Abstract
Both epidemiological and experimental studies have revealed that intake of wine, particularly red wine, in moderation protects cardiovascular health; however, the experimental basis for such an action is not fully understood. Because all types of red wine contain varying amounts of alcohol and antioxidants, it is likely that the cardioprotective effect of red wine is due to both these constituents. In view of its direct action on the vascular smooth muscle cells, alcohol may produce coronary vasodilation in addition to attenuating oxidative stress by its action on the central nervous system. The antioxidant components of red wine may provide cardioprotection by their ability to reduce oxidative stress in the heart under different pathological conditions. Mild-to-moderate red wine consumption improves cardiac function in the ischemic myocardium through the protection of endothelial function, the expression of several cardioprotective oxidative stress-inducible proteins, as well as the activation of adenosine receptors and nitrous oxide synthase mechanisms.
Keywords: Alcohol, Antioxidants, Flavonoids, Polyphenols, Red wine
The beneficial effects of wine can be traced back to the dawn of human civilization. It is a global socioreligious symbol associated with a multitude of therapeutic benefits, including medicinal as well as magical powers. The current popular propositions about the benefits of ‘moderate wine drinking’ in fact date back through history, and were first proposed by the Father of Medicine, Hippocrates of Kos, in Greece. However, the cardiovascular benefits of red wine became the hub of research activity after the observation of ‘French paradox’ by Renaud and de Lorgeril (1) who, in 1992, found that there was a low mortality rate from ischemic heart disease among French people despite their high consumption of saturated fats and the prevalence of other risk factors, such as smoking. This was attributed to their so-called ‘Mediterranean diet’, which includes a large intake of wine. In 1997, a Dutch epidemiological study (2) showed that coronary artery disease in elderly men is inversely proportional to their flavonoids intake.
There is evidence from different sources showing that oxidative stress disturbs the normal balance between the pro-oxidants (oxygen free radicals) and antioxidants either by increasing the formation of free radicals or by decreasing the amount of antioxidants in the myocardium. This oxidative stress plays an important role in cardiovascular diseases, such as ischemic heart disease, arteriosclerosis, congestive heart failure, cardiomyopathy, hypertrophy and arrhythmias (3). Several studies highlight the role of free radicals in myocardial ischemic reperfusion injury (4–6). These studies indicate that the antioxidant reserve and antioxidant enzymes are significantly reduced during ischemia-reperfusion injury. Palm oil-derived polyphenols, such as the mixture of alpha (α), beta (β), gamma (γ) and delta (δ) tocotrienols, as well as tocopherols, have also been shown to significantly reduce the injury caused by ischemia and/or reperfusion through their ability to stabilize proteasomes (7). During ischemia and/or reperfusion injury, there is loss of antioxidant enzymes and antioxidants and thus, the overall antioxidant reserve of the heart is reduced. One of the major functions of antioxidants is to block free radical formation. In the present report, we provide evidence for the cardioprotective effects of red wine and two of its constituents: alcohol and polyphenolic compounds, especially 3,4′,5-trihydroxy-trans-stilbene (resveratrol) and proanthocyanidins.
WHAT IS IN RED WINE THAT AFFECTS THE CARDIOVASCULAR SYSTEM?
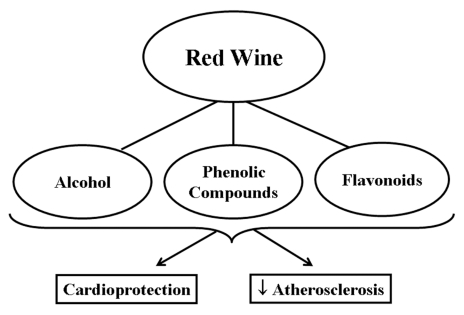
Red wine is rich in a variety of polyphenolic compounds. These compounds are responsible for the colour, bitterness and astringent taste, and act as preservatives that allow for the long aging process used in the manufacturing of wine. These potent antioxidants are found in the solid components of grape berries, such as the skin and seeds. Polyphenols in general are characterized as flavonoids and nonflavonoids: of the flavonoids – catechin, quercetin, proanthocyanidins, condensed tannins and anthocyanins; of the nonflavonoids –hydrolysable tannins, benzene and cinnamate derivatives (8) are found in red wine. Red wine is produced by fermenting grape juice with the pulp, whereas white wine is produced by fermenting grape juice in the absence of the grape pulp. As a result, red wine has a much higher polyphenol content than white wine, as well as a higher level of antioxidant activity (9,10). Besides polyphenols, alcohol constitutes up to 15% of the volume of red wine (Figure 1). A number of epidemiological studies have shown an inverse relationship between moderate alcohol consumption and coronary artery disease; however, high consumption of alcohol leads to increased morbidity and mortality, thus following a J-shaped curve (11–24).
Figure 1.
Red wine and its various components protect the heart from ischemia and/or reperfusion injury as well as attenuate atherosclerosis
Red wine polyphenols act as antioxidants
Arteriosclerosis accounts for almost 40% of all mortality in the United States. According to the oxidative theory, the process of atherogenesis is accelerated by oxidation of low-density lipoprotein (LDL) (25–30). There are a number of biological mechanisms that could lead to LDL oxidation. For instance, oxidation of LDL polyunsaturated lipid components occurs with reactive free radicals and enzyme systems, such as 15-lipoxygenase, cytochrome p450 and myeloperoxidase (31). Red wine polyphenols can reduce LDL sensitivity to lipid peroxidation. In a study (31) in which subjects consumed 375 mL/day of red wine for two weeks, lipid peroxides decreased by 40%, thiobarbituric acid reactive substances by 44% and conjugated dienes by 48%. This study also suggested a pro-oxidant effect of white wine because there was a 21% to 28% increase in thiobarbituric acid reactive substances in the subjects who consumed white wine. Fermenting white wine along with the grape pulp and solids increased its ability to scavenge free radicals and inhibit LDL’s copper ion-induced oxidation. Oxidation properties of this wine were inhibited by up to 87% when the grape juice was allowed to ferment for an additional 18 h resulting in 18% alcohol. The increased inhibition is due to the increased polyphenolic content and is similar to the 94% inhibition seen with red wines (25). Thus, it was demonstrated that it is red wine and not white wine that has important antioxidant activity because of the higher polyphenolic concentration. In another study (32), an alcohol-free powder of red wine phenolic extract was shown to have similar effects as red wine, acting as an antioxidant both in plasma and on LDL. From this study, it can be inferred that the cardioprotective effects of red wine are mainly through its polyphenolic compounds.
The antioxidant effects of red wine polyphenols may result from several mechanisms. Polyphenols can act as free radical scavengers by acting as reducing agents or as hydrogen atom-donating molecules. Chelation of transition metal ions by polyphenols can also diminish the capacity of the metal to generate free radicals (31). Furthermore, reactive copper ion has been found in atherosclerotic plaques and in ceruloplasmin and therefore has been suggested to cause oxidation of LDL (33), whereas polyphenols have been found to strongly inhibit oxidation induced by copper more than that induced by aqueous peroxyls (34). Polyphenols have also been found to reduce macrophage oxidative stress through the inhibition of NADPH oxidase, 15-lipoxygenase, cytochrome p450 and myeloperoxidase (31). Red wine polyphenols are absorbed efficiently in human subjects and bind to LDL, thus protecting it from oxidation (31,32,35). Resveratrol also reduces the synthesis of lipids and eicosanoids, which promote inflammation and atherosclerosis. In another study (36), it was demonstrated that during the digestive process, the action of gastric juice on foodstuffs produces hydroperoxides and malonaldehyde. These free radicals co-oxidized vitamin E, β-carotene and vitamin C, but the lipid peroxidation and co-oxidation of vitamin E and β-carotene were inhibited by red wine polyphenols at acidic pH conditions of the stomach. It was also shown that in the presence of catechin, a well-known polyphenol found in red wine, ascorbic acid worked synergistically to prevent lipid peroxidation and β-carotene co-oxidation (36).
Red wine affects high-density lipoprotein
Red wine is capable of increasing high-density lipoprotein (HDL). This lipid molecule is required for the transport of cholesterol from the arteries and various parts of the body back to the liver for its metabolism and excretion. Therefore, HDL is protective against arteriosclerosis. According to several studies (12,32,37,38), alcohol consumption increases the levels of HDL and thus, may explain a part of red wine’s protective effect. Some studies have shown that moderate consumption of ethanol by itself can increase the plasma concentrations of HDL (39). In a study (35) in which healthy men received 400 mL/day of wine for two weeks, an increase in HDL was found with consumption of red wine although no such beneficial increase was found with white wine. Several studies have shown a dose-dependent increase in HDL and in apolipoprotein A-I levels (37,40). The Enquete Cas-Temoins de L’Infarctus du Myocarde (ECTIM) study (40) involved 561 men with myocardial infarction and 463 healthy men from France and Northern Ireland. It was concluded that there was a significant increase in HDL cholesterol – from 0.47 g/L to 0.59 g/L – in men who consumed 2.3 ounces/day of alcohol as compared with nondrinkers. These men consumed mostly red wine. They also reported an increase in apolipoprotein A-I and A-II (40). In another study (37), it was found that HDL levels increased in people consuming 1.7 ounces/day of wine. Isolated HDL showed an increase of 27% in all molecular species of cholesteryl esters. This effect was also associated with enrichment of the HDL particles in polyunsaturated phospholipids, especially those containing arachidonic acids (+30%) and eicosapentaenoic acids (+90%) and those containing omega-3 fatty acids, which are said to be beneficial against coronary artery disease.
Red wine inhibits vascular smooth muscle cell proliferation and migration
Vascular smooth muscle cell (SMC) proliferation and migration is an important component of atherogenesis (16,41). The abnormal proliferation of vascular SMC in the arterial intima is also an important step in the pathology of restenosis (41). In a rabbit endothelial denudation model, rabbits fed a high-dose resveratrol diet (4 mg/kg/day) developed less intimal hyperplasia than that of control rabbits. The number of SMCs in the thickened intima was reduced in the resveratrol-treated animals (42). In another study (16), bovine aortic SMCs were treated with growth media supplemented with dealcoholized red wine, red wine, or polyphenol extract or resveratrol for 48 h. It was observed that red wine and red wine polyphenols inhibited SMC proliferation in a dose-dependent manner. There are several mechanisms by which red wine could inhibit vascular SMC proliferation. For instance, platelet-derived growth factor is a potent chemo-attractant for SMCs. It induces cellular motility by activation of the phosphatidylinositol 3-kinase (PI3K) and p38 mitogen-activated protein kinase pathways (26). It has been shown that red wine polyphenols inhibit platelet-derived growth factor and serum SMC migration through the inhibition of these signalling pathways (43). Another red wine flavonoid, quercetin, is also said to have antiatherogenic effects on vascular SMCs by inhibiting matrix metallopro-teinase-9, which is responsible for development of intimal formations and plaque rupture in atherogenic lesions (44). Resveratrol has also been shown to inhibit the expression of cell adhesion molecules intracellular cell adhesion molecule 1 (ICAM 1) and vascular cell adhesion molecule 1 (VCAM 1) on the endothelium (45), thus bringing about a reduction in monocyte and granulocyte adhesion.
Red wine polyphenols attenuate platelet aggregation
Many cardioprotective drugs, such as acetylsalicylic acid, act by attenuating platelet aggregability (46). Polyphenols, specifically resveratrol and quercetin, have an antiaggregability effect on human platelets (47). It has also been shown that aggregation in response to ADP and thrombin in human platelets is strongly inhibited by red wine (48). Quercetin has also been demonstrated to decrease the platelet activity by decreasing platelet cytosolic calcium as a consequence of increased cyclic GMP phosphodiesterase activity (47). In a human study (49), subjects who had 375 mL/day of red wine for two to four weeks had decreased ADP-induced platelet aggregation. Red wine, administered intravenously or intra-gastrically at a dose of 1.6 mL/kg and 4.0 mL/kg in stenosed canine coronary arteries, reduced the cyclic blood flow reductions caused by periodic formation of acute platelet-mediated thrombi (46). These same effects were observed when high quantities of grape juice were given, but not with white wine, thus suggesting a role for red wine polyphenols for this attenuation in platelet aggregation (46). Moderate consumption of ethanol by itself is shown to decrease the adhesiveness of platelets (39).
Cardioprotective effects of red wine, resveratrol and proan-thocyanidins
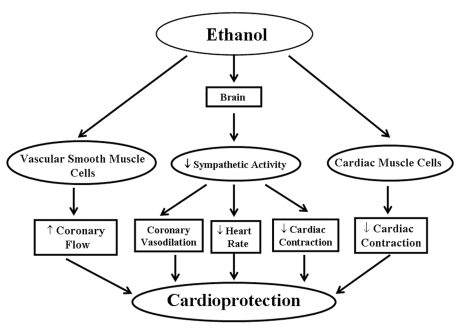
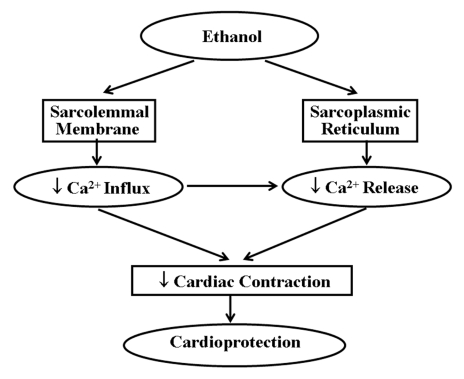
Red wine is composed of more than 500 compounds although only a few are present at concentrations of more than 100 mg/L. These include water, glycerol, ethanol, sugar and organic acids. There is increasing evidence supporting the cardioprotective effects of ethanol (Figure 2), although the mechanisms of cardioprotection remain somewhat obscure. Ethanol decreases sympathetic activity, thereby decreasing heart rate and cardiac contraction while inducing coronary vasodilation; all these effects lead to cardioprotection. On the other hand, Grassi et al (50) showed that increased plasma ethanol level significantly elevates the blood pressure, heart rate and sympathetic nerve activity. Low to moderate concentrations of ethanol are shown to inhibit coronary muscle contraction, increase coronary flow and improve the cardiac output (51). The mechanisms by which ethanol is a vasodilator are still unknown, however, a study on the effects of changes in extracellular Ca2+ on muscle contractions supports a view that ethanol directly activates the coronary vascular smooth muscle by modulating Ca2+ metabolism. The decrease in muscle contraction can arise from decreases in Ca2+ influx through voltage-dependant and receptor-operated Ca2+ channels in the sarcolemmal membrane as well as a decrease in Ca2+ release from the sarcoplasmic reticulum. This impaired intracellular availability of Ca2+ may be responsible for ethanol’s vasodilating effect. As a result, ethanol dilates the coronary arteries and increases the coronary flow. The increased coronary flow improves nutrient and oxygen delivery to the myocardium, maintaining normal cardiac cellular metabolism under stressful situations and resulting in cardioprotection (Figure 3). Other possible causes for the vasodilating effects of ethanol, including changes in cardiac metabolism and generation, as well as release of some intermediate vasodilator vasoactive substance (52), cannot be ruled out. In another study (53), ethanol was found to have cardio-protective effects against ischemia-reperfusion. Low to moderate concentrations of ethanol were shown to significantly increase oxidative stress in the heart. During the first 12 h, malondialdehyde levels in ethanol-perfused hearts increased significantly but decreased after 24 h and returned to baseline levels after 72 h. Ethanol has been shown to induce the expression of the cardioprotective heat shock proteins (HSP), such as HSP-70 and HSP-90 (52,53). These findings suggest that ethanol initially induces oxidative stress, which is then translated into oxidative stress-inducible proteins. Ethanol was also found to inhibit the production of pro-apoptotic factors c-Jun and JNK-1 (53). Miyamae et al (54) showed that ethanol protects the heart from ischemia and/or reperfusion injury by adenosine signalling in guinea pigs. In rats, ethanol also creates ischemic preconditioning which may be mediated predominantly by α1-adrenergic signalling (54).
Figure 2.
Proposed events for ethanol-induced cardioprotection
Figure 3.
Molecular mechanisms of the effect of ethanol leading to cardioprotection where sarcolemmal membrane and Ca2+ play an important role
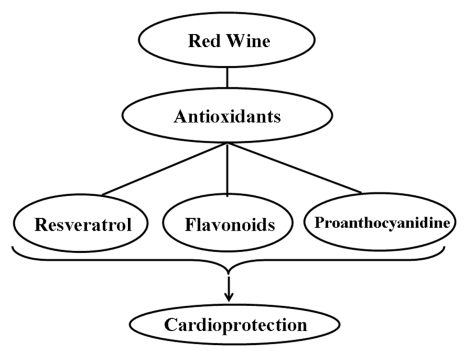
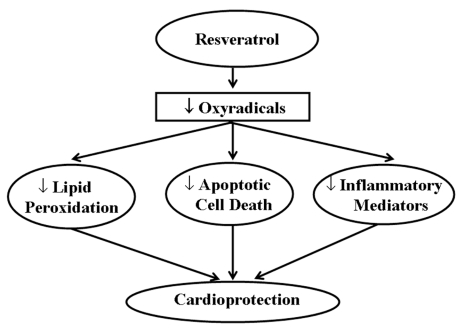
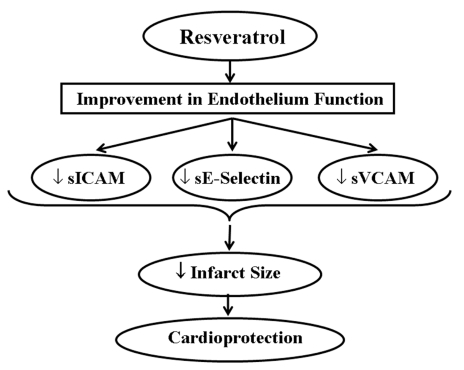
Red wine is considered to exert cardioprotective effects due to the presence of different agents that exhibit antioxidant properties (Figure 4). The concentration of polyphenolic compounds in red wine is approximately 1800 mg/L to 3000 mg/L (55). Of the many polyphenolic compounds in red wine, resveratrol is found to be more cardioprotective. Grape plants do not synthesize resveratrol regularly. It is a defensive molecule, a phyto-alexin, produced to prevent the attack by pathogenic organisms and combat environmental stresses. Recently, a number of studies (56–58) have demonstrated that resveratrol given before ischemic arrest could protect the heart from ischemia and/or reperfusion injury. The role of nitric oxide (NO) is found to be one of the important mechanisms of pharmacological preconditioning by resveratrol (59,60). A recent study (59) suggested that the coordinated up-regulation of inducible NO synthase, vascular endothelial growth factor, kinase insert domain-containing receptor and endothelial NO synthase is one of the resveratrol preconditioning mechanisms. Another study (57) indicated that adenosine receptors have an important function in the resveratrol preconditioning. It suggested that adenosine A1 and A3 receptors, but not A2a or A2b receptors, play a critical role in the pharmacological preconditioning by resveratrol. Resveratrol likely activates both adenosine A1 and A3 receptors, which phosphorylates PI3K, which in turn phosphorylates protein kinase B (Akt), and thus preconditions the heart by producing NO as well as by the activation of antioxidant transcription factor BCl-2. Das et al (58) showed that the activation of adenosine A3 receptors can also precondition the heart by survival signal through the cyclic AMP response element-binding protein phosphorylation via PI3K-Akt and via mitogen-activated extracellular signal-regulated protein kinase-cyclic AMP response element-binding protein pathways. In another study (61), heme oxygenase-I has been reported to play an important role in resveratrol preconditioning. Resveratrol attenuates various soluble intercellular cytokines like ICAM, VCAM and E-selectin through improvement in the endothelium function, which reduces the infarct size (45). Apart from the redox signalling mechanism, resveratrol also protects the heart as a potent antioxidant by scavenging free radicals and inhibiting lipid peroxidation both in vitro and in vivo (53). Thus, resveratrol inhibits apoptotic cell death as well as release and/or generation of inflammatory mediators. Some of the mechanisms for the cardioprotective effects of resveratrol are depicted in Figures 5 and 6.
Figure 4.
Some major polyphenols and flavonoids in red wine which protect the heart
Figure 5.
The cardioprotective effect of resveratrol by scavenging oxyradicals
Figure 6.
Improvement of endothelium function by resveratrol that ultimately leads to cardioprotection. sICAM Soluble intracellular cell adhesion molecule; sVCAM Soluble vascular cell adhesion molecule
Proanthocyanidin, a known flavonoid, is one of the key components found to be cardioprotective in red wine. Proanthocyanidin acts as a potent antioxidant by scavenging both peroxyl and hydroxyl radicals (62), and thus provides protection against myocardial ischemia-reperfusion injury, ventricular fibrillation, ventricular tachycardia and cardiomyocyte apoptosis (63). Proanthocyanidin inhibits cardiomyocyte apoptosis, and downregulates some of the pro-apoptotic genes c-JUN and JNK-1 (64). In another study, Sato et al (62) demonstrated that proanthocyanidin-fed rats showed better post-ischemic ventricular recovery and reduced myocardial infarction compared with those of the control group. A more recent study on chick cardiomyocytes has revealed the effect of proanthocyanidin on reactive oxygen species generation, cell survival, lactate dehydrogenase release and caspase-3 activity (65).
Red wine polyphenols affect vasomotor tone
Red wine polyphenol cardioprotective effects can be seen through various mechanisms. As discussed earlier, polyphenols act as antioxidants. Resveratrol, in particular, improves endothelium function by inhibiting the expression of ICAM and VCAM (45). Also, endothelial NO has vasodilatory effects and therefore is said to be vasoprotective and antiathero-sclerotic (66). Resveratrol can upregulate eNOS expression and consequently increase eNOS-derived NO production. This was observed in an in vitro study (67) when human umbilical vein endothelial cells were incubated for 24 h to 72 h with 10 μmol/L of resveratrol or in the culture medium containing wine. An enhanced endothelial-dependent NO-mediated vasorelaxation was observed (67). When human umbilical vein endothelial cells were exposed to 7.4 μmol/L of resveratrol, eNOS mRNA expression increased by approximately 150%; red wine increased it by 230% compared with the levels in the controls. Because quercetin was found to produce a marked coronary vasorelaxation effect that is endothelial independent, it can be concluded that resveratrol is not the only component responsible for red wine’s vasorelaxation effects. Ethanol is thought to increase polyphenol availability by improving its intestinal absorption, delaying excretion or by affecting its flow through the xenobiotic excretion pathways (39).
CONCLUSION
Red wine attenuates ischemia and/or reperfusion injury; thus, it produces cardioprotection. Components of red wine, such as alcohol and polyphenolic compounds (such as resveratrol and proanthocyanidin), serve equally for its cardioprotective property but through two different mechanisms. Polyphenolic antioxidants scavenge the free radicals while alcohol protects from cellular injury by adapting the heart to oxidative stress. The overall effect of these two components of red wine makes red wine a potent therapeutic agent for the amelioration of myocardial injury associated with ischemia-reperfusion.
ACKNOWLEDGEMENTS
The research work reported in this article was supported by a grant from the Canadian Institutes of Health Research. Dr Dev D Santani was a visiting professor from the Department of Pharmacology, L M College of Pharmacy, Ahmedabad, India.
REFERENCES
- 1.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 2.Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 3.Das DK, Maulik N. Protection against free radical injury in the heart and cardiac performance. In: Sen CK, Packer L, Hänninen O, editors. Exercise and Oxygen Toxicity. Amsterdam: Elsevier Science; 1995. pp. 359–88. [Google Scholar]
- 4.Tosaki A, Bagchi D, Pali T, Cordis GA, Das DK. Comparisons of ESR and HPLC methods for the detection of OH radicals in ischemic/reperfused hearts. A relationship between the genesis of free radicals and reperfusion arrhythmias. Biochem Pharmacol. 1993;45:961–9. doi: 10.1016/0006-2952(93)90182-v. [DOI] [PubMed] [Google Scholar]
- 5.Cordis GA, Maulik N, Das DK. Detection of oxidative stress in heart by estimating the dinitrophenylhydrazine derivative of malonaldehyde. J Mol Cell Cardiol. 1995;27:1645–53. doi: 10.1016/s0022-2828(95)90656-8. [DOI] [PubMed] [Google Scholar]
- 6.Cordis GA, Maulik G, Bagchi D, Riedel W, Das DK. Detection of oxidative DNA damage to ischemic reperfused rat hearts by 8-hydroxydeoxyguanosine formation. J Mol Cell Cardiol. 1998;30:1939–44. doi: 10.1006/jmcc.1998.0752. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Powell SR, Wang P, et al. Cardioprotection with palm tocotrienol: Antioxidant activity of tocotrienol is linked with its ability to stabilize proteasomes. Am J Physiol Heart Circ Physiol. 2005;289:H361–7. doi: 10.1152/ajpheart.01285.2004. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse AL. Wine phenolics. Ann NY Acad Sci. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrman B, Volkova N, Suraski A, Aviram M. White wine with red wine-like properties: Increased extraction of grape skin polyphenols improves the antioxidant capacity of the derived white wine. J Agric Food Chem. 2001;49:3164–8. doi: 10.1021/jf001378j. [DOI] [PubMed] [Google Scholar]
- 10.Vinson JA, Hontz BA. Phenol antioxidant index: Comparative antioxidant effectiveness of red and white wines. J Agric Food Chem. 1995;43:401–3. [Google Scholar]
- 11.Hennekens CH, Rosner B, Cole DS. Daily alcohol consumption and fatal coronary heart disease. Am J Epidemiol. 1978;107:196–200. doi: 10.1093/oxfordjournals.aje.a112525. [DOI] [PubMed] [Google Scholar]
- 12.Yano K, Rhoads GG, Kagan A. Coffee, alcohol and risk of coronary heart disease among Japanese men living in Hawaii. N Engl J Med. 1977;297:405–9. doi: 10.1056/NEJM197708252970801. [DOI] [PubMed] [Google Scholar]
- 13.Teissedre PL, Waterhouse AL. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J Agric Food Chem. 2000;48:3801–5. doi: 10.1021/jf990921x. [DOI] [PubMed] [Google Scholar]
- 14.Howard A, Chopra M, Thurnham D, Strain J, Fuhrman B, Aviram M. Red wine consumption and inhibition of LDL oxidation: What are the important components? Med Hypotheses. 2002;59:101–4. doi: 10.1016/s0306-9877(02)00144-5. [DOI] [PubMed] [Google Scholar]
- 15.Saucier CT, Waterhouse AL. Synergetic activity of catechin and other antioxidants. J Agric Food Chem. 1999;47:4491–4. doi: 10.1021/jf990352t. [DOI] [PubMed] [Google Scholar]
- 16.Araim O, Ballantyne J, Waterhouse A, Sumpio BE. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vasc Surg. 2002;35:1226–32. doi: 10.1067/mva.2002.124358. [DOI] [PubMed] [Google Scholar]
- 17.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA Project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–57. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 18.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–87. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 19.Yarnell JW, Evans AE. The Mediterranean diet revisited – towards resolving the (French) paradox. QJM. 2000;93:783–5. doi: 10.1093/qjmed/93.12.783. [DOI] [PubMed] [Google Scholar]
- 20.Kozararevic D, McGee D, Vojvodic N, et al. Frequency of alcohol consumption and morbidity and mortality: The Yugoslavia Cardiovascular Disease Study. Lancet. 1980;1:613–6. doi: 10.1016/s0140-6736(80)91116-2. [DOI] [PubMed] [Google Scholar]
- 21.Marmot MG, Rose G, Shipley MJ, Thomas BJ. Alcohol and mortality: A U-shaped curve. Lancet. 1981;1:580–3. doi: 10.1016/s0140-6736(81)92032-8. [DOI] [PubMed] [Google Scholar]
- 22.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet. 1988;2:1267–73. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 23.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1:342–8. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Klatsky AL, Friedman GD, Armstrong MA, Kipp H. Wine, liquor, beer, and mortality. Am J Epidemiol. 2003;158:585–95. doi: 10.1093/aje/kwg184. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrman B, Volkova N, Suraski A, Aviram M. White wine with red wine-like properties: Increased extraction of grape skin polyphenols improve the antioxidant capacity of the derived white wine. J Agric Food Chem. 2001;49:3164–8. doi: 10.1021/jf001378j. [DOI] [PubMed] [Google Scholar]
- 26.Aviram M. Interaction of oxidized low density lipoprotein with macrophages in atherosclerosis and the antiatherogenicity of antioxidants. Eur J Clin Chem Clin Biochem. 1996;34:599–608. [PubMed] [Google Scholar]
- 27.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–6. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan M, Aviram M. Oxidized low density lipoprotein: Atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin Chem Lab Med. 1999;37:777–87. doi: 10.1515/CCLM.1999.118. [DOI] [PubMed] [Google Scholar]
- 29.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–27. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 30.Parthasarathy S, Santanam N, Auge N. Oxidized low-density lipoprotein, a two-faced Janus in coronary artery disease? Biochem Pharmacol. 1998;56:279–84. doi: 10.1016/s0006-2952(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 31.Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001;12:41–8. doi: 10.1097/00041433-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Nigdikar SV, Williams NR, Griffin BA, Howard AN. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am J Clin Nutr. 1998;68:258–65. doi: 10.1093/ajcn/68.2.258. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenwald E, Chisolm GM, Fox PL. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J Clin Invest. 1994;93:1493–501. doi: 10.1172/JCI117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Amsha R, Croft KD, Puddey IB, Proudfoot JM, Beilin LJ. Phenolic content of various beverages determines the extent of inhibition of human serum and low-density lipoprotein oxidation in vitro: Identification and mechanism of action of some cinnamic acid derivatives from red wine. Clin Sci (Lond) 1996;91:449–58. doi: 10.1042/cs0910449. [DOI] [PubMed] [Google Scholar]
- 35.Fuhrman B, Lavy A, Aviram M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am J Clin Nutr. 1995;61:549–54. doi: 10.1093/ajcn/61.3.549. [DOI] [PubMed] [Google Scholar]
- 36.Gorelik S, Lapidot T, Shaham I, et al. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: Health implications. J Agric Food Chem. 2005;53:3397–402. doi: 10.1021/jf040401o. [DOI] [PubMed] [Google Scholar]
- 37.Perret B, Ruidavets JB, Vieu C, et al. Alcohol consumption is associated with enrichment of high-density lipoprotein particles in polyunsaturated lipids and increased cholesterol esterification rate. Alcohol Clin Exp Res. 2002;26:1134–40. doi: 10.1097/01.ALC.0000026101.76701.12. [DOI] [PubMed] [Google Scholar]
- 38.Araya J, Rodrigo R, Orellana M, Rivera G. Red wine raises plasma HDL and preserves long-chain polyunsaturated fatty acids in rat kidney and erythrocytes. Br J Nutr. 2001;86:189–95. doi: 10.1079/bjn2001369. [DOI] [PubMed] [Google Scholar]
- 39.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–39. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Marques-Vidal P, Cambou JP, Nicaud V, et al. Cardiovascular risk factors and alcohol consumption in France and Northern Ireland. Atherosclerosis. 1995;115:225–32. doi: 10.1016/0021-9150(94)05517-m. [DOI] [PubMed] [Google Scholar]
- 41.Rivard A, Andres V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histol Histopathol. 2000;15:557–71. doi: 10.14670/HH-15.557. [DOI] [PubMed] [Google Scholar]
- 42.Zou J, Huang Y, Cao K, et al. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 2000;68:153–63. doi: 10.1016/s0024-3205(00)00925-5. [DOI] [PubMed] [Google Scholar]
- 43.Iijima K, Yoshizumi M, Hashimoto M, et al. Red wine polyphenols inhibit proliferation of vascular smooth muscle cells and downregulate expression of cyclin A gene. Circulation. 2000;101:805–11. doi: 10.1161/01.cir.101.7.805. [DOI] [PubMed] [Google Scholar]
- 44.Moon SK, Cho GO, Jung SY, et al. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem Biophys Res Commun. 2003;301:1069–78. doi: 10.1016/s0006-291x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 45.Das S, Bertelli AA, Bertelli A, Maulik N, Das DK. Anti-inflammatory action of resveratrol: A novel mechanism of action. Drugs Exp Clin Res. 2006 not published yet. [Google Scholar]
- 46.Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–8. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 47.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–19. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 48.Pace-Asciak CR, Rounova O, Hahn SE, Diamandis EP, Goldberg DM. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta. 1996;246:163–82. doi: 10.1016/0009-8981(96)06236-5. [DOI] [PubMed] [Google Scholar]
- 49.Seigneur M, Bonnet J, Dorian B, et al. Effect of the consumption of alcohol, white wine, and red wine on platelet function and serum lipids. J Appl Cardiol. 1990;5:215–22. [Google Scholar]
- 50.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: A preliminary report. J Hypertens Suppl. 1989;7:S20–1. doi: 10.1097/00004872-198900076-00007. [DOI] [PubMed] [Google Scholar]
- 51.Altura BM, Zou LY, Altura BT, Jelicks L, Wittenberg BA, Gupta RK. Beneficial vs. detrimental actions of ethanol on heart and coronary vascular muscle: Roles of Mg2+ and Ca2+ Alcohol. 1996;13:499–513. doi: 10.1016/0741-8329(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 52.Su CY, Chong KY, Owen OE, Dillmann WH, Chang C, Lai CC. Constitutive and inducible hsp70s are involved in oxidative resistance evoked by heat shock or ethanol. J Mol Cell Cardiol. 1998;30:587–98. doi: 10.1006/jmcc.1997.0622. [DOI] [PubMed] [Google Scholar]
- 53.Sato M, Maulik N, Das DK. Cardioprotection with alcohol: Role of both alcohol and polyphenolic antioxidants. Ann NY Acad Sci. 2002;957:122–35. doi: 10.1111/j.1749-6632.2002.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 54.Miyamae M, Camacho SA, Zhou HZ, Diamond I, Figueredo VM. Alcohol consumption reduces ischemia-reperfusion injury by species-specific signaling in guinea pigs and rats. Am J Physiol. 1998;275:H50–6. doi: 10.1152/ajpheart.1998.275.1.H50. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg DM, Tsang E, Karumanchiri A, Diamandis E, Soleas G, Ng E. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal Chem. 1996;68:1688–94. doi: 10.1021/ac951083i. [DOI] [PubMed] [Google Scholar]
- 56.Ray PS, Maulik G, Cordis GA, Bertelli AAE, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999;27:160–9. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 57.Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: Role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005;288:H328–35. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 58.Das S, Tosaki A, Bagchi D, Maulik N, Das DK. Resveratrol-mediated activation of cAMP response element-binding protein through adenosine A3 receptor by Akt-dependent and -independent pathways. J Pharmacol Exp Ther. 2005;314:762–9. doi: 10.1124/jpet.105.084285. [DOI] [PubMed] [Google Scholar]
- 59.Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: A potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005;42:281–9. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: Role of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;282:H1988–95. doi: 10.1152/ajpheart.01012.2001. [DOI] [PubMed] [Google Scholar]
- 61.Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol. 2005;69:41–8. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J Mol Cell Cardiol. 1999;31:1289–97. doi: 10.1006/jmcc.1999.0961. [DOI] [PubMed] [Google Scholar]
- 63.Bagchi D, Sen CK, Ray SD, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523–524:87–97. doi: 10.1016/s0027-5107(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 64.Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–37. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 65.Shao ZH, Vanden Hoek TL, Xie J, et al. Grape seed proanthocyanidins induce pro-oxidant toxicity in cardiomyocytes. Cardiovasc Toxicol. 2003;3:331–9. doi: 10.1385/ct:3:4:331. [DOI] [PubMed] [Google Scholar]
- 66.Sumpio BE, Oluwole B, Wang X, Awolesi M. The Pathophysiology and Clinical Applications of Nitric Oxide. United Kingdom: Hardwood Academic Publishers; 1998. Regulation of nitric oxide synthase expression and activity by hemodynamic forces; pp. 171–93. [Google Scholar]
- 67.Wallerath T, Deckert G, Ternes T, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–8. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]