Abstract
OBJECTIVE
To investigate the effects of atorvastatin on long-term prognosis in diabetic patients with high plasma levels of non-high-density lipoprotein cholesterol (non-HDL-C) after percutaneous coronary intervention (PCI).
METHODS
A total of 648 diabetic patients with high plasma levels of non-HDL-C who had undergone successful PCI were randomly assigned to therapy group (n=327, atorvastatin, 20 mg/day) or control group (n=321, without any lipid-modifying therapy). Study end points included all-cause death, fatal or nonfatal myocardial infarction (MI), and revascularization.
RESULTS
The median follow-up was 21±2.9 months. Rates of MI (6.4% versus 12.3%, P=0.013), revascularization (19.2% versus 26.6%, P=0.029) and composite end points (26.9% versus 41.5%, P<0.001) were significantly lower in the atorvastatin group compared with those of the control group, although mortality rate (5.1% versus 7.9%, P=0.196) was not. Patients treated with atorvastatin had significantly improved adjusted event-free survival rate than controls (hazard ratio 0.52, 95% CI 0.30 to 0.91, P=0.022).
CONCLUSION
Diabetic patients with high plasma levels of non-HDL-C should receive long-term lipid-modifying drugs after PCI to reduce MI and revascularization rates.
Keywords: : Atherosclerosis, Catheterization, Cholesterol, Diabetes mellitus, Statins
Recent research (1) showed that non-high-density lipoprotein cholesterol (non-HDL-C) is superior to low-density lipoprotein cholesterol (LDL-C) for predicting cardiac mortality and has been introduced as a secondary target of lipid-modifying therapy other than LDL-C. It is not known what role lipid-modifying therapy plays in high plasma non-HDL-C level patients with both diabetes and coronary artery disease after percutaneous coronary intervention (PCI). The aim of the present study is to investigate effects of atorvastatin on long-term prognosis in those patients.
METHODS
Study population
The study population consisted of 648 consecutive patients with both diabetes and coronary artery disease who had undergone successful PCI between January 2002 and May 2003 at General Hospital of ShenYang Military Command (China). Patients were eligible for enrolment if they had a plasma non-HDL-C concentration greater than 3.4 mmol/L. The criteria were determined according to therapy target of Adult Treatment Panel (ATP) III guidelines (1), and diabetes was diagnosed according to 1999 World Health Organization criteria. Exclusion criteria were systolic blood pressures greater than 140 mmHg and diastolic blood pressures greater than 90 mmHg despite drug treatment; active liver disease or sustained elevated plasma alanine aminotransferase without identified cause; other severe diseases (such as carcinoma or disease of the lung, kidney or hematological system); pregnancy or breastfeeding; use of contraceptive drugs; or secondary hyperlipidemia caused by thyroid hypofunction, gout, pancreatitis, alcoholism or medicine.
Laboratory analysis
Venous blood was collected the day after admission. Plasma triglyceride (TG), total cholesterol, LDL-C and HDL-C were measured by the CHOD-PAP method. Apolipoprotein A and apolipoprotein B were measured by the immunoturbidimetry method. Non-HDL-C is calculated as total cholesterol minus HDL-C (1).
PCI procedure
PCI procedure was completed by conventional method, and successful criteria were residual stenosis 30% or less, grade three Thrombolysis In Myocardial Infarction (TIMI) flow, and without severe complications (2).
Medication protocol
All patients were randomly assigned to therapy group (n=327) or control group (n=321) after PCI procedure. Atorvastatin (Lipitor, Pfizer, USA) 20 mg, was taken every night by patients in the therapy group, and no lipid-modifying medicine was taken by the control group. There was no difference in the use of other medicine between the two groups. All patients provided informed written consent and the ethics committee at the hospital approved the trial. During the study, diet habit, life style and treatment of concomitant disease for all patients were maintained as before. Blood pressure of hypertensive patients was controlled lower than 140/90 mmHg after drug treatment. Treatment of diabetes was maintained as before, and glycosylated hemoglobin was maintained below 7% according to the American Heart Association/American College of Cardiology guidelines (3).
End points and follow-up
End points included all-cause death, fatal or nonfatal myocardial infarction (MI) and revascularization. Patients were scheduled for clinical re-evaluation at six and 12 months after hospital discharge and then annually at the outpatient ward until January 2005. The occurrence of clinical events as well as plasma lipid levels were recorded.
Statistical analysis
SPSS version 12.0 (SPSS, USA) was used. Discrete variables or continuous variables were compared with the χ2 or Student’s t tests. Event-free survival distribution was estimated according to the Kaplan-Meier method, and the corresponding P value was obtained from the log rank test. Cox proportional hazards model was used to analyze the correlations between atorvastatin therapy and clinical events, and results were expressed with hazards ratios and 95% CIs. Statistical significance was considered to be P<0.05.
RESULTS
Patient characteristics
Fifteen cases in the therapy group and five cases in the control group were lost during follow-up. Rates of follow-up were comparable between the two groups (95.4% versus 98.4%, P>0.05). The median follow-up was 21±2.9 months. The average duration of diabetes was 9.4 years. Treatment for diabetes was with insulin (n=267), sulfonylureas (n=101), alpha-glucosidase inhibitors (n=137), nateglinide (n=107) and lifestyle modification alone (n=63). Some patients received a combination of medications. There were no notable differences in age, ejection fraction, sex, incidence of smoking, hypertension and stroke between the two groups. The proportion of patients taking other cardiovascular drugs, such as acetylsalicylic acid, clopidogrel, beta-blockers, calcium antagonists, nitrates and angiotensin-converting enzyme inhibitors after PCI was similar between the groups (data not shown). Other baseline data are shown in Table 1.
TABLE 1.
Demographic data and clinical characteristics at baseline
| Atorvastatin (n=327) | Control (n=321) | P | |
|---|---|---|---|
| Emergent PCI | 60 | 75 | 0.122 |
| Type I diabetes | 116 | 128 | 0.257 |
| OMI | 144 | 132 | 0.465 |
| Revascularization | 44 | 60 | 0.086 |
| Number of diseased vessels | 690 | 715 | |
| 1 | 108 | 88 | |
| 2 | 75 | 72 | 0.224 |
| 3 | 144 | 161 | |
| Lesion type (AHA/ACC) | 725 | 757 | |
| A/B1 | 105 | 138 | |
| B2 | 288 | 271 | 0.096 |
| C | 332 | 348 | |
| Stent | |||
| Cypher (Cordis, USA) | 30 | 39 | |
| Taxus (Boston Scientific, USA) | 22 | 33 | 0.991 |
| Bare metal | 275 | 249 | |
| Baseline lipid concentration | |||
| TC (mmol/L) | 5.85±1.29 | 5.72±1.27 | 0.186 |
| TG (mmol/L) | 3.33±0.76 | 3.44±0.66 | 0.091 |
| ApoA (g/L) | 1.38±0.17 | 1.36±0.11 | 0.063 |
| ApoB (g/L) | 1.04±0.26 | 1.02±0.22 | 0.264 |
| HDL-C (mmol/L) | 1.40±0.29 | 1.39±0.28 | 0.461 |
| LDL-C (mmol/L) | 3.21±0.65 | 3.29±0.69 | 0.129 |
AHA/ACC American Heart Association/American College of Cardiology; ApoA Apolipoprotein A; ApoB Apolipoprotein B; HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; OMI Old myocardial infarction; PCI Percutaneous coronary intervention; TC Total cholesterol; TG Triglyeride
Changes in plasma non-HDL-C concentrations
Baseline plasma non-HDL-C concentrations were similar in the two groups. Plasma non-HDL-C concentrations were lowered significantly in the atorvastatin group after an average of 21 months of therapy (4.42±1.06 mmol/L versus 3.35±1.08 mmol/L, P<0.001), and non-HDL-C concentrations in the control group did not change (4.39±1.12 mmol/L versus 4.31±1.09 mmol/L, P>0.05).
Effects of lipid-modifying therapy on clinical events
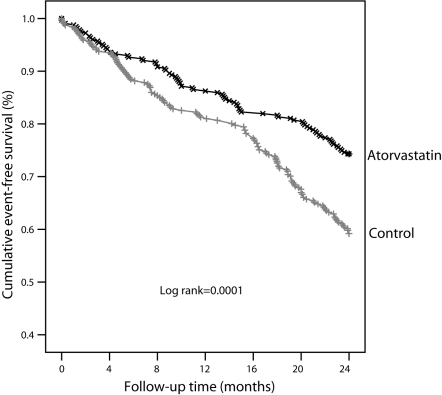
The incidence of MI and revascularization was significantly lower in the therapy group that in the control group after an average of 21 months of atorvastatin therapy, as was the overall incidence of combined end points including death, MI and revascularization. There were no notable differences in incidence of all-cause death between the two groups (Table 2). Kaplan-Meier curves showed that there were notable differences in survival time free of adverse clinical events between the groups (Figure 1).
TABLE 2.
Comparision of major adverse cardiac events between two groups
| Atorvastatin (n=327) | Control (n=321) | P | |
|---|---|---|---|
| Myocardial infarction*, n (%) | 20 (6.4) | 39 (12.3) | 0.013 |
| Revascularization, n (%) | 60 (19.2) | 84 (26.6) | 0.029 |
| All-cause death, n (%) | 16 (5.1) | 25 (7.9) | 0.196 |
| Combined end points, n (%) | 84 (26.9) | 131 (41.5) | <0.001 |
Including fatal and nonfatal myocardial infarction
Figure 1.
Cumulative survival curves for composite end point events (death, myocardial infarction and revascularization)
Correlation between lipid-modifying therapy and clinical events
Multivariate Cox proportional hazards models showed that an average of 21 months of atorvastatin therapy can significantly improve event-free survival rates adjusting for other possible confounding factors (hazard ratio 0.52, 95% CI 0.30 to 0.91, P=0.022).
DISCUSSION
Plasma non-HDL-C encompasses the apolipoprotein B-containing particles that promote atherosclerosis, including LDL and lipoprotein(a) (4). Many large clinical trials had demonstrated that non-HDL-C was a strong predictor of cardiovascular mortality (5–7). A 20% increase in cardiovascular mortality was reported in patients with non-HDL-C concentrations of 5.7 mmol/L or greater (8). Based on those reports, diabetic patients with non-HDL-C 3.4 mmol/L or greater were chosen as our study subjects. No restriction criteria were imposed with regard to the TG level of study subjects, although ATP III guidelines (1) recommend that non-HDL-C should be a secondary target of lipid-modifying therapy only if TG is more than 2.27 mmol/L (200 mg/dL). Our aim is to elucidate whether plasma non-HDL-C is an independent risk factor indicative of the need for lipid-modifying therapy after PCI in high-risk patients with both diabetes and coronary artery disease. Our results showed that lipid-modifying therapy was beneficial to those patients with high plasma non-HDL-C, thus demonstrating that non-HDL-C is a strong predictor of coronary artery disease events. In fact, average plasma TG levels in our study subjects were higher than normal values, which suggests that there is a close correlation between non-HDL-C and TG in dyslipidemic patients with both diabetes and coronary artery disease.
Dyslipidemia, hyperglycemia, insulin resistance, endothelial dysfunction and hypercoagulation are common concomitant states in diabetic patients, and these factors result in a worse prognosis for diabetic patients than for nondiabetic patients after PCI (9).The recent Lescol Intervention Prevention Study (LIPS) showed that fluvastatin reduces the incidence of major adverse cardiac events among patients with unstable and stable angina after PCI (10). However, there is relatively little information regarding the effect of lipid-modifying therapy for secondary prevention in patients with both diabetes and coronary artery disease after PCI. Our study population were all dyslipidemic patients with both coronary artery disease and diabetes, and most of them were high-risk patients because of their advanced age and high blood pressure. Multivessel disease and type B2/C lesions were common in these patiens, so their coronary lesions were severe. An average of 21 months of therapy with traditional dose of atorvastatin (20 mg/day) reduced incidence of MI, revascularization and combined end points. Our study shows that atorvastatin is beneficial to patients with both diabetes and coronary artery disease after PCI and should be a chief lipid-modifying drug in patients with multiple high-risk factors, especially those with high plasma concentrations of non-HDL-C.
Because few patients died, differences in mortality between the groups were not significant. With regard to differences in other major adverse cardiac events between the groups, active lipid-modifying therapy is beneficial to patients with high plasma concentrations of non-HDL-C and diabetes, as well as in patients with coronary artery disease after PCI.
REFERENCES
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–21. [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Dove JT, Jacobs AK, et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines) – executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) J Am Coll Cardiol. 2001;37:2215–39. doi: 10.1016/s0735-1097(01)01344-4. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Blair SN, Bonow RO, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 2001;104:1577–9. doi: 10.1161/hc3801.097475. [DOI] [PubMed] [Google Scholar]
- 4.Franzini C, Valente C, Luraschi P. Low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol and apolipoprotein B: Relationships among the different measurements. Scand J Clin Lab Invest. 2004;64:703–7. doi: 10.1080/00365510410003011. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V, Hardison R, Kelsey SF, et al. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–42. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 6.Lu W, Resnick HE, Jablonski KA, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type II diabetes: The strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Rallidis LS, Pitsavos C, Panagiotakos DB, et al. Non-high density lipoprotein cholesterol is the best discriminator of myocardial infarction in young individuals. Atherosclerosis. 2005;179:305–9. doi: 10.1016/j.atherosclerosis.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–9. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 9.Morgan KP, Kapur A, Beatt KJ. Anatomy of coronary disease in diabetic patients: An explanation for poorer outcomes after percutaneous coronary intervention and potential target for intervention. Heart. 2004;90:732–8. doi: 10.1136/hrt.2003.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CH, de Feyter P, Serruys PW, et al. Beneficial effects of fluvastatin following percutaneous coronary intervention in patients with unstable and stable angina: Results from the Lescol intervention prevention study (LIPS) Heart. 2004;90:1156–61. doi: 10.1136/hrt.2003.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]