Abstract
A case of influenza A myopericarditis is presented in a patient with cardiac tamponade and immunomediated cardiac failure. Myocarditis is a rare and potentially lethal complication of infection with influenza A, and may manifest with a wide spectrum of cardiac involvement, from mild myocarditis to cardiogenic shock. Even with full supportive therapy, mortality is significant, and normalization of left ventricular function may occur in up to 50% of cases. In the present report, previous literature regarding cardiac involvement following influenza A infection is reviewed, and potential immunological mechanisms involved in the progression of the cardiomyopathy following influenza A infection are discussed. Cardiovascular involvement in influenza infection should be considered in all patients presenting with hemodynamic compromise.
Keywords: Cardiac failure, Cardiac tamponade, Influenza A, Myocarditis
Influenza A infection is a debilitating respiratory illness, which accounted for 50 to 100 million deaths worldwide in the 1918 pandemic. Rarely, it can affect the cardiovascular system. We report a case of influenza A myopericarditis presenting with cardiac tamponade and immunomediated cardiac failure. Cardiovascular involvement in influenza should be considered in patients presenting with hemodynamic compromise.
CASE PRESENTATION
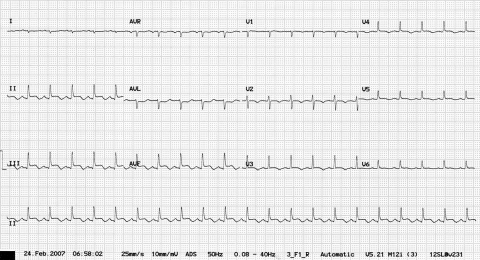
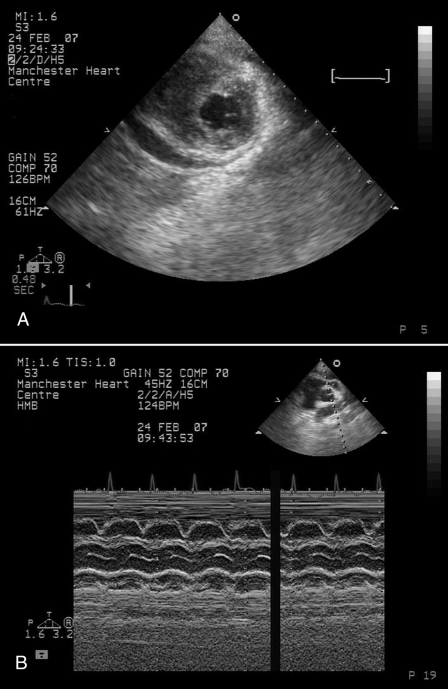
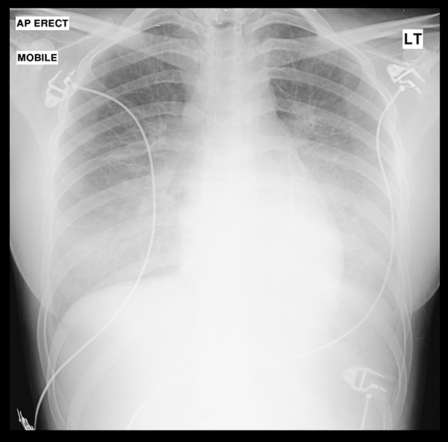
A 17-year-old Chinese student presented to the emergency room with a three-day history of flu-like symptoms associated with an upper respiratory tract illness and dizziness. While awaiting assessment, she collapsed. She was previously well with no other relevant medical history and had been in the United Kingdom studying for three months. On examination, she was pale, clammy and pyrexial at 38.1°C. She also had tachycardia of 126 beats/min, a blood pressure of 81/60 mmHg and an oxygen saturation on room air of 94%. Her chest examination did not reveal any abnormalities, but her cardiovascular examination revealed a raised jugular venous pressure with soft heart sounds but no audible murmurs. Electrocardiogram (ECG) demonstrated a sinus tachycardia with inferior ST-segment elevation, diffuse anterolateral T wave inversion and small voltage complexes (Figure 1). Her chest x-ray was reported as being clear. An urgent echocardiogram was performed, which demonstrated a 1.5 cm pericardial effusion with right ventricular diastolic collapse and echocardiographic evidence of cardiac tamponade (Figures 2A and 2B). Left ventricular (LV) function was reported as good, with no regional wall motion abnormalities demonstrated. Blood cultures were taken, and an autoimmune screen, serum virology and routine blood samples were analyzed. Serum electrolytes were normal, but her white blood cell count was elevated at 17.6×109/L, C-reactive protein was elevated at 35 mg/L and erythrocyte sedimentation rate was normal at 5 mm/h. Her creatine kinase (CK) level was elevated at 585 U/L (normal range from 0 U/L to 165 U/L) and her troponin I level was 1.40 μg/L (normal range from 0 μg/L to 0.01 μg/L). An urgent computed tomography scan of the chest was performed to exclude aortic dissection, which demonstrated a patchy ground glass appearance in lung parenchyma consistent with pneumonitis and a small pleural effusion. There was no evidence of aortic dissection. Pericardiocentesis was performed, and a pericardial drain was inserted. A total of 170 mL of straw-coloured fluid was aspirated and sent for culture. Blood pressure responded well to pericardiocentesis and rose to 113/84 mmHg. She was stable for a further 24 h, in which serial echocardiograms demonstrated good LV function, with no recurrence of the pericardial effusion, and decreasing CK levels to 285 U/L. The working diagnosis was a myopericarditis of a viral etiology. However, at 36 h postadmission, the patient became unwell, her oxygen saturation dropped to 86% on room air and she became profoundly hypotensive at 70/50 mmHg. Repeat echocardiography demonstrated no evidence of recurrence of the pericardial effusion, but she had severely impaired LV function, with an ejection fraction of less than 30%. Repeat chest x-ray demonstrated widespread pulmonary infiltrates (Figure 3), and a repeated CK measurement was within normal range. She was treated initially with the broad-spectrum antibiotics meropenem (500 mg three times a day) and clarithromycin (500 mg twice a day) to cover atypical pneumonias, and methylprednisolone (1 g once a day) in case of an autoimmune etiology. A Swan-Ganz catheter was inserted to aid fluid management, and the patient was transferred to an intensive care unit, where continuous positive airway pressure was commenced. The patient remained hypotensive; therefore, inotropic support was initiated with enoximone and noradrenaline. Over the subsequent five days, her requirements for inotropic support declined, and respiratory support with continuous positive airway pressure was stopped. LV function returned to normal, and she was discharged from hospital one week later. She remained well after six weeks of follow-up, with normal LV function on echocardiography.
Figure 1.
Electrocardiography
Figure 2.
Echocardiogram showing pericardial effusion in two-dimensional short-axis parasternal view (A) and diastolic collapse of right ventricle with M-mode (B)
Figure 3.
Repeat chest x-ray demonstrating widespread pulmonary infiltrates
Serum antinuclear antibody, antineutrophil cytoplasmic antibody, anti-double-stranded DNA, and C3 and C4 levels were normal. Repeated blood cultures were negative, and an atypical pneumonia screen was negative. Serologies for Pneumocystis carinii pneumonia, Coxsackie virus, Epstein-Barr virus, echovirus, cytomegalovirus, adenovirus, enterovirus and influenza B viruses were negative. Serum influenza A testing was positive, with a titre greater than 640, and polymerase chain reaction testing for influenza A from a throat swab was also positive. Diagnosis was, therefore, influenza A myopericarditis with cardiac tamponade, cardiac failure and pneumonitis.
DISCUSSION
Influenza A and B viruses are enveloped viruses with a segmented genome made up of eight single-stranded RNA segments of 890 to 2341 nucleotides each (1). They can be further subdivided on the basis of the antigenicity of the surface proteins hemagglutinin and neuraminidase. Thus, influenza A is further subdivided into 16 hemagglutinin (H1 to H16) and nine neuraminidase (N1 to N9) subtypes. It is estimated that 50% of those infected with influenza virus have no clinical symptoms, while in the remaining 50%, clinical presentation varies from afebrile respiratory symptoms (similar to the common cold) to febrile illnesses causing disorders affecting the lung, heart, brain, kidneys and liver (2). Every year, the global burden of influenza is believed to be three to five million cases of severe illness and up to 300,000 deaths annually. Myocardial involvement is a rare complication of influenza. In a case series of 505 children admitted with influenza from 2003 to 2004 in nine tertiary centres in Canada, only two had cardiac involvement with myocarditis (3), although in a United States case series of 47 patients who died following influenza infection, myocarditis was identified in six cases. In a Japanese cohort (4) of 96 patients with influenza A, 11 were identified to have cardiac involvement. In contrast, in a United Kingdom cohort of 152 patients with serologically confirmed influenza, no cardiac involvement was identified by means of CK-MB or troponin measurements (5). In the largest case series to date of myocardial involvement in influenza A infection from Japan (6) involving nine patients with influenza A myocarditis, cardiac involvement occurred between four and seven days after the onset of influenza, and the most common symptom was worsening dyspnea. ECG changes were common. Three patients had ST elevation, and one patient had new-onset left bundle branch block. Cardiac enzymes were elevated and global LV function was depressed in all nine patients, and of these, four had normalization of LV function and one patient died. There have been previous case reports of influenza myocarditis, including cases of cardiogenic shock treated aggressively with inotropic agents, ventricular assist devices and mechanical support.
Several studies using a molecular biological approach have reported that different cardiotropic viruses are implicated in most cases of human myocarditis. It is uncertain whether myocyte damage in the early phase of the disease is linked primarily to the viral presence or to immunomediated damage. However, it is now widely accepted that progression of the disease is mainly sustained by immunomechanisms. Proinflammatory cytokines, including tumour necrosis factor-alpha (TNF-α), have been recognized as important factors in the initiation and development of the pathophysiology of inflammatory cardiomyopathies. Indeed, it has been demonstrated in human studies (7) that infection of myocardium with influenza A is associated with an increased expression of TNF-α and its receptors (TNFRI and TNFRII) in the myocardium. An association between depressed myocardial function and elevated TNF-α messenger RNA and protein levels in myocardium has been previously demonstrated in patients with myocarditis (8). TNF-α may depress myocardial contractility through a number of mechanisms, including NO-mediated mechanisms and direct actions on intracellular calcium handling in the myocardium. Spontaneous resolution in cardiac function has been reported in up to 40% of patients with viral myocarditis (9), although the role of immunomodulation remains uncertain in these cases.
CONCLUSIONS
Myocardial involvement in influenza infection is well recognized and must be considered in patients presenting with raised cardiac markers or an abnormal ECG. An echocardiogram in these patients is warranted at an early stage to look for potentially fatal complications, such as cardiac tamponade, and to assess LV function to further guide treatment. Furthermore, cardiac involvement should be considered in any patient who becomes acutely more breathless or hypotensive during infection with influenza, and further cardiac investigations in these patients are warranted.
REFERENCES
- 1.Noda T, Sagara H, Yen A, et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006;439:490–2. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DL, Vaudry W, Scheifele DW, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics. 2006;118:e610–9. doi: 10.1542/peds.2005-2744. [DOI] [PubMed] [Google Scholar]
- 4.Kaji M, Kuno H, Sato Y, Oizumi K. Elevated serum myosin light chain I in influenza patients. Intern Med. 2001;40:594–7. doi: 10.2169/internalmedicine.40.594. [DOI] [PubMed] [Google Scholar]
- 5.Greaves K, Oxford JS, Price CP, Clarke GH, Crake T. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: Measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med. 2003;163:165–8. doi: 10.1001/archinte.163.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Onitsuka H, Imamura T, Miyamoto N, et al. Clinical manifestations of influenza a myocarditis during the influenza epidemic of winter 1998–1999. J Cardiol. 2001;37:315–23. [PubMed] [Google Scholar]
- 7.Calabrese F, Carturan E, Chimenti C, et al. Overexpression of tumor necrosis factor (TNF)alpha and TNFalpha receptor I in human viral myocarditis: Clinicopathologic correlations. Mod Pathol. 2004;17:1108–18. doi: 10.1038/modpathol.3800158. [DOI] [PubMed] [Google Scholar]
- 8.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72:561–6. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dec GW, Jr, Palacios IF, Fallon JT, et al. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985;312:885–90. doi: 10.1056/NEJM198504043121404. [DOI] [PubMed] [Google Scholar]