Abstract
BACKGROUND
The pleiotropic antiatherosclerotic effects of statins are believed to be associated with the inhibition of Rho-kinase. However, a systematic analysis of Rho-kinase activation in atherosclerotic lesions is missing.
OBJECTIVES
To analyze the distribution and phosphorylation of target proteins of Rho-kinase, such as myosin light chain (MLC) and ezrin-radixin-moesin (ERM) proteins, in the apolipoprotein E (ApoE) knockout model of accelerated atherosclerosis, as well as the effects of treatment with the Rho-kinase inhibitor Y-27632.
METHOD
Western diet-fed ApoE-deficient mice underwent carotid ligation and were sacrificed 14 days after surgery. One group of ligated mice was treated with the Rho-kinase inhibitor Y-27632. Nonligated C57Bl6/J mice on normal chow and ApoE-deficient mice on Western diet were used as controls. Lesion structure and size were analyzed using Masson-elastic stained cross-sections. The distribution and phosphorylation of Rho-kinase target proteins were studied immunohistochemically.
RESULTS
Two weeks after surgery, atherosclerotic plaque-like lesions developed in ligated carotids. Lesion development was inhibited by Y-27632. ERM was expressed ubiquitously, but in the intact arteries, it was phosphorylated exclusively in the endothelium and periadventitial adipocytes. In the atherosclerotic lesions, foamy macrophages also exhibited a strong phospho-ERM signal. Y-27632 inhibited ERM phosphorylation in the plaques. MLC and phospho-MLC were associated with smooth muscle cells and did not respond to the Y-27632 treatment.
CONCLUSIONS
A cell type-selective distribution and phosphorylation of target proteins of Rho-kinase were demonstrated in the carotid artery of the normal mouse model, as well as in the ApoE-knockout model of accelerated atherosclerosis. Various downstream targets of the same enzyme may be differentially involved in specific pathological processes in a cell type-specific manner.
Keywords: Atherosclerosis, Endothelium, Macrophages, Phosphorylation, Rho-kinase
Statins exert their vascular protective effects as a result of their primary lipid-lowering activity and, presumably, in a ‘pleiotropic’, cholesterol-independent manner (1). Because statins inhibit an early step in the cholesterol biosynthesis, they also inhibit the synthesis of isoprenoids, which are important post-translation lipid attachments for intracellular signalling molecules such as the Rho GTPases (2).
It was therefore suggested that Rho-kinase itself can be a direct vascular target based on its role in endothelial nitric oxide biology and monocyte migration, as well as in smooth muscle cell (SMC) contraction, migration and proliferation (3). Multiple studies (4–10) have demonstrated the efficacy of Rho-kinase inhibitors in several animal models related to arteriosclerosis. However, a systematic analysis of Rho-kinase activation and drug-induced inhibition in atherosclerotic lesions is missing.
Rho-kinases phosporylate various protein substrates (11), including the myosin light chain (MLC) phosphatase, which is responsible for the dephosphorylation of MLC. Because the phosphorylation of MLC phosphatase is associated with the inactivation of this enzyme, the net effect of Rho-kinase activation is consistent with increased phosphorylation of MLC. Moreover, Rho-kinase can phosphorylate MLC directly. Thus, MLC phosporylation is believed to be a hallmark of Rho-kinase activation. Other Rho-kinase substrates include LIM kinase and ezrin-radixin-moesin (ERM) proteins.
In the present study, we analyzed the distribution and phosphorylation of target proteins of Rho-kinase, such as MLC and ERM proteins, in the apolipoprotein E knockout (ApoE-KO) model of accelerated atherosclerosis, as well as the effects of treatment with the Rho-kinase inhibitor Y-27632.
METHODS
Animal experiments
Eight-week-old ApoE-KO mice and C57Bl6/J mice (genetic background strain of ApoE-KO mice) were obtained from Taconic (USA). The ApoE-KO mice were pre-fed a Western diet containing 0.21% cholesterol and 21% fat for 14 days. To accelerate lesion formation, the left common carotid artery was ligated under isofluorane anesthesia as described previously (12). Sham-operated, nonligated ApoE-KO mice were used as controls. The mice were kept on the same diet post surgery. Mice were treated with Rho-kinase inhibitor Y-27632 at a dose of 10 mg/kg and 30 mg/kg twice a day (vehicle in a control group) by oral gavage started 1 h before surgery. The doses for Y-27632 were chosen based on the range of effective doses reported in the literature, which extends from 5 mg/kg/day (7,9) to 30 mg/kg/day (5,6), with 100 mg/kg/day being the maximum reported (8). ApoE-KO mice were distributed between experimental and control groups as shown in Table 1. In addition, a group of intact, age-matched C57Bl6/J mice (n=6) were kept on a standard mouse chow diet and did not undergo any surgical or pharmacological manipulations. This group served as an ultimate negative control representing the distribution and phosphorylation of Rho-kinase target proteins in the normal mouse carotid artery.
TABLE 1.
Distribution of apolipoprotein E knockout mice between experimental and control groups
| Group | Sham operation | Carotid ligation |
|---|---|---|
| Vehicle | n=6 | n=6 |
| Y-27632, 10 mg/kg, Bid | – | n=6 |
| Y-27632, 30 mg/kg, Bid | – | n=6 |
Bid Twice a day; Y-27632 A specific Rho-kinase inhibitor
The mice were sacrificed 14 days after carotid ligation. At necropsy, blood was collected via cardiac puncture, and plasma lipids were analyzed with a Hitachi analyzer. For histology end points, the arteries were perfused-fixed with zinc-Tris fixative. All of the procedures and protocols involving the use of animals were approved by the Eli Lilly Animal Care and Use Committee and conformed to the “Guide for the Care and Use of Laboratory Animals” published by the United States National Institutes of Health (National Institutes of Health publication No 85 to 23, revised 1996).
Histology and immunohistochemistry
Carotid arteries were embedded in paraffin and sectioned. Ten equally spaced (200 μm) cross-sections were stained with Masson’s trichrome. For immunohistochemistry, the sections were incubated overnight with primary antibody at 4°C. Rabbit antihuman ERM, phospho-ezrin (Thr567)/radixin (Thr564)/moesin (Thr558) (pERM), and phospho-MLC (pMLC) 2 (Ser19) antibodies were obtained from Cell Signaling Technology (USA). Rabbit antihuman MLC antibody was obtained from Santa Cruz Biotechnology (USA). For rat antimouse Mac-2 (Accurate Chemical, USA) staining, sections were incubated for 30 min at room temperature. After primary antibody incubation, sections were incubated with biotinylated goat antirabbit or rabbit antirat immunoglobulin G (Vector Laboratories, USA), followed by streptavidin-biotin complex (Dako, USA) and development with diaminobenzidine. Rabbit or rat immunoglobulin G staining was used as a negative control. Alpha smooth muscle actin EPOS was obtained from Dako (USA) . Sections were incubated for 60 min at room temperature followed by development with diaminobenzidine.
Image analysis and statistics
The intimal (lesion) area, defined as an area between the lumen and the internal elastic lamina, was calculated using Masson-stained sections. ERM-, pERM-, MLC- and pMLC-immunostained sections were used to measure the intensity of cytoplasmic immunoperoxidase staining in the intimal, medial and adventitial cells. Images were captured and analyzed using Image-Pro Plus version 5.0.1 (Media Cybernetics, USA). Data are presented as mean ± SEM. The differences between groups were determined using one-way ANOVA with post-hoc Dunnett’s t test. A value of P<0.05 was regarded as a statistically significant difference.
RESULTS
Lesion development and effects of Y-27632
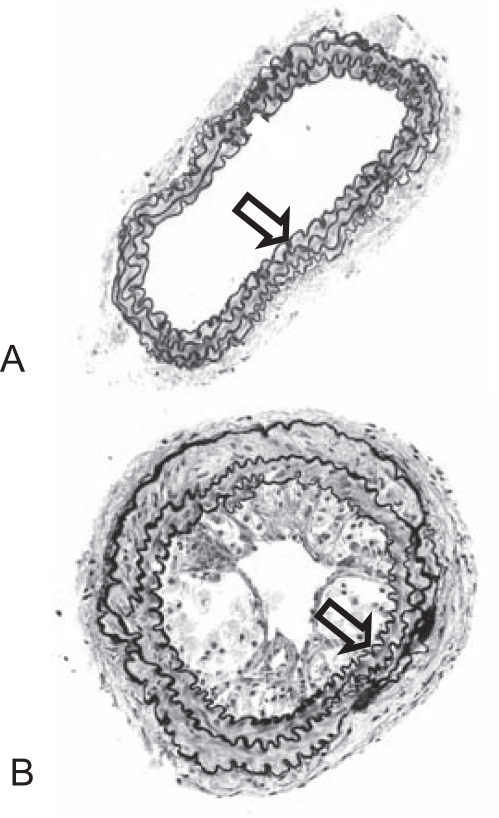
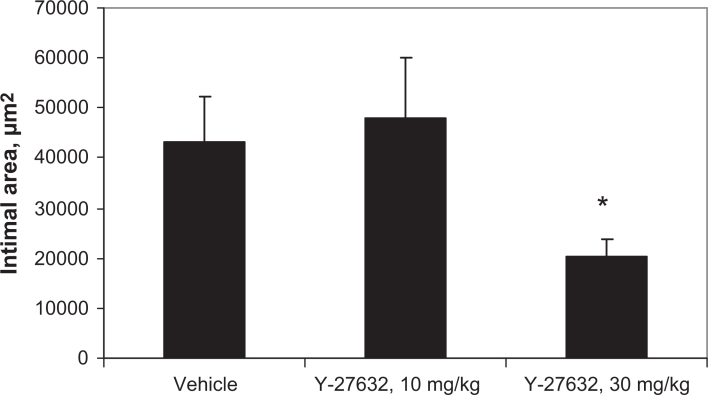
Fourteen days after ligation of the left common carotid artery, near the area of bifurcation, atherosclerotic plaque-like lesions had developed along the length of the artery (Figure 1). No spontaneous lesions occurred in the carotid arteries of the control mice. Intimal lesions consisted of the fibrous cap, rich in SMCs and extracellular matrix, and the more centrally located core area enriched with lipids and macrophages. Y-27632 treatment at the highest dose (30 mg/kg twice a day) significantly inhibited lesion formation, while the lower dose (10 mg/kg twice a day) was not efficacious (Figure 2).
Figure 1.
Lesion development in the carotid arteries of apolipoprotein E knockout mice. Masson-elastin staining of (A) the nonligated artery and (B) 14 days after ligation. Arrow denotes internal elastic lamina. Original magnification ×20
Figure 2.
Intimal area in ligated carotid arteries treated with Rho-kinase inhibitor Y-27632. *P<0.05
Distribution and phosphorylation patterns of Rho-kinase targets
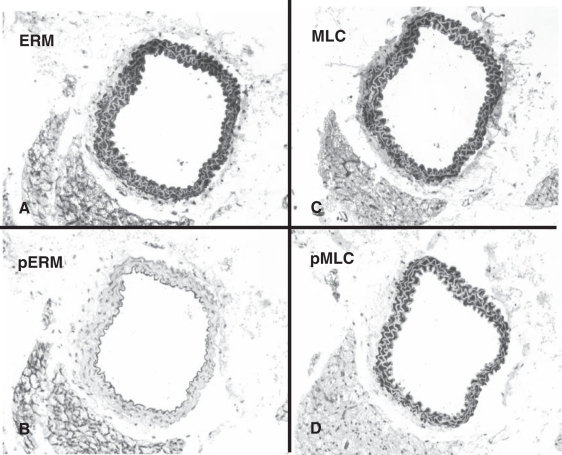
In the intact carotid arteries of both ApoE-KO and C57Bl6/J mice (Figure 3), ERM immunostaining was ubiquitous. Remarkably, ERM was phosphorylated exclusively in endothelial cells (both the luminal endothelium of the carotid artery and the adventitial capillary endothelium) as well as in periadvenitial adipocytes. MLC immunostaining was predominantly associated with the medial SMC. Phophorylated MLC immunostaining exhibited a very similar pattern.
Figure 3.
Distribution and phosporylation patterns of target proteins of Rho-kinase in the nonligated carotid arteries of apolipoprotein E knockout mice. Ezrin-radixin-moesin (ERM) immunostaining is ubiquitous (A), while phospho-ERM (pERM) staining is confined to endothelial cells (B). Myosin light chain (MLC) immunostaining is predominantly associated with the medial smooth muscle cell (C). Phospho-MLC (pMLC) immunostaining exhibits a very similar pattern (D). Original magnification ×20
The observed patterns suggested a broad expression of ERM but very tight cell-specific regulation of ERM phosphorylation. In contrast, cell type specificity of pMLC seemed to be dependent on the pattern of MLC expression rather than its phosphorylation.
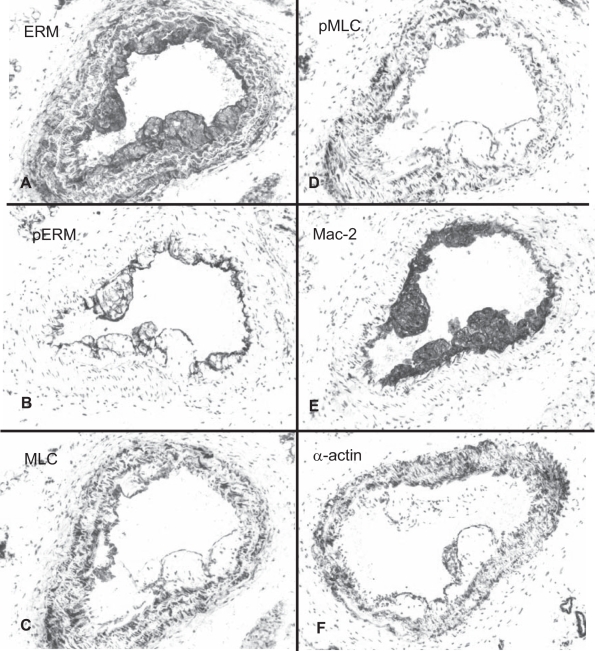
Fourteen days after carotid ligation (Figure 4), virtually every cell, including the macrophages and the SMCs in the lesions, was ERM-positive. Endothelial cells in the ligated arteries, as in the intact arteries, were strongly pERM-positive. As a distinct feature of the plaques, ERM phosphorylation was prominent in the lipid-laden macrophages (foam cells), while lesional SMCs were pERM-negative. Noticeably, pERM staining in the foam cells was predominantly associated with the cell surface. MLCs, as in the intact arteries, were associated with SMCs. In the present study, however, both medial and intimal SMCs expressed MLCs. Otherwise, there was no difference in MLC immunostaining pattern between the control and ligated arteries. As in the intact arteries, the immunostaining pattern of pMLC was very similar to MLC.
Figure 4.
Distribution and phosporylation patterns of Rho-kinase target proteins in the carotid lesions of apolipoprotein E knockout mice 14 days after ligation. Ezrin-radixin-moesin (ERM) immunostaining is ubiquitous (A). Phospho-ERM (pERM) detected in endothelial cells and macrophages but not in smooth muscle cells (SMCs) (B). Myosin light chain (MLC) and phospho-MLC (pMLC) immunostaining (C and D, respectively) are associated predominantly with SMCs. Mac-2 (E) and alpha (α)-actin immunostaining (F) illustrate the location of macrophages and SMCs, respectively. Original magnifications ×20
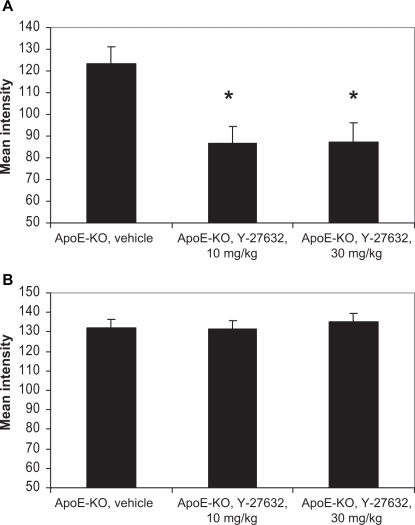
Y-27632 treatment did not have any significant effect on the intensity of ERM immunostaining. However, mean intensity of the pERM signal was lowered at both doses of the compound (Figures 4 and 5). It was significantly different from control arteries (P<0.05), but no difference between the doses had been found. We did not detect any changes in the MLC and pMLC immunostaining in the plaque (Figure 5).
Figure 5.
Effects of Rho-kinase inhibitor Y-27632 on the mean intensity of phospho-ezrin-radixin-moesin (A) and phospho-myosin light chain (B) immunostaining in the intimal cells of apolipoprotein E knockout (ApoE-KO) mouse carotid lesions. *P<0.05
Thus, atherosclerotic plaques featured ERM phosphorylation in association with the luminal endothelium and lesion macrophages, while MLC phosphorylation was associated with SMCs. Of all the target proteins of Rho-kinase studied, only pERM in the intima demonstrated decreased intensity of the staining in the animals treated with Y-27632.
DISCUSSION
We have demonstrated a cell type-selective distribution and phosphorylation of Rho-kinase target proteins ERM and MLC in the normal mouse carotid artery, as well as in the ApoE-KO model of accelerated atherosclerosis. ERM was expressed ubiquitously, but in the control arteries it was phosphorylated exclusively in the endothelium and periadventitial adipocytes. In the atherosclerotic lesions, foamy macrophages exhibited a strong pERM signal associated with the cell surface. ERM was also phosphorylated in the adventitial fibroblasts of ligated carotids. Y-27632 inhibited ERM phosphorylation in the plaques but, however, failed to demonstrate dose dependence. MLC and pMLC were associated with SMC (medial in normal arteries plus intimal in ligated arteries) and did not respond to the Y-27632 treatment.
Y-27632 efficacy has been demonstrated in animal models related to vascular injury and arteriosclerosis in general (4,6–10). Surprisingly, actual efficacy data on atherosclerosis per se are scarce. Mallat et al (5) reported that Y-27632 inhibited formation of early atherosclerotic lesions in low-density lipoprotein receptor-deficient mice. However, Rho-kinase inhibition was documented by functional changes in contractility of denuded aortic rings, and molecular mechanisms of these changes have not been addressed. Our efficacy data, obtained in a flow cessation model of accelerated atherosclerosis in ApoE-KO mice, are consistent with those reported by Mallat et al (5). Pearce et al (8) used a flow cessation approach in the normocholesterolemic C57Bl6/J mice and demonstrated prevention of myointimal thickening formation in the mice treated with Rho-kinase inhibitor. No attempt to characterize the effects on the target proteins of Rho-kinase was made. Thus, our results are in agreement with published efficacy data in similar animal models, but the molecular effectors of such an efficacy in each individual case cannot be directly compared.
In our experiments, Y-27632 treatment was associated with apparent inhibition of ERM phosphorylation. These changes were detected at both doses of the compound, but only the highest dose was efficacious. The precise cause of this discrepancy is unclear. It is possible that Y-27632 possesses pleiotropic Rho-kinase independent activities that may have contributed to antiatherosclerotic efficacy. Alternatively, other Rho-kinase substrates or downstream signal transduction mechanisms can play important vascular protective roles. Studies that include additional markers of Rho-kinase activation are needed to establish tight correlations between pharmacodynamic and efficacy end points. Once the appropriate markers are found, it will be important to establish associations between those markers and plaque features other than size. In particular, it will be intriguing to unravel the potential involvement of Rho-kinase, not only in the plaque development, but also in plaque vulnerability. Animal models of accelerated atherosclerosis that were used in the present study have been recently modified to induce plaque rupture (13). A comparison of Rho-kinase activation in the stable and vulnerable lesions is likely to yield novel useful information.
Finally, it is important to note that many proteins may be phosphorylated by more than one kinase. Therefore, the lack of tight correlation between the drug-induced inhibition of ERM phosphorylation and its efficacy may be partly explained by the involvement of the other kinases that are also activated in atherosclerotic plaques.
In general, systematic molecular data on Rho-kinase activation and inhibition in atherosclerosis are lacking. To the best of our knowledge, this is the first demonstration of differential expression and phosphorylation of target proteins of Rho-kinase by various cell types in normal and diseased blood vessels. The biological underpinnings of these patterns are unknown. It has been reported that pERM is responsible for interactions between actin filaments and the plasma membrane (11). Therefore, pERM may be needed in cases of ‘active’ cell surfaces (maintenance of endothelial integrity, phagocytosis in macrophages, etc). Phospho-MLC is necessary for cell contraction (2). This may explain the association of pMLC with SMC and the involvement of MLC phosphorylation in vasospasm and hypertension. It is unknown which substrate(s) is primarily driving Rho-kinase inhibitor efficacy in atherosclerosis. Our data suggest ERM phosphorylation-mediated macrophage infiltration and foam cell formation play a critical role, rather than MLC phosphorylation-mediated SMS contraction, although further studies are needed.
It is conceivable that various downstream targets of Rho-kinase may be differentially involved in specific pathological processes in a cell type-specific manner. Therefore, target inhibition may have different molecular and functional consequences in various disease contexts. Accordingly, markers of target inhibition by a drug may be disease-related, ie, different for hypertension and atherosclerosis, and may be presented by different cell types.
REFERENCES
- 1.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–5. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–38. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 3.Hu E, Lee D. Rho kinase inhibitors as potential therapeutic agents for cardiovascular diseases. Curr Opin Investig Drugs. 2003;4:1065–75. [PubMed] [Google Scholar]
- 4.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 5.Mallat Z, Gojova A, Sauzeau V, et al. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–8. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto Y, Uwatoku T, Oi K, et al. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: Involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:181–6. doi: 10.1161/01.ATV.0000105053.46994.5B. [DOI] [PubMed] [Google Scholar]
- 7.Miyata K, Shimokawa H, Kandabashi T, et al. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–8. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 8.Pearce JD, Li J, Edwards MS, English WP, Geary RL. Differential effects of Rho-kinase inhibition on artery wall mass and remodeling. J Vasc Surg. 2004;39:223–8. doi: 10.1016/s0741-5214(03)01037-1. [DOI] [PubMed] [Google Scholar]
- 9.Shimokawa H, Morishige K, Miyata K, et al. Long-term inhibition of Rho-kinase induces a regression of arteriosclerotic coronary lesions in a porcine model in vivo. Cardiovasc Res. 2001;51:169–77. doi: 10.1016/s0008-6363(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang YX, Martin-McNulty B, da Cunha V, et al. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–26. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 11.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 12.Ivan E, Khatri JJ, Johnson C, et al. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: The murine model of macrophage-rich carotid artery lesions. Circulation. 2002;105:2686–91. doi: 10.1161/01.cir.0000016825.17448.11. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki T, Kuzuya M, Nakamura K, et al. A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1304–9. doi: 10.1161/01.ATV.0000219687.71607.f7. [DOI] [PubMed] [Google Scholar]