
In diabetic patients
Diabetic patients have a higher prevalence of SMI and unrecognized myocardial infarction than patients without diabetes (38). Patients with diabetes and SMI have a very poor prognosis, as reflected by adverse cardiac events or death (39). The prevalence of silent CAD is 6% to 23% in low-risk diabetic patients. In high-risk diabetic patients, the prevalence may be as high as 60% (40). Early detection of silent ischemia is important in diabetics, as well as in patients with impaired fasting glucose levels (41). Silent ischemia is also associated with higher hemoglobin A1C levels, reflecting the importance of achieving and maintaining euglycemia (42). The prevalence of silent ischemia in middle-aged patients with new-onset type 2 diabetes but without additional cardiovascular risk factors (assessed by exercise stress testing and coronary angiography) is similar to that observed in studies of subjects with longstanding diabetes who had additional cardiovascular risk factors (43). There is an independent association of the metabolic syndrome and insulin resistance with silent CAD in patients with type 2 diabetes mellitus (44). The criterion of two or more risk factors for CAD did not help to identify asymptomatic type 2 diabetic patients with a higher prevalence of CAD and is only related to more severe CAD with unfavourable coronary anatomy (45). Erectile dysfunction, which is widely prevalent in diabetic and hypertensive patients, has recently been shown to be a warning sign of underlying, silent CAD (46). Cardiovascular autonomic neuropathy and microalbu-minuria (in addition to traditional risk factors for CAD) have both been associated with an increased risk of SMI and mortality in diabetic patients older than 60 years of age (47). Polymorphisms of the angiotensin-converting enzyme gene, more specifically the D allele, increases the risk of SMI in type 2 diabetes mellitus (48).
Sleep apnea
Obstructive sleep apnea is also associated with myocardial ischemia (silent or symptomatic), acute coronary events, transient ischemic attack, stroke, cardiac arrhythmias, pulmonary hypertension and heart failure (49).
SHORTCOMINGS OF AVAILABLE MODALITIES IN THE DIAGNOSIS OF SMI
Bedside ECG monitoring
Continuous ECG monitoring is the mainstay of cardiac monitoring in patients admitted to the hospital for cardiac or non-cardiac causes. The sensitivity and accuracy of detecting ST-T changes suggestive of ischemia depend on the number of leads used during ECG monitoring (50). Accurate ST-segment monitoring requires expertise in ECG interpretation, as well as knowledge of the functions and limitations of continuous ST-segment monitoring (51). Routine ICU surveillance shows a low sensitivity of 3% for detecting ECG changes suggestive of prolonged myocardial ischemia, with a specificity of 90% per monitoring interval, a positive predictive value of 17% and a negative predictive value of 95% compared with frequent 12-lead ECG monitoring. In addition, there are other disease states commonly seen in critically ill patients, such as drug overdose or poisoning, and electrolyte and metabolic disturbances, which may cause false-positive ECG changes suggestive of ischemia (52).
Exercise stress testing
Exercise stress testing combined with ECG testing has been used for the detection of ST-T changes secondary to myocardial ischemia and infarction. The average reported sensitivity of exercise ECG testing is 68% (range 23% to 100%), with a specificity of 77% (range 17% to 100%) (53,54). In studies (55) in which referral bias was minimized, however, lower sensitivity values were reported, falling in the range of 45% to 60%. Differences in results of stress tests between men and women have been the subject of considerable controversy. A false-positive response, frequently seen in women, is one of the limitations of this technique used to diagnose SMI (56), which often leads to additional testing for CAD, causing unnecessary anxiety to the patient. A wide variety of miscellaneous situations have been associated with false-positive ST-segment responses to exercise. These include digitalis administration, hypokalemia (57), normal postprandial changes (58), hyper-ventilation (59), postural changes (60), vasoregulatory abnormalities (61), mitral valve prolapse (62), pectus excavatum (63), and intraventricular conduction defects, including bundle branch blocks, Wolff-Parkinson-White syndrome (64,65) and syndrome X (66). In addition to limitations in the sensitivity of exercise testing for the detection of myocardial ischemia, certain drugs such as beta-blockers and calcium channel blockers reduce the heart rate and maximum systolic arterial blood pressure during exercise, thus reducing or eliminating ST-segment depression. In the process of increasing exercise capacity, nitrates may also prevent or minimize changes in exercise-induced ST-segment changes (67,68).
Ambulatory ECG monitoring
Ambulatory ECG (AECG) monitoring offers important diagnostic information in patients with SMI and helps stratify the risk of serious events in patients with various forms of heart disease. AECG monitoring is insufficient in detecting silent ischemia in patients with chronic stable CAD (69), but it is useful in documenting characteristics of both painful myocardial infarctions and SMI occurring during out-of-hospital activities. Few data are available concerning SMI during AECG monitoring in asymptomatic patients with CAD (70). The relationships among the measures of ischemia severity during exercise testing, AECG monitoring during daily activities, and even the frequency of angina and nitroglycerin consumption are also very poor (71). Although AECG monitoring identifies a subset of patients with unstable angina at risk of adverse cardiac events, the low incidence of AECG abnormalities in the current therapeutic era and the higher incidence of abnormalities using other simple risk-stratifying modalities suggest that AECG monitoring should not be routinely recommended for clinical risk assessment (6). The incidence of ischemia on AECG monitoring is often quite low, and its positive predictive value may also be low. Resting QRS and ST-T wave abnormalities postmyocardial infarction also often preclude meaningful interpretation of additional ST-segment deviation (6). In the evaluation of daily life physical and mental triggers of painful and painless myocardial ischemic episodes in patients with CAD (assessed using AECG monitoring), it was ascertained that 85% of ischemic episodes occur without chest pain and 66% of angina reports are unaccompanied by ST-segment depression. The data revealed an uncoupling of anginal symptoms from ambulatory ischemic episodes in patients with CAD during daily life activities (72).
The sensitivity and specificity of ST-segment deviations for detecting CAD by AECG monitoring have been reported to be 79% and 75%, respectively (73). The diagnostic accuracy of 24 h AECG monitoring in patients within 72 h of coronary angiography has been shown to be poor (33%), and ischemic ST-segment shifts are not predictive of future coronary events (74). In approximately 30% of patients with stable CAD, 24 h AECG monitoring is often insufficient to detect silent ischemia (69). Continuous AECG monitoring is uncomfortable to the patient, is difficult to conduct in the home setting and is difficult to interpret because of a large number of artifacts. Even when ischemic ST-segment depression on AECG monitoring is interpreted with reference to the presence or absence of hemodynamically significant coronary artery stenosis at angiography, one should keep in mind that myocardial ischemia may occur in the absence of coronary stenosis, as seen in microvascular angina or subocclusive vasospastic angina (75).
Radionuclide imaging techniques
Ischemia may also be detected by radionuclide perfusion scanning. A major limitation of myocardial perfusion imaging (MPI) is the high false-positive rate observed in many laboratories, which is attributed to image attenuation artifacts. The false-positive rate remains high in obese subjects and women, who may demonstrate defects reflecting breast attenuation artifacts. Other limitations include long imaging protocols, which may take many hours; high equipment expense and the necessity of injecting radiopharmaceutical agents, with exposure to radiation; inability to visualize the heart in a real-time approach; and high cost to patients. For many patients, an ECG stress test has poor sensitivity and specificity, and is therefore unlikely to be the technique of choice. MPI, which provides evidence of ischemia by directly measuring the ‘total ischemic burden’, is clearly the method of choice in low- and intermediate-risk patients (76). Although the sensitivity and specificity of MPI for detecting CAD is better than that of ECG stress testing, false-negative and false-positive results do occur. In a population with a low prevalence of CAD, a positive result is of little predictive value, whereas in a population with a high prevalence of CAD, a negative result is of little practical diagnostic value. MPI has optimal discriminative value in a patient population with a pretest probability of CAD ranging from approximately 40% to 70%. This population includes patients with atypical chest pain, asymptomatic patients with major risk factors and asymptomatic patients with a positive ECG stress test (77,78).
THE ROLE OF CONTINUOUS INTRACARDIAC MONITORING
In patients with ACS, the goal of therapeutic intervention is to reverse ongoing ischemia and to interrupt or prevent myocardial cell death.
Continuous monitoring of the status of the culprit artery is mandatory to tailor appropriate therapy for each patient. Coronary angiography reveals vessel anatomy only at a brief moment in time, and continuous ECG monitoring may reflect only ST-segment changes. Hence, both methods have shortcomings and limitations in terms of myocardial physiology monitoring and efficient detection of ischemic myocardium (79,80). Although the ECG provides a more accurate assessment of the myocardium than angiography in situations in which vessel patency has been restored but ischemia persists because of ‘no reflow’ or reperfusion injury, ECG monitoring is currently not a comprehensive tool for measuring real physiological parameters of ischemic myocardium (81). Underuse of ST-segment monitoring appears to stem from three sources: technical problems with noise levels, high frequency of false ST-segment alarms and lack of adequate equipment for accurate ST-segment analysis; a lack of clarity as to how information about changes in the ST segment, especially in asymptomatic patients, should be used to determine clinical therapy; and a lack of confidence in the technology by many physicians and nurses (79).
Who should have continuous intracardiac monitoring?
Intracardiac monitoring should be given to patients with chronic CAD, especially those with diabetes; patients with an abnormal baseline ECG, including left bundle branch block, accessory pathways, left ventricular hypertrophy and electrolyte abnormalities; patients on medications such as digitalis; patients with permanent pacemakers; patients with an altered level of consciousness or who, for any reason, are unable to report their pain; high-risk postoperative patients undergoing noncardiac surgery or other high-risk patients admitted to the ICU; and patients undergoing high-risk PCI or coronary artery bypass graft surgery. These patients should be given the highest priority for continuous intracardiac monitoring, which provides uninterrupted, real-time information about the occurrence, frequency and severity of ischemic episodes over the course of the dynamic occlusive process (82).
What are the goals for continuous intracardiac monitoring in various diagnostic settings?
Future cardiac monitors should incorporate a versatile, comprehensive intracardiac monitoring system to continuously detect metabolic, ECG and hemodynamic derangements precisely and reliably. An alarm system may also be incorporated to signal any deterioration in the hemodynamic and physiological parameters to the patient, the health care providers and the emergency medical services through a preset warning system on detection of myocardial ischemia. Suitable noise reduction strategies should be developed to reduce the number of false alarms.
Which intracardiac physiological and hemodynamic parameters should be monitored continuously?
Newer and more reliable physiological and hemodynamic parameters (such as myocardial and coronary sinus temperature), metabolic indicators of ischemia in the coronary sinus (including coronary sinus pH, partial pressure of CO2, partial pressure of O2, lactate and troponin) and hemodynamic factors (such as heart rate, cardiac output, pulmonary artery pressure, and ventricular end-diastolic pressures and volumes) should be measured continuously.
What are the requirements for accurate and continuous intracardiac monitoring?
The intracardiac monitoring device includes an integrated, implantable lead and electronic processing box implanted in the subclavian area. The lead may be positioned in the right ventricular apex and can continuously measure more reliable physiological and hemodynamic parameters, as well as intra-cardiac electrograms relevant to the detection of myocardial ischemia. Using specialized leads with incorporated sensors, intracardiac monitoring can be used to constantly monitor myocardial temperature for any decrease in right ventricular apex temperature, which often precedes ECG or hemodynamic changes, and thus may be used as a reliable marker of ischemia. In addition, cardiac output may be monitored with the ther-modilution technique. Intracardiac pressures and volumes may also be measured constantly with this technique. The collected data can be uploaded and transmitted over the Internet to a central processing unit for review. The coronary sinus can be used as a new gateway to access the heart for the implementation of continuous intracardiac monitoring. An increase in temperature in the coronary sinus can be used as a reliable marker of myocardial ischemia.
EMERGING TECHNOLOGIES FOR THE DETECTION OF SMI
The available strategies that best detect and treat SMI, the clinical implications of SMI, the prognostic significance in various clinical settings and the available diagnostic tools, all represent limitations. To overcome all of these limitations, it is time that we advanced and improved our knowledge to implement new technologies using reliable determinants, targeted for continuous monitoring, detection and treatment of SMI.
Intramyocardial temperature monitoring
Among different determinants and physiological markers, temperature is a reliable factor that can be measured and monitored continuously in all clinical settings.
Intramyocardial temperature monitoring has been used as a marker of myocardial perfusion, ischemia and metabolic status during cardioplegia for cardiac surgery, and its correlation with the level of myocardial protection and with other physiological factors has been investigated in several studies (83,84).
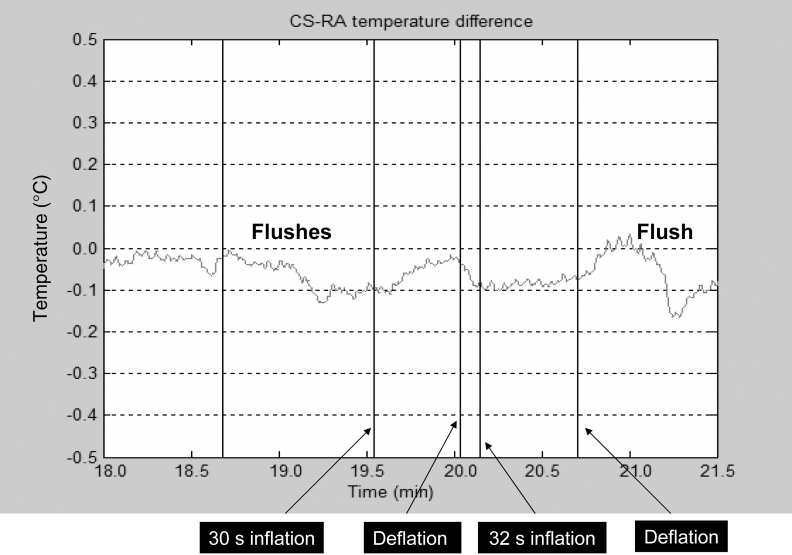
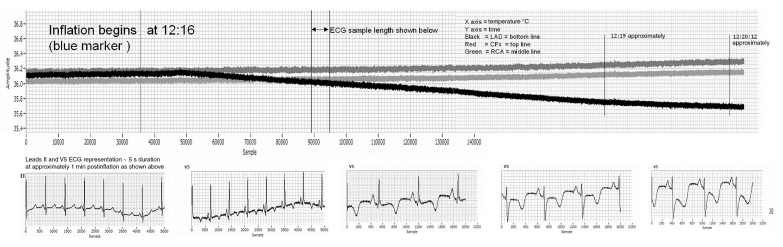
Previous studies (85,86) have shown that widespread inflammation has been observed in patients with CAD. The inflammatory process may result in an increased temperature in the coronary sinus (87). Stefanadis et al (88) measured blood temperature in the coronary sinus and the right atrium of patients with significant narrowing of the left coronary or right coronary arteries and in subjects without CAD. Coronary sinus temperature was increased in patients with CAD compared with controls and was found to be a prognostic factor for mid-term clinical outcomes. Coronary sinus and intramyocardial temperature monitoring were demonstrated by Eftekhari et al (89) in human and animal studies to be the two appropriate modalities for measuring temperature changes to detect myocardial ischemia. These researchers monitored temperature changes, along with other biomarkers of ischemia, in the coronary sinus during PCI in their human study, and they demonstrated that transient myocardial ischemia during balloon inflation was associated with an increased coronary sinus temperature (Figure 1). They also showed a correlation between the magnitude of ischemic myocardial burden and the degree of temperature change in the coronary sinus (89). In another study (90), experimental acute coronary occlusion in a swine model produced an average intramyocardial temperature drop of 0.1±0.03°C (P<0.05) in all three major epicardial coronary artery distributions. The temperature changes preceded ECG changes of ischemia by an average duration of 1 min to 2 min (Figure 2). Further studies are being proposed to demonstrate the effect of chronic coronary occlusion on intramyocardial temperature in an ovine model. Hence, intramyocardial and coronary sinus temperature changes may be a reliable marker of acute and chronic ischemia, especially SMI (90).
Figure 1.
Coronary sinus (CS) and right atrial (RA) temperature changes with percutaneous transluminal coronary angioplasty. Note that the temperature rises with balloon inflation and returns to baseline after balloon deflation
Figure 2.
Experimental left anterior descending (LAD) artery occlusion lasting for 1 h. The temperature decrease begins approximately 20 s post-occlusion. CFx Circumflex; ECG Electrocardiogram; RCA Right coronary artery
Tissue O2 tension
Monitoring tissue O2 tension has become clinically feasible with the development of miniaturized implantable Clark electrodes. The polarographic O2 sensors enable the measurement of O2 partial pressure in tissues, organs and body fluids, directly and continuously. Tissue O2 tension has been measured successfully in the ICU, as well as during neurosurgical procedures (91).The partial pressure of O2 in the muscle may prove to be an early and reliable indicator of stagnant blood flow and tissue hypoxia. Limiting factors in the use of polarographic O2 probes are the dependence of electrode currents on tissue temperature, errors in O2 partial pressure readings due to tissue trauma and edema related to electrode insertion, and accidental intravascular placement of the O2 sensors (92).
Near-infrared spectroscopy
Intravascular near-infrared spectroscopy is another emerging tool for the detection of myocardial ischemia. The intravascular near-infrared spectral analysis of the coronary sinus blood with a fibre optic catheter can be a reliable diagnostic tool for the on-line detection and follow-up of acute myocardial ischemia (93). Monitoring of myocardial tissue impedance with silicon-based microprobes is being characterized and developed. Incorporation of additional sensors to detect tissue pH and electrolyte concentrations will further enhance the ability of these devices to provide an accurate and early diagnosis of ischemia.
Computed tomography
Recently, multislice computed tomography (CT) has been shown to be capable of visualizing not only the coronary arteries (wall and lumen), but also the cardiac muscle, with high spatial resolution. Multislice CT provides high-quality three-dimensional images of coronary arteries. Initial results of four-, 16- and more recently 64-slice CT, compared with conventional angiograms, are very promising. Noninvasive visualization of the coronary arteries and accurate detection of stenosis are now possible with ECG-gated 16-slice CT. Multislice CT can also detect nonstenotic coronary plaques. Finally, visualization of cardiac muscle with multislice CT makes it possible to detect myocardial ischemia. Electron beam tomography coronary calcium imaging is an evolving technique for the early detection of coronary atherosclerosis, and recent studies have established its prognostic value in asymptomatic individuals. The relationship of coronary artery calcium scores to obstructive CAD is an evolving area of interest and is clinically relevant because it determines which individuals are likely to benefit from revascularization procedures (94).
CONCLUSIONS
SMI has arrived at an exciting crossroad where newer technologies for continuous monitoring, such as measurement of intramyocardial temperature and other local metabolic parameters, will help to identify vulnerable patients in various settings, improve diagnostic capabilities, and help categorize patients into low- and high-risk groups. Silent ischemia represents only the tip of the iceberg of the ischemic spectrum. Newer technologies will help identify a larger subset of patients who can benefit from continuous monitoring.
Footnotes
DISCLOSURES: This research is currently funded by a United States Army Grant, the Texas Training and Technology for Trauma and Terrorism (T5) program.
REFERENCES
- 1.Colbeck EH. Angina pectoris: A criticism and a hypothesis. Lancet. 1903;i:793–5. [Google Scholar]
- 2.Hollenberg NK. Controversies in cardiovascular care: Silent myocardial ischemia. Complicat Card Patient. 1987;1:24–30. [PubMed] [Google Scholar]
- 3.Cohn PF. Silent myocardial ischemia. Ann Intern Med. 1988;109:312–7. doi: 10.7326/0003-4819-109-4-312. [DOI] [PubMed] [Google Scholar]
- 4.Cohn PF. Silent myocardial ischemia: Classification, prevalence, and prognosis. Am J Med. 1985;79:2–6. doi: 10.1016/0002-9343(85)90486-3. [DOI] [PubMed] [Google Scholar]
- 5.Canto JG, Shilpak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–9. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 6.Stone PH. Asymptomatic myocardial ischemia in stable angina, unstable angina, and myocardial infarction: Current status and future directions. Cardiol Rounds. 1998;2:1–8. [Google Scholar]
- 7.Maseri A. Ischemic Heart Disease: A Rational Basis for Clinical Practice and Clinical Research. New York: Churchill Livingstone; 1995. [Google Scholar]
- 8.Stern S, Cohn PF, Pepine CJ. Silent myocardial ischemia. Curr Probl Cardiol. 1993;18:301–59. doi: 10.1016/0146-2806(93)90010-y. [DOI] [PubMed] [Google Scholar]
- 9.Glazier JJ, Chierchia S, Brown MJ, Maseri A. Importance of generalized defective perception of painful stimuli as a cause of silent myocardial ischemia in chronic stable angina pectoris. Am J Cardiol. 1986;58:667–72. doi: 10.1016/0002-9149(86)90335-8. [DOI] [PubMed] [Google Scholar]
- 10.Falcone C, Auguadro C, Sconocchia R, et al. Susceptibility to pain during coronary angioplasty: Usefulness of pulpal test. J Am Coll Cardiol. 1996;28:903–9. doi: 10.1016/s0735-1097(96)00252-5. [DOI] [PubMed] [Google Scholar]
- 11.Crea F, Gaspardone A. New look to an old symptom: Angina pectoris. Circulation. 1997;96:3766–73. doi: 10.1161/01.cir.96.10.3766. [DOI] [PubMed] [Google Scholar]
- 12.Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: Regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med. 1996;124:939–49. doi: 10.7326/0003-4819-124-11-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Nihoyannopoulos P, Marsonis A, Joshi J, Athanassopoulos G, Oakley CM. Magnitude of myocardial dysfunction is greater in painful than in painless myocardial ischemia: An exercise echocardiographic study. J Am Coll Cardiol. 1995;25:1507–12. doi: 10.1016/0735-1097(95)00096-m. [DOI] [PubMed] [Google Scholar]
- 14.Mittleman MA, Maclure M, Sherwood JB, et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–5. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 15.Gullette EC, Blumenthal JA, Babyak M, et al. Effects of mental stress on myocardial ischemia during daily life. JAMA. 1997;277:1521–6. [PubMed] [Google Scholar]
- 16.Jiang W, Babyak M, Krantz DS, et al. Mental stress – induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 17.Hikita H, Kurita A, Takase B, Nakamura H. Re-examination of the roles of beta-endorphin and cardiac autonomic function in exercise-induced silent myocardial ischemia. Ann Noninvasive Electrocardiol. 1997;2:319–25. [Google Scholar]
- 18.Mazzone A, Mazzucchelli I, Vezzoli M, et al. Increased expression of peripheral benzodiazepine receptors on leukocytes in silent myocardial ischemia. J Am Coll Cardiol. 2000;36:746–50. doi: 10.1016/s0735-1097(00)00778-6. [DOI] [PubMed] [Google Scholar]
- 19.Mazzone A, Cusa C, Mazzucchelli I, et al. Increased production of inflammatory cytokines in patients with silent myocardial ischemia. J Am Coll Cardiol. 2001;38:1895–901. doi: 10.1016/s0735-1097(01)01660-6. [DOI] [PubMed] [Google Scholar]
- 20.Ozhan H, Akdemir R, Duran S, et al. Transient silent ischemia after percutaneous transluminal coronary angioplasty manifested with a bizarre electrocardiogram. J Electrocardiol. 2005;38:206–9. doi: 10.1016/j.jelectrocard.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Cohn PF. Silent myocardial ischemia: Recent developments. Curr Atheroscler Rep. 2005;7:155–63. doi: 10.1007/s11883-005-0039-8. [DOI] [PubMed] [Google Scholar]
- 22.Anand DV, Lim E, Raval U, Lipkin D, Lahiri A. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004;11:450–7. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi AC, Dovellini EV, Marchi F, Pucci P, Santoro GM, Fazzini PF. Silent myocardial ischemia during ambulatory electrocardiographic monitoring in patients with effort angina. J Am Coll Cardiol. 1983;1:934–9. doi: 10.1016/s0735-1097(83)80213-7. [DOI] [PubMed] [Google Scholar]
- 24.Deanfield JE, Shea M, Ribiero P, et al. Transient ST-segment depression as a marker of myocardial ischemia during daily life. Am J Cardiol. 1984;54:1195–200. doi: 10.1016/s0002-9149(84)80066-1. [DOI] [PubMed] [Google Scholar]
- 25.Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation. 2003;108:1263–77. doi: 10.1161/01.CIR.0000088001.59265.EE. [DOI] [PubMed] [Google Scholar]
- 26.Langer A, Freeman MR, Armstrong PW. ST segment shift in unstable angina: Pathophysiology and association with coronary anatomy and hospital outcome. J Am Coll Cardiol. 1989;13:1495–502. doi: 10.1016/0735-1097(89)90338-0. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb SO. Association between silent myocardial ischemia and prognosis: Insensitivity of angina pectoris as a marker of coronary artery disease activity. Am J Cardiol. 1987;60:33J–8J. doi: 10.1016/0002-9149(87)90681-3. [DOI] [PubMed] [Google Scholar]
- 28.Nademanee K, Intarachot V, Josephson MA, Rieders D, Vaghaiwalla Mody F, Singh BN. Prognostic significance of silent myocardial ischemia in patients with unstable angina. J Am Coll Cardiol. 1987;10:1–9. doi: 10.1016/s0735-1097(87)80152-3. [DOI] [PubMed] [Google Scholar]
- 29.Currie P, Ashby D, Saltissi S. Prognostic significance of transient myocardial ischemia on ambulatory monitoring after acute myocardial infarction. Am J Cardiol. 1993;71:773–7. doi: 10.1016/0002-9149(93)90822-t. [DOI] [PubMed] [Google Scholar]
- 30.Jereczek M, Andresen D, Schröder J, et al. Prognostic value of ischemia during Holter monitoring and exercise testing after acute myocardial infarction. Am J Cardiol. 1993;72:8–13. doi: 10.1016/0002-9149(93)90210-4. [DOI] [PubMed] [Google Scholar]
- 31.Brieger D, Eagle KA, Goodman SG, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: Insights from the Global Registry of Acute Coronary Events. Chest. 2004;126:461–9. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 32.Booker KJ, Holm K, Drew BJ, et al. Frequency and outcomes of transient myocardial ischemia in critically ill adults admitted for noncardiac conditions. Am J Crit Care. 2003;12:508–16. [PubMed] [Google Scholar]
- 33.Arquès S, Roux E, Stolidi P, et al. Silent ischemia and acute cardiac insufficiency with normal systolic function: Diagnostic value of troponin I measurement. Arch Mal Coeur Vaiss. 2003;96:854–8. [PubMed] [Google Scholar]
- 34.Guest TM, Ramanathan AV, Tuteur PG, Schechtman KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA. 1995;273:1945–9. [PubMed] [Google Scholar]
- 35.Yousif H, Davies G, Westaby S, Prendiville OF, Sapsford RN, Oakley CM. Preoperative myocardial ischaemia: Its relation to perioperative infarction. Br Heart J. 1987;58:9–14. doi: 10.1136/hrt.58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner MJ, Johnstone DE, Lalonde L, et al. Perioperative myocardial infarction with coronary artery surgery: Diagnosis, incidence and consequences. Can J Cardiol. 1987;3:336–41. [PubMed] [Google Scholar]
- 37.Pasternack PF, Grossi EA, Baumann FG, et al. The value of silent myocardial ischemia monitoring in the prediction of perioperative myocardial infarction in patients undergoing peripheral vascular surgery. J Vasc Surg. 1989;10:617–25. doi: 10.1067/mva.1989.15572. [DOI] [PubMed] [Google Scholar]
- 38.DeLuca AJ, Kaplan S, Aronow WS, et al. Comparison of prevalence of unrecognized myocardial infarction and of silent myocardial ischemia detected by a treadmill exercise sestamibi stress test in patients with versus without diabetes mellitus. Am J Cardiol. 2006;98:1045–6. doi: 10.1016/j.amjcard.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Grover M, Talwalkar S, Casbard A, et al. Silent myocardial ischaemia and haemoglobin concentration: A randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang. 2006;90:105–12. doi: 10.1111/j.1423-0410.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 40.Zellweger MJ. Prognostic significance of silent coronary artery disease in type 2 diabetes. Herz. 2006;31:240–5. doi: 10.1007/s00059-006-2790-1. [DOI] [PubMed] [Google Scholar]
- 41.Kokot T, Nowakowska-Zajdel E, Muc-Wierzgon M, et al. Impaired fasting glucose and silent myocardial ischemia. Pol Arch Med Wewn. 2005;114:1066–71. [PubMed] [Google Scholar]
- 42.DeLuca AJ, Saulle LN, Aronow WS, Ravipati G, Weiss MB. Prevalence of silent myocardial ischemia in persons with diabetes mellitus or impaired glucose tolerance and association of hemoglobin A1c with prevalence of silent myocardial ischemia. Am J Cardiol. 2005;95:1472–4. doi: 10.1016/j.amjcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Fornengo P, Bosio A, Epifani G, Pallisco O, Mancuso A, Pascale C. Prevalence of silent myocardial ischaemia in new-onset middle-aged type 2 diabetic patients without other cardiovascular risk factors. Diabet Med. 2006;23:775–9. doi: 10.1111/j.1464-5491.2006.01910.x. [DOI] [PubMed] [Google Scholar]
- 44.Gazzaruso C, Solerte SB, De Amici E, et al. Association of the metabolic syndrome and insulin resistance with silent myocardial ischemia in patients with type 2 diabetes mellitus. Am J Cardiol. 2006;97:236–9. doi: 10.1016/j.amjcard.2005.07.133. [DOI] [PubMed] [Google Scholar]
- 45.Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2006;47:65–71. doi: 10.1016/j.jacc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Jackson G, Rosen RC, Kloner RA, Kostis JB. The second Princeton consensus on sexual dysfunction and cardiac risk: New guidelines for sexual medicine. J Sex Med. 2006;3:28–36. doi: 10.1111/j.1743-6109.2005.00196.x. [DOI] [PubMed] [Google Scholar]
- 47.Chico A, Tomás A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27:213–7. doi: 10.1385/ENDO:27:3:213. [DOI] [PubMed] [Google Scholar]
- 48.Xing G, Zeng X, Wang Y, Zhao L. Angiotensin converting enzyme gene and exercise-induced silent myocardial ischemia in type 2 diabetes mellitus. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:206–8. [PubMed] [Google Scholar]
- 49.Bounhoure JP, Galinier M, Didier A, Leophonte P. Sleep apnea syndromes and cardiovascular disease. Bull Acad Natl Med. 2005;189:445–59. [PubMed] [Google Scholar]
- 50.Klootwijk P, Meij S, von Es GA, et al. Comparison of usefulness of computer assisted continuous 48-h 3-lead with 12-lead ECG ischaemia monitoring for detection and quantitation of ischaemia in patients with unstable angina. Eur Heart J. 1997;18:931–40. doi: 10.1093/oxfordjournals.eurheartj.a015381. [DOI] [PubMed] [Google Scholar]
- 51.Drew BJ, Wung SF, Adams MG, Pelter MM. Bedside diagnosis of myocardial ischemia with ST-segment monitoring technology: Measurement issues for real-time clinical decision making and trial designs. J Electrocardiol. 1998;30(Suppl):157–65. doi: 10.1016/s0022-0736(98)80067-8. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein B. Intensive care unit ECG monitoring. Card Electrophysiol Rev. 1997;1:308–10. doi: 10.1023/a:1016300202560. [DOI] [PubMed] [Google Scholar]
- 53.Gianrossi R, Detrano R, Mulvihill D, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80:87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 54.Froelicher VF, Lehmann KG, Thomas R, et al. The electrocardiographic exercise test in a population with reduced workup bias: Diagnostic performance, computerized interpretation, and multivariable prediction. Veterans Affairs Cooperative Study in Health Services #016 (QUEXTA) Study Group. Quantitative Exercise Testing and Angiography. Ann Intern Med. 1998;128:965–74. doi: 10.7326/0003-4819-128-12_part_1-199806150-00001. [DOI] [PubMed] [Google Scholar]
- 55.Tavel ME, Shaar C. Relation between the electrocardiographic stress test and degree and location of myocardial ischemia. Am J Cardiol. 1999;84:119–24. doi: 10.1016/s0002-9149(99)00219-2. [DOI] [PubMed] [Google Scholar]
- 56.Sketch MH, Mohiuddin SM, Lynch JD, Zencka AE, Runco V. Significant sex differences in the correlation of electrocardiographic exercise testing and coronary arteriograms. Am J Cardiol. 1975;36:169–73. doi: 10.1016/0002-9149(75)90521-4. [DOI] [PubMed] [Google Scholar]
- 57.Sketch MH, Mooss AN, Butler ML, Nair CK, Mohiuddin SM. Digoxin-induced positive exercise tests: Their clinical and prognostic significance. Am J Cardiol. 1981;48:655–9. doi: 10.1016/0002-9149(81)90143-0. [DOI] [PubMed] [Google Scholar]
- 58.Riley CP, Oberman A, Sheffield LT. Electrocardiographic effects of glucose ingestion. Arch Intern Med. 1972;130:703–7. [PubMed] [Google Scholar]
- 59.Jacobs WF, Battle WE, Ronan JA., Jr False-positive ST-T-wave changes secondary to hyperventilation and exercise. A cineangiographic correlation. Ann Intern Med. 1974;81:479–82. doi: 10.7326/0003-4819-81-4-479. [DOI] [PubMed] [Google Scholar]
- 60.Lachman AB, Semler HJ, Gustafson RH. Postural ST-T wave changes in the radioelectrocardiogram simulating myocardial ischemia. Circulation. 1965;31:557–63. doi: 10.1161/01.cir.31.4.557. [DOI] [PubMed] [Google Scholar]
- 61.Friesinger GC, Biern RO, Likar I, Mason RE. Exercise electrocardiography and vasoregulatory abnormalities. Am J Cardiol. 1972;30:733–40. doi: 10.1016/0002-9149(72)90147-6. [DOI] [PubMed] [Google Scholar]
- 62.Engel PJ, Alpert BL, Hickman JR., Jr The nature and prevalence of the abnormal exercise electrocardiogram in mitral valve prolapse. Am Heart J. 1979;98:716–24. doi: 10.1016/0002-8703(79)90468-x. [DOI] [PubMed] [Google Scholar]
- 63.Kattus AA. Exercise electrocardiography: Recognition of the ischemic response, false positive and negative patterns. Am J Cardiol. 1974;33:721–31. doi: 10.1016/0002-9149(74)90212-4. [DOI] [PubMed] [Google Scholar]
- 64.Feil H, Brofman BL. The effect of exercise on the electrocardiogram of bundle branch block. Am Heart J. 1953;45:665–75. doi: 10.1016/0002-8703(53)90308-1. [DOI] [PubMed] [Google Scholar]
- 65.Strasberg B, Ashley WW, Wyndham CR, et al. Treadmill exercise testing in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1980;45:742–8. doi: 10.1016/0002-9149(80)90116-2. [DOI] [PubMed] [Google Scholar]
- 66.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276:1063–6. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 67.Gianelly RE, Treister BL, Harrison DC. The effect of propranolol on exercise-induced ischemic S-T segment depression. Am J Cardiol. 1969;24:161–5. doi: 10.1016/0002-9149(69)90398-1. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein RE, Rosing DR, Redwood DR, Beiser GD, Epstein SE. Clinical and circulatory effects of isosorbide dinitrate. Comparison with nitroglycerin. Circulation. 1971;43:629–40. doi: 10.1161/01.cir.43.5.629. [DOI] [PubMed] [Google Scholar]
- 69.Caussé C, Allaert FA, Marcantoni JP, Wolf JE. Frequency and detection rate of silent myocardial ischemia by Holter monitoring in patients with stable coronary insufficiency under treatment. Study of 95,725 recorded hours. Arch Mal Coeur Vaiss. 2001;94:779–84. [PubMed] [Google Scholar]
- 70.Cohn PF, Lawson WE. Characteristics of silent myocardial ischemia during out-of-hospital activities in asymptomatic angiographically documented coronary artery disease. Am J Cardiol. 1987;59:746–9. doi: 10.1016/0002-9149(87)91085-x. [DOI] [PubMed] [Google Scholar]
- 71.Borzak S, Fenton T, Glasser SP, et al. Discordance between effects of anti-ischemic therapy on ambulatory ischemia, exercise performance and anginal symptoms in patients with stable angina pectoris. The Angina and Silent Ischemia Study Group (ASIS) J Am Coll Cardiol. 1993;21:1605–11. doi: 10.1016/0735-1097(93)90375-b. [DOI] [PubMed] [Google Scholar]
- 72.Krantz DS, Hedges SM, Gabbay FH, et al. Triggers of angina and ST-segment depression in ambulatory patients with coronary artery disease: Evidence for an uncoupling of angina and ischemia. Am Heart J. 1994;128:703–12. doi: 10.1016/0002-8703(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 73.Kunkes SH, Pichard A, Meller J, Gorlin R, Herman MV, Kupersmith J. Use of the ambulatory ECG to diagnose coronary artery disease. J Electrocardiol. 1980;13:341–6. doi: 10.1016/s0022-0736(80)80085-9. [DOI] [PubMed] [Google Scholar]
- 74.Nair CK, Khan IA, Esterbrooks DJ, Ryschon KL, Hilleman DE. Diagnostic and prognostic value of Holter-detected ST-segment deviation in unselected patients with chest pain referred for coronary angiography: A long-term follow-up analysis. Chest. 2001;120:834–9. doi: 10.1378/chest.120.3.834. [DOI] [PubMed] [Google Scholar]
- 75.Lanze G, Sestito A. Silent ischemia detection by long-term ECG recording. Int J Bioelectrom. 2002;4:2. [Google Scholar]
- 76.Gibbons RJ, Hodge DO, Berman DS, et al. Long-term outcome of patients with intermediate-risk exercise electrocardiograms who do not have myocardial perfusion defects on radionuclide imaging. Circulation. 1999;100:2140–5. doi: 10.1161/01.cir.100.21.2140. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy HL. Importance of the standard electrocardiogram in ambulatory (Holter) electrocardiography. Am Heart J. 1992;123:1660–77. doi: 10.1016/0002-8703(92)90820-l. [DOI] [PubMed] [Google Scholar]
- 78.Stern S. State of the art in stress testing and ischaemia monitoring. Card Electrophysiol Rev. 2002;6:204–8. doi: 10.1023/a:1016364622124. [DOI] [PubMed] [Google Scholar]
- 79.Drew B, Krucoff MW. Multilead ST-segment monitoring in patients with acute coronary syndromes: A consensus statement for healthcare professionals. ST-Segment Monitoring Practice Guideline International Working Group. Am J Crit Care. 1999;8:372–86. [PubMed] [Google Scholar]
- 80.Klootwijk P, Cobbaert C, Fioretti P, Kint PP, Simoons ML. Noninvasive assessment of reperfusion and reocclusion after thrombolysis in acute myocardial infarction. Am J Cardiol. 1993;72:75G–84G. doi: 10.1016/0002-9149(93)90111-o. [DOI] [PubMed] [Google Scholar]
- 81.Gottlieb SO, Gerstenblith G. Assessing the total ischemic burden in the management of unstable angina. A review. Am J Med. 1986;81:7–11. doi: 10.1016/0002-9343(86)90972-1. [DOI] [PubMed] [Google Scholar]
- 82.Langer A, Krucoff MW, Klootwijk P, et al. Noninvasive assessment of speed and stability of infarct-related artery reperfusion: Results of the GUSTO ST segment monitoring study. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995;25:1552–7. doi: 10.1016/0735-1097(95)00110-p. [DOI] [PubMed] [Google Scholar]
- 83.Dearani JA, Axford TC, Patel MA, Healey NA, Lavin PT, Khuri SF. Role of myocardial temperature measurement in monitoring the adequacy of myocardial protection during cardiac surgery. Ann Thorac Surg. 2001;72:S2235–43. doi: 10.1016/s0003-4975(01)03320-3. [DOI] [PubMed] [Google Scholar]
- 84.Matsui Y, Yoshida T, Miyama M, Gohda T, Yasuda K, Tanabe T. Correlation of myocardial temperature, intramyocardial pH, and myocardial electrical activity during hyperkalemic hypothermic cardioplegic arrest to functional recovery after reperfusion. Nippon Kyobu Geka Gakkai Zasshi. 1993;41:1452–9. [PubMed] [Google Scholar]
- 85.Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347:5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 86.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 87.Libby P. Vascular biology of atherosclerosis: Overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 88.Stefanadis C, Tsiamis E, Vaina S, et al. Temperature of blood in the coronary sinus and right atrium in patients with and without coronary artery disease. Am J Cardiol. 2004;93:207–10. doi: 10.1016/j.amjcard.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 89.Eftekhari H, Payvar S, Ahmed AH, et al. Coronary sinus temperature changes during myocardial ischemia. Am J Cardiol. 2005;96(Suppl 1):105H. (Abst) [Google Scholar]
- 90.Shankar KJ, Ahmed AH, Munir MS, et al. Myocardial ischemia produces detectable temperature changes in the swine myocardium. Am J Cardiol. 2006;98(Suppl 1):68M. (Abst) [Google Scholar]
- 91.Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–50. doi: 10.1097/00003246-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 92.Boldt J. Clinical review: Hemodynamic monitoring in the intensive care unit. Crit Care. 2002;6:52–9. doi: 10.1186/cc1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baykut D, Gebhard MM, Bölükoglu H, et al. Online detection of myocardial ischemia by near infrared spectroscopy with a fiberoptic catheter. Thorac Cardiovasc Surg. 2001;49:162–6. doi: 10.1055/s-2001-14294. [DOI] [PubMed] [Google Scholar]
- 94.Paul JF. Non-invasive coronary artery imaging using multislice computed tomography. Bull Acad Natl Med. 2005;189:657–70. [PubMed] [Google Scholar]