Abstract
BACKGROUND
Atrial conduction delay and its association with left atrial dimension, left atrial pressure and left ventricular (LV) diastolic dysfunction in patients at risk of atrial fibrillation (AF) may be assessed by high-resolution electrocardiography of P wave.
OBJECTIVES
To determine how left atrial size, left atrial pressure and LV diastolic dysfunction, measured noninvasively by transthoracic echocardiography, influence atrial conduction time.
METHODS
Signal-averaged electrocardiography of P wave and echocardiogram were performed on 70 patients (average age of 63±10 years; 37 male and 33 female), divided into three groups: group A, patients with paroxysmal AF (n=29); group B, patients with type 2 diabetes mellitus and arterial hypertension, but without AF (n=23); and group C, healthy control patients (n=18). Standard statistical methods were used.
RESULTS
Filtered P wave duration, measured by signal-averaged electrocardiography, was significantly prolonged in group A and group B compared with control group C (138±12 ms and 125±9 ms versus 117±8 ms; P ≤ 0.001 and P ≤ 0.01, respectively). Left atrial diameter, area and volume were significantly increased in group A compared with group C (P ≤ 0.01, P ≤ 0.05 and P ≤ 0.001, respectively), but no significant differences were found in these dimensions between group B and group C. Left atrial pressure, determined with the Doppler echocardiographic parameter ratio of early diastolic transmitral velocity to mitral annular early diastolic velocity, was significantly higher in group A, as well in group B, than group C (P ≤ 0.05, P ≤ 0.01). As well, impaired LV relaxation was present more often in group A (42%) and group B (50%).
CONCLUSION
Atrial conduction delay in patients at risk of AF (patients with diabetes and hypertension in group B) was associated with increased left atrial pressure and impaired LV relaxation. Patients with paroxysmal AF (group A) presented left atrial dilation, increased left atrial pressure and impaired LV relaxation, and these factors were associated with more significantly prolonged atrial conduction in group A than in group B.
Keywords: Atrial fibrillation, Diastolic dysfunction, Left atrial pressure, Left atrial size, Signal-averaged P wave
A trial fibrillation (AF) is the most common sustained cardiac arrhythmia, and its prevalence is increasing (1). AF occurs in 0.3% to 0.4% of the adult population. The impact of AF on morbidity and mortality is substantial, as are the socioeconomic consequences. AF is most commonly seen in patients with underlying structural heart disease, including hypertensive heart disease, cardiomyopathy, coronary artery disease, valvular heart disease and congestive heart failure (2).
The substrate, trigger and modulator are included in AF formation. Several factors may contribute to the permanence of AF, such as slow atrial conduction, shortening of atrial refractoriness and increased size of heart atria. Experimental data from Allessie et al (3) strongly support the concept originally proposed by Moe (4) that AF is based on the simultaneous random activation of multiple wavelets – the multiple wavelet hypothesis. The wavelength is a product of refractory period and conduction velocity. The reentry is more likely to occur in conditions of slow conduction velocity and short refractory periods. The mapping of AF has shown that at least three to six separate wavelets are necessary for AF maintenance (3). Enlarged atria are more inclined to AF because a larger area allows for more re-entry circuits.
Left ventricular (LV) diastolic dysfunction is an important risk factor of AF, because LV diastolic dysfunction has fundamental influences on left atrial properties. We attempted to determine how LV diastolic dysfunction increases left atrial pressure and left atrial diameter (LAD), both of which influence atrial conduction times measured noninvasively by signal-averaged electrocardiography (SAECG) of P wave.
Background of SAECG
SAECG has become an important tool in the evaluation of patients with ventricular arrhythmias (5). Signal averaging of the QRS complex from the surface electrocardiogram eliminates random noise, thus permitting the visualization of otherwise undetectable electrocardiographic signals. Recently, in an effort to identify substrates of atrial arrhythmias, similar signal-averaging technology has been applied to the P wave from the surface electrocardiogram. SAECG of P wave has been shown, in retrospective and prospective studies, to discriminate between patients at risk of AF and those without risk of AF. SAECG of P wave may improve the low sensitivity of standard ECG of P wave in the detection of patients at risk of AF. However, its role in the clinical management of patients still remains unclear, partly because standards for P wave measurement are not yet available (6). SAECG is a noninvasive test that takes 30 min to record. Averaging followed by amplification after proper filtering of the electrical signal allows more precise measurements of duration and amplitude of the P wave. A prolonged filtered P wave duration (FPD) is one of the best predictors of AF. The main contribution of SAECG is the decrease in noise. The noise is reduced by a factor of 1/N, in which N is the number of averaged signals. The advantage of the SAECG over the standard ECG is its ability to record low-amplitude, high-frequency electrical signals, responsible for delayed myocardial conduction, a prerequisite component for re-entrant arrhythmias. Minimizing the value of an alignment error by the ‘template creation’ technique permits the accurate determination of the onset and termination of the P wave. SAECG of P wave has demonstrated good immediate and short-term reproducibility, and this is an important concern in the applicability of P wave SAECG in clinical practice.
PATIENTS AND METHODS
Patients
Twenty-nine patients with documented paroxysmal AF (group A), 23 patients with type 2 diabetes mellitus and essential arterial hypertension (grade 1 according to the European Society of Cardiology/European Society of Hypertension classification) but without documented AF (group B), and 18 healthy control subjects (group C) were evaluated. Basic characteristics are presented in Table 1. Exclusion criteria included patients with congestive heart failure, significant valvular abnormalities, coronary artery disease, thyropathy and chronic obstructive pulmonary disease. None of the patients were taking amiodarone within three months of evaluation or other antiarrhythmic agents within one week before the evaluation. All the patients in group A had at least seven days of sinus rhythm without paroxysm of AF.
TABLE 1.
Basic characteristics of evaluated groups
| Group A (n=29) | Group B (n=23) | Group C (n=18) | All patients (n=70) | P* | |
|---|---|---|---|---|---|
| Men, n (%) | 14 (48.3) | 12 (52.2) | 11 (61.1) | 37 (52.9) | NS, NS |
| Age, years | 66.2±10.6 | 61.5±11.4 | 60.4±5.7 | 63.7±10.2 | NS, NS |
| Weight, kg | 82.4±14.2 | 78.0±13.0 | 74.5±12.7 | 78.7±13.5 | NS, NS |
| Height, cm | 169.4±8.5 | 169.8±8.2 | 171.4±10.1 | 170.1±8.8 | NS, NS |
| BMI, kg/m2 | 28.7±4.0 | 27.0±3.7 | 25.4±3.9 | 27.2±4.0 | ≤ 0.05, NS |
| BSA, m2 | 1.93±0.19† | 1.89±0.18† | 1.87±0.19† | 1.90±0.19† | NS, NS |
Values are expressed as mean ± SD unless otherwise specified. Group A consists of patients with paroxysmal atrial fibrillation. Group B consists of patients with type 2 diabetes mellitus and arterial hypertension, but without atrial fibrillation. Group C consists of control healthy patients.
P values of differences between groups A and C and between groups B and C, respectively;
Values are estimated according to reference 26. BMI Body mass index; BSA Body surface area; NS Not significant
SAECG of P wave
SAECG recordings of P wave were performed using a MAC 5000 ECG machine (GE Medical Systems, USA) according to a previously described technique (7). The P wave SAECG was recorded while the patient relaxed in the supine position in a quiet room. After at least 10 min of complete rest, recordings were obtained at a paper speed of 200 mm/s using a 40 Hz to 250 Hz bidirectional filter. Data were converted from analogue to digital with 12-bit accuracy at a sampling rate of 1 kHz. At least 300 cycles were averaged, provided that noise level was maintained at less than 0.5 μV. The filtered X, Y and Z orthogonal Frank leads were combined into a vector magnitude: √(X2 + Y2 + Z2). The FPD of the vector magnitude was defined as the interval between the onset and offset point. P wave onset was defined as the first atrial deflection from baseline noise level, and offset as the return of the atrial signal to baseline. Root-mean-square (RMS) voltages, representing amplitudes in different segments of the P wave, were also measured.
Transthoracic echocardiography
M-mode and two-dimensional echocardiography (SSD 5000, Aloka, Japan) were performed in all the patients to determine left atrial size, LAD in parasternal long-axis projection, left atrial area (LAarea) in apical four-chamber (A4C) projection and left atrial volume (LAvol). LAvol was calculated as LAvol = 4/3 × π × (LAD × LAt × LAl /8), in which LAt and LAl are transversal and longitudinal diameter of left atrium, respectively, in A4C projection. Pulsed Doppler parameters of early (E) and late (A) transmitral velocities, deceleration time (dT), mitral annular early (Em) and late (Am) diastolic velocities, systolic (S) and diastolic (D) forward flow velocities of pulmonary veins, and atrial reversal flow velocity were performed to estimate LV diastolic function and left atrial pressure. Criteria for LV diastolic dysfunction were impaired relaxation (E/A ratio of less than 0.75, dT greater than 240 ms); pseudo-normalization (E/A ratio of 0.75 to 1.5, dT 150 ms to 240 ms, LAvol greater than 28 mL/m2); and restrictive filling (E/A ratio greater than 1.5, dT less than 150 ms).
Statistical analysis
Data are expressed as mean ± SD. Differences between groups were assessed by unpaired Student’s t test. χ2 test was performed for categorical variables. Tests of the strength of association between linear variables were assessed using Pearson correlations (r, correlation coefficient). Statistical inferences were significant if the two-tailed probability statistic was less than or equal to 0.05. All statistical analyses were computed with SPSS version 10.0 (SPSS Inc, USA) for Windows.
RESULTS
P wave SAECG results are presented in Table 2. FPD was significantly prolonged in patients with documented paroxysmal AF (group A) compared with the control patients (group C). RMS voltage of terminal 20 ms of P wave (RMS 20), best among the parameters for expressing terminal low amplitude signals (RMS 40, RMS 30, RMS 20), was decreased in group A compared with group C, but the difference was not significant. FPD was also significantly prolonged in patients with diabetes and hypertension (group B), but not so markedly as seen in group A. However, P wave voltages were more reduced in group B than in group A, especially in the terminal 30 ms and 20 ms segments of P wave. These differences in RMS 30 and RMS 20 were significant.
TABLE 2.
Results of signal-averaged electrocardiography of P wave
| Group A (mean ± SD) | Group B (mean ± SD) | Group C (mean ± SD) | P* | |
|---|---|---|---|---|
| PD, ms | 126.8±14.5 | 114.5±8.2 | 109.6±9.4 | ≤ 0.001, NS |
| FPD, ms | 138.4±12.4 | 125.3±9.1 | 117.2±7.7 | ≤ 0.001, ≤ 0.01 |
| RMS 40, μV | 5.4±2.0 | 4.3±1.8 | 4.9±1.6 | NS, NS |
| RMS 30, μV | 4.3±1.8 | 3.4±1.6 | 4.8±1.5 | NS, ≤ 0.01 |
| RMS 20, μV | 3.6±1.6 | 2.9±1.3 | 4.1±1.7 | NS, ≤ 0.05 |
| RMSP, μV | 7.1±2.2 | 5.2±1.5 | 5.7±1.6 | ≤ 0.05, NS |
| Integral P, μVms | 735±232 | 499±160 | 499±141 | ≤ 0.001, NS |
| Noise, μV | 0.39±0.14 | 0.38±0.17 | 0.42±0.19 | NS, NS |
Group A consists of patients with paroxysmal atrial fibrillation. Group B consists of patients with type 2 diabetes mellitus and arterial hypertension, but without atrial fibrillation. Group C consists of control healthy patients.
P values of differences between groups A and C and between groups B and C, respectively. FPD Filtered P wave duration; Integral P Integral of P wave; NS Not significant; PD P wave duration; RMS 20 Root-mean-square voltage of terminal 20 ms of P wave; RMS 30 Root-mean-square voltage of terminal 30 ms of P wave; RMS 40 Root-mean-square voltage of terminal 40 ms of P wave; RMSP Root-mean-square voltage of total P wave
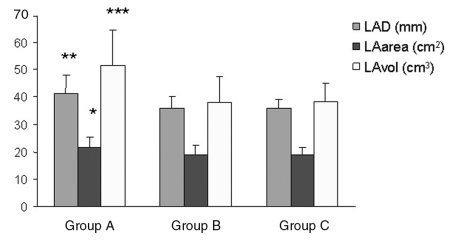
In group A compared with group C, all left atrial dimensions were significantly increased, except for transversal diameter of the left atrium (Table 3). The most significant differences were presented in the LAvol, and the lowest differences were in the LAarea. There were no significant differences in atrial dimensions between groups B and C (Table 3). The comparison of left atrial dimensions is also demonstrated in graphic form (Figure 1).
TABLE 3.
Left atrial dimensions
| Group A (mean ± SD) | Group B (mean ± SD) | Group C (mean ± SD) | P* | |
|---|---|---|---|---|
| LAD, cm | 4.1±0.7 | 3.6±0.4 | 3.6±0.3 | ≤ 0.01, NS |
| LAD/BSA, cm/m2 | 2.1±0.3 | 1.9±0.3 | 1.9±0.2 | ≤ 0.05, NS |
| LAarea, cm2 | 21.6±3.9 | 18.7±3.6 | 18.8±2.9 | ≤ 0.05, NS |
| LAarea/BSA, cm/m2 | 10.9±2.1 | 10.0±1.4 | 10.1±1.4 | NS, NS |
| LAl, cm | 5.2±0.6 | 4.7±0.5 | 4.8±0.5 | ≤ 0.05, NS |
| LAt, cm | 4.5±0.5 | 4.2±0.4 | 4.2±0.5 | NS, NS |
| LAvol, cm3 | 51.5±12.8 | 37.8±9.5 | 38.3±6.7 | ≤ 0.001, NS |
| LAvol/BSA, cm3/m2 | 26.7±7.3 | 20.3±4.9 | 20.6±3.2 | ≤ 0.01, NS |
Group A consists of patients with paroxysmal atrial fibrillation. Group B consists of patients with type 2 diabetes mellitus and arterial hypertension, but without atrial fibrillation. Group C consists of control healthy patients.
P values of differences between groups A and C and between groups B and C, respectively. BSA Body surface area; LAarea Left atrial area; LAD Left atrial diameter; LAl Longitudinal diameter of left atrium; LAt Transversal diameter of left atrium; LAvol Left atrial volume; NS Not significant
Figure 1.
Left atrial dimensions in each group of patients. Group A consists of patients with paroxysmal atrial fibrillation. Group B consists of patients with type 2 diabetes mellitus and arterial hypertension, but without atrial fibrillation. Group C consists of control healthy patients. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. LAarea Left atrial area; LAD Left atrial diameter; LAvol Left atrial volume; NS Not significant
LV diastolic dysfunction, assessed by early (E wave) and late (A wave) diastolic transmitral velocity, as well as by dT, was present in 42% of patients in group A and 50% of patients in group B. All patients with LV diastolic dysfunction had impaired relaxation characteristics. All subjects in control group C demonstrated normal diastolic function of the left ventricle. Tissue Doppler Em, a preload-independent index of LV relaxation, was lower in groups A and B than in group C, but the difference was only significant in group B. Decrease of the Em/Am ratio may be a reflection of an increase of LV end-diastolic pressure. Also, tissue Doppler Em/Am ratio was significantly decreased only in group B. Enhance of E/Em ratio strongly correlated with higher left atrial pressure. This ratio was again increased significantly in group B, but insignificantly in group A, compared with group C. Doppler velocities of pulmonary flow were not significantly different between evaluating groups, except reduced D velocity in group B. Pulsed and tissue Doppler parameters are shown in Table 4.
TABLE 4.
Pulsed Doppler and tissue Doppler echocardiographic parameters
| Group A (mean ± SD) | Group B (mean ± SD) | Group C (mean ± SD) | P* | |
|---|---|---|---|---|
| E, m/s | 0.65±0.14 | 0.69±0.17 | 0.66±0.17 | NS, NS |
| A, m/s | 0.76±0.21 | 0.91±0.30 | 0.67±0.13 | NS, ≤ 0.01 |
| E/A | 0.92±0.38 | 0.81±0.25 | 1.04±0.35 | NS, ≤ 0.05 |
| dT, ms | 216±35 | 227±46 | 193±40 | NS, ≤ 0.05 |
| Em, cm/s | 10.3±3.4 | 8.8±2.6 | 11.8±3.0 | NS, ≤ 0.01 |
| Am, cm/s | 10.9±2.3 | 12.5±2.1 | 12.8±3.3 | NS, NS |
| Em/Am | 0.98±0.35 | 0.71±0.22 | 0.99±0.37 | NS, ≤ 0.05 |
| E/Em | 7.18±1.99 | 8.39±3.28 | 5.70±1.05 | ≤ 0.05, ≤ 0.01 |
| A/Am | 7.07±2.46 | 7.54±3.08 | 5.04±1.94 | ≤ 0.05, ≤ 0.05 |
| S, cm/s | 49.7±11.7 | 49.1±8.3 | 53.7±7.7 | NS, NS |
| D, cm/s | 37.5±6.8 | 34.4±4.2 | 42.4±6.2 | NS, ≤ 0.001 |
| S/D | 1.37±0.37 | 1.45±0.29 | 1.29±0.23 | NS, NS |
| Ar, cm/s | 22.5±2.9 | 22.9±3.8 | 23.3±4.3 | NS, NS |
Group A consists of patients with paroxysmal atrial fibrillation. Group B consists of patients with type 2 diabetes mellitus and arterial hypertension, but without atrial fibrillation. Group C consists of control healthy patients.
P values of differences between groups A and C and between groups B and C, respectively. A Late diastolic transmitral velocity; Am Mitral annular late diastolic velocity; Ar Atrial reversal flow velocity of pulmonary veins; D Diastolic forward flow velocity of pulmonary veins; dT Deceleration time; E Early diastolic transmitral velocity; Em Mitral annular early diastolic velocity; S Systolic forward flow velocity of pulmonary veins; NS Not significant
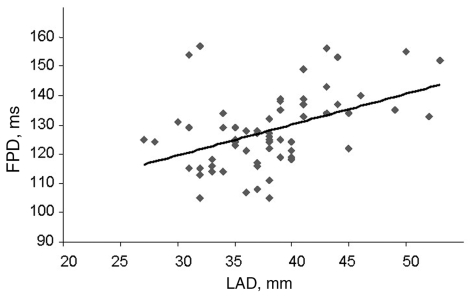
A positive correlation was also demonstrated between FPD and LAD (r=0.445; P=0.001), LA area (r=0.327; P=0.015) LAvol (r=0.423; P=0.001), dT (r=0.290; P=0.033), S/D ratio (r=0.310; P=0.41) and age (r=0.282; P=0.018). On the other hand, FPD negatively correlated with the D wave (r= –0.318, P=0.036). Figure 2 shows positive correlation between FPD and LAD.
Figure 2.
Positive correlation of filtered P wave duration (FPD) and left atrial diameter (LAD). r=0.445; P=0.001
DISCUSSION
AF may be caused by abnormalities in atrial conduction. The relationship between atrial conduction time and P wave duration indicate that the signal-averaged P wave duration may serve as a prognostic index for the development of AF. Yamada et al (8) published a preliminary study in which the P wave-triggered SAECG was used. This method demonstrated significant differences in the total duration of the P wave, with 82% specificity and 88% sensitivity for identifying patients at risk of developing AF. Fukunami et al (9) showed that combining P wave duration and RMS voltage of the last 20 ms measurement increased the sensitivity and specificity of P wave SAECG to 91% and 76%, respectively, for the development of AF. Moreover, the presence or absence of organic heart disease appeared to have no effect on the duration of the P wave or the presence of low-amplitude terminal potentials. P wave SAECG has also been evaluated prospectively as a predictor of recurrence of AF after successful cardioversion of paroxysmal and chronic AF (10). Most studies have shown that a prolonged P wave duration can identify patients at risk of postoperative AF with a reasonable degree of accuracy (11). The signal-averaged P wave duration depends on several factors such as age (12), autonomic tone (13), left atrial dimension and left atrial pressure.
We evaluated two different groups of patients. Group A consisted of patients with documented paroxysmal AF. The recurrence of AF was very frequent in these patients. Patients in group B had diseases that were predisposing to AF (diabetes mellitus and arterial hypertension) without documented AF. Östgren et al (14) showed a novel association between AF and the combined occurrence of type 2 diabetes and hypertension.
It is known that left atrial enlargement is a risk predictor of nonrheumatic AF (15). LAvol is also a predictor of severity of LV diastolic dysfunction (16). The risk of AF enhances with the severity of LV diastolic dysfunction (17) Also, Tsang et al (18) demonstrated that the presence and severity of diastolic dysfunction are independent predictors of first-documented nonvalvular AF in elderly patients. According to data in another study by Tsang et al (19), abnormal diastolic function of the left ventricle was a risk factor for the first episode of AF, only if atrial dilation (greater than 27 mL/m2) was present.
E/Em ratio and B-type natriuretic peptide have been shown to correlate with LV filling pressures. Dokainish et al (20) even demonstrated that mitral E/Em ratio has a better correlation than B-type natriuretic peptide with pulmonary capillary wedge pressure (PCWP). Tissue Doppler echocardiography is a novel technique for directly measuring myocardial velocities. Em has been shown to be a relatively load-independent measure of myocardial relaxation in patients with cardiac disease (21). Faggiano et al (22) tried to determine the contribution of left atrial pressure (PCWP by right-sided heart catheterization) and dimension (echocardiography) to signal-averaged P wave duration in patients with congestive heart failure. They found that in patients with chronic heart failure, P wave duration in SAECG seems to depend more on the level of atrial pressure than on left atrial dimension.
Our results confirmed that high left atrial pressure, expressed by increased E/Em ratio, might play a role in prolonging P wave duration, as was shown by Doppler parameters in group B. Prolonged atrial conduction in group B was not associated with left atrial enlargement. However, other factors could have contributed to atrial conduction delay in group B, such as diabetic cardiac autonomic neuropathy. Insulin resistance and impaired glucose control are plausible mechanisms for development of AF. Furthermore, insulin resistance has been shown to be associated with LV hypertrophy (23). LV diastolic dysfunction, which is often associated with LV hypertrophy, can, through increase of filling pressure, lead to left atrial enlargement. This could be seen in group A but not in group B. In interpretating this fact, we should not forget that structural atrial remodelling is frequent in patients with paroxysmal AF, and that this remodelling is usually connected with left atrial dilation. Because the left atrium is a complex structure, it is not sufficient to measure only LAD in parasternal long-axis projection, but measurements of LAarea and LAvol may also have benefits. We also demonstrated significant differences in LAvol compared with LAD. Assessment of LAarea provided only little benefit, probably because of problems in identifying the endocardial borderline.
According to our results of E wave velocities, A wave velocities and dT, LV diastolic dysfunction was present in approximately one-half of the patients in group A (42%) and group B (50%). Nagueh et al (22) found that Em was a preload-independent index of LV relaxation. Mitral E velocity, corrected for the influence of relaxation (ie, the E/Em ratio), is strongly related to mean PCWP and may be used to estimate LV filling pressures. Em/Am ratio was found to be useful in the estimation of LV end-diastolic pressure (24). The patients with AF following acute myocardial infarction had reduced Sm and Em, as well as increased E/Em ratio, compared with those without AF. The E/Em ratio appears to be a useful parameter for assessing the risk of AF occurrence after anterior acute myocardial infarction (25). Also, our results of tissue Doppler parameters can be interpreted in this meaning – Em and the ratio of Em/Am were reduced, and E/Em increased in group A and especially in group B.
The relationship between S/D ratio and LV diastolic dysfunction is nonlinear. Impaired relaxation leads to a decrease of the D wave and the S/D ratio to remain higher than one. Pseudonormalization or restrictive filling is associated with decreases in S wave; therefore, S/D ratio decreases below one. In our study, patients with LV diastolic dysfunction presented only impaired relaxation. Our results (enhanced S/D ratio) corresponded with this message. Increase of left atrial pressure is also associated with higher velocity of atrial reversal flow velocity, but we did not find any difference in this parameter.
Study limitations
Other factors, apart from left atrial pressure and volume, may determine the electrocardiographic P wave, such as atrial hypertrophy, atrial fibrosis, autonomic tone and age, etc. It is known that a paroxysm of AF can be asymptomatic, so we cannot completely exclude AF in groups B and C.
CONCLUSION
Early recognition of patients at risk of AF may help to minimize potential health risks, costs and other complications. P wave SAECG has been shown to discriminate between patients at risk of AF and those without risk of AF. FPD prolongation on SAECG may be connected with LV diastolic dysfunction and following an increase of left atrial pressure and left atrial enlargement; therefore, FPD should be assessed in patients at risk of AF. Combining P wave duration with other predictors of AF (including echocardiography) may improve the diagnostic value of P wave SAECG.
REFERENCES
- 1.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: The Framingham study. N Engl J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 3.Allessie MA, Konings K, Kirchhof CJ, Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol. 1996;77:10A–23A. doi: 10.1016/s0002-9149(97)89114-x. [DOI] [PubMed] [Google Scholar]
- 4.Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–8. [Google Scholar]
- 5.Steinberg JS, Regan A, Sciacca RR, Bigger JT, Jr, Fleiss JL. Predicting arrhythmic events after acute myocardial infarction using the signal-averaged electrocardiogram. Am J Cardio1. 1992;69:13–21. doi: 10.1016/0002-9149(92)90669-p. [DOI] [PubMed] [Google Scholar]
- 6.Darbar D, Jahangir A, Hammill S, Gersh BJ. P wave signal-averaged electrocardiography to identify risk for atrial fibrillation. Pacing Clin Electrophysiol. 2002;25:1447–53. doi: 10.1046/j.1460-9592.2002.01447.x. [DOI] [PubMed] [Google Scholar]
- 7.Jordaens L, Tavernier R, Gorgov N, Kindt H, Dimmer C, Clement DL. Signal-averaged P wave: Predictor of atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9(8 Suppl):S30–4. [PubMed] [Google Scholar]
- 8.Yamada T, Fukunami M, Ohmori M, et al. Clinical significance of atrial signal-averaged electrocardiogram for detection of patients with paroxysmal atrial fibrillation during sinus rhythm. Circulation. 1989;80(Suppl II):636. doi: 10.1161/01.cir.83.1.162. (Abst) [DOI] [PubMed] [Google Scholar]
- 9.Fukunami M, Yamada T, Ohmori M, et al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–9. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 10.Aytemir K, Aksoyek S, Yildirir A, Ozer N, Oto A. Prediction of atrial fibrillation recurrence after cardioversion by P wave signal-averaged electrocardiography. Int J Cardiol. 1999;70:15–21. doi: 10.1016/s0167-5273(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation. 1993;88:2618–22. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 12.Babaev AA, Vloka ME, Sadurski R, Steinberg JS. Influence of age on atrial activation as mesasured by the P-wave signal-averaged electrocardiogram. Am J Cardiol. 2000;86:692–5. doi: 10.1016/s0002-9149(00)01056-0. [DOI] [PubMed] [Google Scholar]
- 13.Cheema AN, Ahmed MW, Kadish AH, Goldberger JJ. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. J Am Coll Cardiol. 1995;26:497–502. doi: 10.1016/0735-1097(95)80028-f. [DOI] [PubMed] [Google Scholar]
- 14.Östgren CJ, Merlo J, Råstam L, Lindblad U. Atrial fibrillation and its association with type 2 diabetes and hypertension in a Swedish community. Diabetes Obes Metab. 2004;6:367–74. doi: 10.1111/j.1462-8902.2004.00358.x. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation: The Framingham Heart Study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 16.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 18.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–44. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 19.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–8. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Dokainish H, Zoghbi WA, Lakkis NM, et al. Optimal noninvasive assessment of left ventricular filling pressures: A comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–9. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi Wi, Quiñones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 22.Faggiano P, D’Aloia A, Zanelli E, Gualeni A, Musatti P, Giordano A. Contribution of left atrial pressure and dimension to signal-averaged P-wave duration in patients with chronic congestive heart failure. Am J Cardiol. 1997;79:219–22. doi: 10.1016/s0002-9149(96)00720-5. [DOI] [PubMed] [Google Scholar]
- 23.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: Sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 24.Dagdelen S, Eren N, Karabulut H, et al. Estimation of left ventricular end-diastolic pressure by color M-mode Doppler echocardiography and tissue Doppler imaging. J Am Soc Echocardiogr. 2001;14:951–8. doi: 10.1067/mje.2001.113544. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz R, Kasap H, Baykan M, et al. Assessment of left ventricular function by Doppler tissue imaging in patients with atrial fibrillation following acute myocardial infarction. Int J Cardiol. 2005;102:79–85. doi: 10.1016/j.ijcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [PubMed] [Google Scholar]