Abstract
BACKGROUND
Long-term atrial fibrillation changes in many regulatory and structural processes may result in stabilization of the arrhythmia. There is evidence that decreased amplitude of L-type Ca2+ current – probably a key mechanism of atrial remodelling –resulting from changes in expression of regulatory proteins is at least jointly responsible.
OBJECTIVES
To assess the expressions of protein phosphatases PP1 and PP2A, as well as the effect of verapamil pretreatment (VPT), in the early phases of atrial fibrillation in a rabbit model.
METHODS
Four groups, each consisting of six animals, were studied: ‘not paced, no drug’ group; ‘paced, no drug’ group (rapid atrial pacing [RAP] 600 beats/min for 24 h); ‘not paced, verapamil’ (NPV) group (duration of VPT was seven days, verapamil 7.5 mg/kg was administered every 12 h); and ‘paced, verapamil’ (PV) group (pacemaker stimulation after VPT). Protein expression was evaluated by Western blot analysis.
RESULTS
RAP resulted in an augmented (32%) PP1 expression (not paced, no drug group versus paced, no drug group). The increase in PP1 expression was prevented with VPT (NPV group versus PV group). Expression of PP2A was not influenced by RAP. However, VPT led to an increase of PP2A expression (16%) after RAP (NPV group versus PV group).
CONCLUSIONS
Fortified expression of protein phosphatases might be – besides transcriptional downregulation of channel subunits – another important cause of reduced L-type Ca2+ current after RAP. Blocking L-type Ca2+ channels with verapamil to prevent tachycardia-induced changes of PP1 expression might be expedient.
Keywords: Atrial fibrillation, Atrial remodelling, L-type Ca2+ channel, PP1, PP2A
Atrial fibrillation (AF) is the most common sustained arrhythmia in humans (1), characterized by a variety of electrophysiological, mechanical and structural alterations caused by the arrhythmia itself (2). This process is termed ‘atrial remodelling in AF’ as described by Wijffels et al (3). Electrophysiological alterations of ionic current level are well studied; for example, during experimental and clinical AF, L-type Ca2+ current (ICa,L) amplitude decreases (4–7).
On the one hand, multiple findings suggest that the down-regulation of ICa,L is transcription-mediated (8–10), and on the other hand, several groups report that there is no change in messenger RNA or protein expression (11,12).
A recent study by Christ et al (13) shows increased protein phosphatase expression in patients with chronic AF (13). Because balanced activity between phosphorylating protein kinases (protein kinase A, protein kinase C, Ca2+/calmodulin-dependent kinase II) (14,15) and dephosphorylating protein phosphatases (PP1 and PP2A) (16) is responsible for the actual amplitude of basal ICa,L, this relationship could be an additional explanation for the consistent finding of AF-induced reduction of ICa,L amplitude.
Using a rapid atrial pacing (RAP) model of experimental AF (RAPMAF), Bosch et al (5) showed that electrophysiological changes induced by RAP in a rabbit’s atrium could be observed after a few hours and to a similar extent as in the chronic state.
The present study examined potential changes in protein expression of protein phosphatases PP1 and PP2A after short-term RAP.
If changes in protein phosphatase expression were an additional explanation for reduced ICa,L amplitude, circumventing these alterations might be therapeutically useful. Therefore, the effect of verapamil on the expression of PP1 and PP2A, before and after RAP, was studied.
METHODS
All animal care procedures were in accordance with the institutional guidelines of the University of Tübingen, Tübingen, Germany. Animals were instrumented as described previously (5). Altogether, 24 animals were used. Rabbits were randomly divided into four groups of six animals each. Animals in the ‘not paced, no drug’ (NPND) group received atrial pacemaker probes, but no atrial stimulation was applied. After successful lead implantation, RAP was applied in the ‘paced, no drug’ (PND) group. The ‘not paced, verapamil’ (NPV) group was treated similar to the NPND group but with additional verapamil pretreatment (VPT) (subcutaneous injection of verapamil 7.5 mg/kg, dissolved in isotonic NaCl, was administered every 12 h for seven days). Finally, the ‘paced, verapamil’ (PV) group was treated similar to the PND group but with additional verapamil medication (dosage was the same as in the NPV group).
Removal of the heart and sample preparation were performed as described previously (5).
Protein concentration was determined by a modified Lowry assay. Proteins were fractionated on 8% sodium dodecyl sulphate polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Amersham Biosciences, Germany) according to standard protocols. Commercialized antibodies were used to detect the catalytic α subunit of PP1 (Sigma, USA), the catalytic α subunit of PP2A (Pharmingen, Germany) and glyceraldehyde-3-phosphate dehydrogenase (Biotrend, Germany). Protein bands were visualized with horseradish peroxidase-conjugated antirabbit immunoglobulin G (Dako, Germany) and antimouse immunoglobulin G (Sigma, Germany) by enhanced chemiluminescence reagents and Hyperfilm enhanced chemiluminescence (Amersham Biosciences, Germany). The films were densitometrically evaluated using ‘Quantity One’ software (Bio-Rad Laboratories, Germany). The expressions of PP1α and PP2Aα were normalized to that of glyceraldehyde-3-phosphate dehydrogenase as a standard.
Data analysis
In each animal, protein measurements were repeated three times, and the average value and SEM were calculated. Data are expressed as mean ± SEM. Statistical comparisons between groups were performed by one-way ANOVA. A two-tailed P<0.05 was considered to be statistically significant.
RESULTS
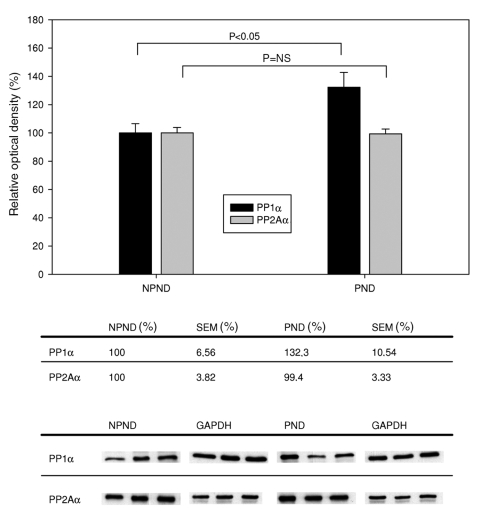
Differences in expression of PP1α und PP2Aα between the NPND and PND groups are shown in Figure 1. RAP led to a 32% increase (P=0.031) of PP1α expression, whereas no significant change of PP2Aα expression (P=0.901) was observed.
Figure 1.
Expression of protein phosphatases PP1 and PP2A (catalytic alpha [α] subunits) with and without rapid atrial pacing. Top Ordinate indicates relative optical density (OD), determined by comparing the absolute OD of the band with the OD of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Beneath the graph are the tabulated data. Bottom Representative Western blots of each group. NPND Not paced, no drug; NS Not significant; PND Paced, no drug
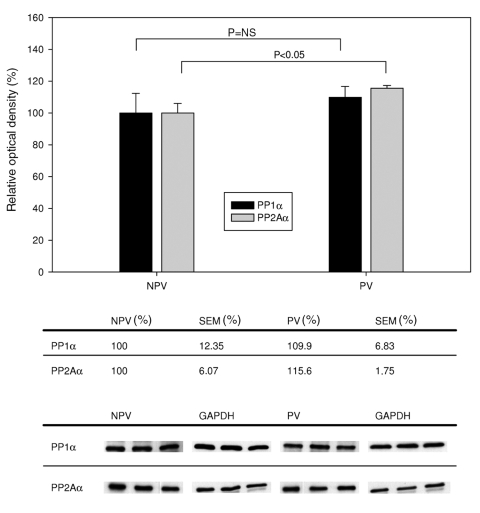
Figure 2 shows the differences in expression of PP1α and PP2Aα between the NPV and PV groups. After VPT, augmentation of PP1α expression after RAP was absent; no significant change (P=0.50) was observed. However, after VPT a significant increase (16%; P=0.033) of PP2Aα expression (NPV versus PV) was observed after RAP.
Figure 2.
Expression of protein phosphatases PP1 and PP2A (catalytic alpha [α] subunits) after verapamil pretreatment with and without rapid atrial pacing. Top Ordinate indicates relative optical density (OD), determined by comparing the absolute OD of the band with the OD of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Beneath the graph are the tabulated data. Bottom Representative Western blots of each group. NPV Not paced, verapamil; NS Not significant; PV Paced, verapamil
DISCUSSION
Effects of RAP on expression of PP1 and PP2A
Phosphorylation and dephosphorylation are key mechanisms for the functional regulation of L-type Ca2+ channels. The aim of the present study was to evaluate whether decreased ICa,L amplitude in the early phases of AF in the RAPMAF is accompanied by alterations of these mechanisms. In the heart, PP1 and PP2A counteract phosphorylation of L-type Ca2+ channels by protein kinase A and protein kinase C, respectively (14–16).
We showed that even in the early phases of RAP there are changes in protein expression of phosphatases: after 24 h, expression of the catalytic α subunit of PP1 is upregulated, whereas no significant changes of the α subunit of PP2A are observed.
These results indicate that upregulation of PP1α might be part of the pathomechanisms in the very early phases of atrial remodelling and a mechanism for rapid downregulation of L-type Ca2+ channels, which is caused by AF-induced intracellular Ca2+ overload. It was reported that the activity of several kinases and phosphatases depend on intracellular Ca2+ concentration (17–19).
Christ et al (13) found that augmented expression of the catalytic subunit of PP2A was associated with no changes in PP1 expression (α subunit). They concluded that diminished phosphorylation of L-type Ca2+ channels was at least jointly responsible for decreased ICa,L amplitude in patients with chronic AF.
In the RAPMAF, 24 h of RAP led to diminished ICa,L with associated changes in protein and messenger RNA expression (5). Our results – comparable with those from human chronic AF (hCAF) – suggest that decreased phosphorylation of L-type Ca2+ channels due to increased expression of protein phosphatases is at least partly responsible for the diminished current amplitude.
The protein expressions of protein phosphatases in hCAF and RAPMAF are oppositional: on the one hand, there is an upregulation of PP1α with no change of PP2Aα (RAPMAF), and on the other hand, no change of PP1α is associated with an increase of catalytic subunit PP2γ (hCAF) (3).
There is a large variety of species-specific denotation of respective protein phosphatases (16). Ono and Fozzard (20) demonstrated that in rabbit hearts, PP1 interacts with the phosphorylation site of the L-type Ca2+ channel, mediating the closing rate of the channel, whereas PP2A interacts with the site of the L-type Ca2+ channel that adjusts the probability of the channel opening. The former mechanism is of greater importance for regulation of current amplitude in rabbits. Therefore, it seems possible that both – apparently different –changes of expression (hCAF versus RAPMAF) are concepts of the same remodelling processes caused by rapid atrial frequencies in different species.
Another explanation for this oppositional observation might be different time points of examination, because atrial remodelling is a fluent, time-dependent process (3). In our study, we examined early phases of remodelling, whereas chronic AF was studied by Christ et al (13).
Effects of verapamil on expression of PP1 and PP2A with and without RAP
In our second experimental setting, we examined the effect of VPT on protein phosphatase expression.
As indicated in Figure 2, there was no statistically significant difference in PP1α expression between the NPV and PV groups; therefore, verapamil obviously prevents atrial tachycardia-induced changes of PP1α expression (NPND group versus PND group).
However, we also showed that VPT and subsequent RAP elevates PP2Aα expression, even though that increase was less pronounced (16%; P=0.033). Because PP1 has more influence on ICa,L amplitude than PP2A in rabbits (see above), this observation is probably of minor importance.
We also previously showed that VPT prevented expected reductions in ICa,L amplitude caused by RAP in rabbit atrium (21). Because we were able to show that verapamil affects protein phosphatase expression, we cannot exclude the possibility that the prevention of pacing-induced PP1 upregulation is –besides probable direct effects of verapamil on Ca2+ channel subunits – at least partly responsible for this observation.
Besides the effects on L-type Ca2+ channel regulation, increased activity of phosphatases may be associated with augmented dephosphorylation of other proteins. Electromechanical coupling is of special interest in this regard. Because contractile dysfunction promotes atrial blood clots, improvement of atrial contraction by blockade of phosphatases could lead to a decreased risk of thromboembolism in patients with intermittent AF.
In summary, according to our data, the isolated effects of verapamil on protein phosphatases make this drug a candidate for the treatment of AF; thus, a key mechanism of atrial remodelling (reduction of ICa,L) can be prevented and the beneficial effects of contractile function can be expected.
Potential limitations
Verapamil leads to multiple alterations of atrial electrophysiology; therefore, the beneficial isolated effect of VPT on protein phophatases might be diminished. Results of clinical studies (22–24) that evaluated the prevention of postoperative AF by verapamil were contradictory.
Changes in atrial refractory period and inducibility of AF were diminished by VPT in several experimental models of AF (25–28) and humans (29). Simultaneously, two other studies showed a loss of effect of verapamil after longer periods of atrial tachycardias (30,31). Even more controversially, verapamil leads to increased duration of AF both in goats and in humans (32,33).
Additionally, only early phases (24 h) of atrial remodelling were examined in the present study, and changes might not represent clinicial AF in all aspects.
Further experiments with sustained atrial pacing are needed to ascertain whether verapamil treatment either leads only to a delay of tachycardia-induced atrial remodelling or can diminish long-term pathophysiological alterations. In addition, effects of VPT on many other mechanisms that maintain AF need to be studied.
CONCLUSIONS
In the present study, we showed that fortified expression of protein phosphatases might be – besides transcriptional down-regulation of channel subunits – another important cause of reduced ICa,L in the early phases of AF.
Alterations in the expression of protein phosphatases by VPT were affected beneficially. Therefore, the use of verapamil to prevent tachycardia-induced changes might be expedient. However, verapamil leads to multiple alterations in atrial electrophysiology; hence, the significance of the benefits of this isolated effect of verapamil on protein phosphatases remains unknown.
ACKNOWLEDGEMENTS
This work was supported by the Bundesministerium für Bildung und Forschung Germany (BMBF)/University of Tübingen (IZKF) (No 01KS9602) and the ‘Kompetenznetz Vorhofflimmern’ (No 01GI0204). The authors thank Jeannette Gogel for expert technical assistance.
REFERENCES
- 1.Hennersdorf MG, Perings C, Kelm M, Strauer BE. Atrial fibrillation. Internist (Berl) 2001;42:1631–40. doi: 10.1007/s001080170015. [DOI] [PubMed] [Google Scholar]
- 2.Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol. 2003;98:137–48. doi: 10.1007/s00395-003-0409-8. [DOI] [PubMed] [Google Scholar]
- 3.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 4.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 5.Bosch RF, Scherer CR, Rüb N, et al. Molecular mechanisms of early electrical remodeling: Transcriptional downregulation of ion channel subunits reduces I(Ca,L) and I(to) in rapid atrial pacing in rabbits. J Am Coll Cardiol. 2003;41:858–69. doi: 10.1016/s0735-1097(02)02922-4. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 7.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–35. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 8.Brundel BJ, Van Gelder IC, Henning RH, et al. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res. 1999;42:443–54. doi: 10.1016/s0008-6363(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 9.Lai LP, Su MJ, Lin JL, et al. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: An insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–7. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder IC, Brundel BJ, Henning RH, et al. Alterations in gene expression of proteins involved in the calcium handling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:552–60. doi: 10.1111/j.1540-8167.1999.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 11.Grammer JB, Zeng X, Bosch RF, Kühlkamp V. Atrial L-type Ca2+-channel, beta-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- 12.Schotten U, Haase H, Frechen D, et al. The L-type Ca2+-channel subunits alpha1C and beta2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J Mol Cell Cardiol. 2003;35:437–43. doi: 10.1016/s0022-2828(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 13.Christ T, Boknik P, Wöhrl S, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–7. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 14.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 15.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994;91:9659–63. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann F, Lacinová L, Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 19.Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–74. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 20.Ono K, Fozzard HA. Two phosphatase sites on the Ca2+ channel affecting different kinetic functions. J Physiol. 1993;470:73–84. doi: 10.1113/jphysiol.1993.sp019848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessel RE, Wöhrl S, Gogel J, et al. Prevention of tachycardia induced cellular electrical remodelling by verapamil in rabbit atria. Eur Heart J. 2004;25(Suppl 1):478. (Abst) [Google Scholar]
- 22.Davison R, Hartz R, Kaplan K, Parker M, Feiereisel P, Michaelis L. Prophylaxis of supraventricular tachyarrhythmia after coronary bypass surgery with oral verapamil: A randomized, double-blind trial. Ann Thorac Surg. 1985;39:336–9. doi: 10.1016/s0003-4975(10)62626-4. [DOI] [PubMed] [Google Scholar]
- 23.Seitelberger R, Hannes W, Gleichauf M, Keilich M, Christoph M, Fasol R. Effects of diltiazem on perioperative ischemia, arrhythmias, and myocardial function in patients undergoing elective coronary bypass grafting. J Thorac Cardiovasc Surg. 1994;107:811–21. [PubMed] [Google Scholar]
- 24.Smith EE, Shore DF, Monro JL, Ross JK. Oral verapamil fails to prevent supraventricular tachycardia following coronary artery surgery. Int J Cardiol. 1985;9:37–44. doi: 10.1016/0167-5273(85)90401-2. [DOI] [PubMed] [Google Scholar]
- 25.Tieleman RG, Van Gelder IC, Crijns HJ, et al. Early recurrences of atrial fibrillation after electrical cardioversion: A result of fibrillation-induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–73. doi: 10.1016/s0735-1097(97)00455-5. [DOI] [PubMed] [Google Scholar]
- 26.Kurita Y, Mitamura H, Shiroshita-Takeshita A, et al. Daily oral verapamil before but not after rapid atrial excitation prevents electrical remodeling. Cardiovasc Res. 2002;54:447–55. doi: 10.1016/s0008-6363(02)00269-9. [DOI] [PubMed] [Google Scholar]
- 27.Kinebuchi O, Mitamura H, Shiroshita-Takeshita A, et al. Oral verapamil attenuates the progression of pacing-induced electrical and mechanical remodeling of the atrium. Circ J. 2004;68:494–500. doi: 10.1253/circj.68.494. [DOI] [PubMed] [Google Scholar]
- 28.Moriguchi M, Niwano S, Yoshizawa N, Kojima J, Inuo K, Izumi T. Verapamil suppresses the inhomogeneity of electrical remodeling in a canine long-term rapid atrial stimulation model. Pacing Clin Electrophysiol. 2003;26:2072–82. doi: 10.1046/j.1460-9592.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 29.Daoud EG, Knight BP, Weiss R, et al. Effect of verapamil and procainamide on atrial fibrillation-induced electrical remodeling in humans. Circulation. 1997;96:1542–50. doi: 10.1161/01.cir.96.5.1542. [DOI] [PubMed] [Google Scholar]
- 30.Fareh S, Bénardeau A, Nattel S. Differential efficacy of L- and T-type calcium channel blockers in preventing tachycardia-induced atrial remodeling in dogs. Cardiovasc Res. 2001;49:762–70. doi: 10.1016/s0008-6363(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Yu WC, Cheng JJ, et al. Effect of verapamil on long-term tachycardia-induced atrial electrical remodeling. Circulation. 2000;101:200–6. doi: 10.1161/01.cir.101.2.200. [DOI] [PubMed] [Google Scholar]
- 32.Duytschaever MF, Garratt CJ, Allessie MA. Profibrillatory effects of verapamil but not of digoxin in the goat model of atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:1375–85. doi: 10.1046/j.1540-8167.2000.01375.x. [DOI] [PubMed] [Google Scholar]
- 33.Shenasa M, Kus T, Fromer M, LeBlanc RA, Dubuc M, Nadeau R. Effect of intravenous and oral calcium antagonists (diltiazem and verapamil) on sustenance of atrial fibrillation. Am J Cardiol. 1988;62:403–7. doi: 10.1016/0002-9149(88)90967-8. [DOI] [PubMed] [Google Scholar]