Abstract
Natural IgM antibodies (Abs) play an important role in clearing pathogens, enhancing immune responses, and preventing autoimmunity. However, the molecular mechanisms that mediate the functions of natural IgM Abs are understood only to a limited degree. This shortcoming is largely due to the fact that isolated natural IgM Abs are commonly polyspecific and recognize a variety of antigens (Ags) with no apparent structural homology. It is generally believed that polyspecificity is an inherent property of natural Abs. However, there is increasing evidence that polyspecificity may be induced by mild denaturing conditions. In this study, we compared the specificity of three polyspecific IgM Abs in conventional buffers and undiluted sera deficient in immunoglobulins. All three Abs lost their polyspecificity in serum. They no longer reacted with conventional screening Ags, including hapten-BSA conjugates, ssDNA, thyroglobulin and myosin, but fully retained their reactivity with cognate peptide Ags selected from a T7 phage library. The acquisition of narrow specificity by polyspecific IgM in serum was also observed with muscle tissue sections used as a source of endogenous Ags. The loss of polyspecificity by different Abs was apparently dependent on the presence of different serum constituents. The results of this study suggest that the seemingly inherent polyspecificity of many natural IgM Abs may be largely an in vitro phenomenon related to the lack of normal serum components in the medium. Potential mechanisms underlying the loss of polyreactivity are discussed.
Keywords: Natural antibody, IgM, polyspecificity, T7 phage display
1. Introduction
Natural IgM Abs are important components of the innate immune system that participate in clearing pathogens, enhancing immune responses and preventing autoimmunity (Baumgarth et al., 2000; Boes, 2000; Ehrenstein et al., 2000; Ehrenstein et al., 1998; Ochsenbein et al., 1999). The molecular recognition mechanisms underlying the functions of natural IgM Abs are only understood to a limited degree (Notkins, 2004). This shortcoming is largely due to the fact that the majority of monoclonal natural IgM Abs with detectable binding activity in vitro are polyspecific and recognize a variety of Ags that share no obvious structural similarity (Dighiero et al., 1983; Notkins, 2004). Polyspecificity correlates with the encoding of Abs by germline genes and the structure of the CDR3H region (Chen et al., 1991; Diaw et al., 1997). Kinetic and crystallographic data indicate that polyspecific Abs may accommodate unrelated Ags due to the high conformational plasticity of their antigen-combining sites (Foote and Milstein, 1994; James et al., 2003).
It is generally believed that polyspecificity is an inherent property of natural Abs, which enables a limited set of immunoglobulins to react with a large variety of pathogens and degraded or modified endogenous Ags. An alternative view is that natural Abs with originally narrow specificity but high conformational plasticity may become polyspecific as a result of exposure to mild denaturing conditions. Such conditions include the presence of chaotropic agents, low pH, high salt, and oxidative physiological compounds (Bouvet et al., 2001; Dimitrov et al., 2006; McIntyre, 2004; McMahon and O'Kennedy, 2000). The concept of induced polyspecificity suggests that polyspecific Abs may be generated in the inflammatory environment induced by pathogen invasion or tissue destruction (Bouvet et al., 2001; Dimitrov et al., 2006; McIntyre, 2004).
The polyspecificity of natural Abs was historically studied using simple buffer media, such as buffered saline containing BSA or gelatin and non-ionic detergent (Dighiero et al., 1983). In view of the high conformational plasticity of natural Abs, it would appear that the absence of normal serum components, including those contributing to the maintenance of the physiological redox potential, might by itself lead to Ab conformational changes supporting polyspecificity (Foote and Milstein, 1994; James et al., 2003). It is also conceivable that low-affinity natural IgM Abs could more effectively react with surface Ags in buffer than in undiluted plasma or serum. IgM molecules are highly sensitive to “molecular crowding” conditions due to their large size and disk-like shape (Ellis, 2001). Therefore, even weak reactivity of surface Ags with plasma macromolecules could potentially create a “crowded” environment that would restrict IgM penetration (Sebestyen et al., 2006).
To examine the relationship between the medium composition and polyreactivity, we compared the binding specificities expressed by polyspecific IgM Abs in conventional buffer media and undiluted sera deficient in immunoglobulins. The crucial element of this study was the use of small peptide Ags that were recognized by tested IgM along with conventional screening Ags. We have shown previously that the vast majority of random C-terminal peptides displayed on T7 phage react with natural IgM Abs present in undiluted serum and blood (Sokoloff et al., 2001; Sokoloff et al., 2000; Sokoloff et al., 2004). The IgM recognition of these peptides is strikingly specific (Sokoloff et al., 2001; Sokoloff et al., 2000; Sokoloff et al., 2004). Based on these observations, we set out to select analogous peptide Ags recognized by polyspecific IgM and use such medium-independent Ags as “standards” in testing IgM binding to common screening Ags. We have found that the presence of undiluted serum led to the acquisition of virtually monoreactive properties by polyspecific IgM Abs.
2. Materials and methods
2.1 Antibodies and Antigens
AP- and biotin-conjugated goat IgG specific for the mouse IgM μ-chain and rat Abs (clones EM34.1 and 9A8) specific for the mouse κ- and λ-chains (Sigma, St. Louis, MO); C3 goat antiserum (Bethyl Laboratories, Montgomery, TX); goat IgG specific for the IgM Fc5μ region and rabbit IgG directed against the goat IgG γ-chain (Pierce, Rockford, IL); Fab Abs specific for the mouse Fab region (Jackson ImmunoResearch, West Grove, PA); anti-phage T7 rabbit IgG (Sokoloff et al., 2004); Flu(6)-BSA, TNP(14)-BSA, TNP(15)-BSA, NP(24)-BSA, NIP(31)-BSA, DNP(10)-BSA and PC(15)-BSA (Biosearch Technologies, Novato, CA); rabbit muscle myosin, bovine thyroglobulin and calf thymus ssDNA and dsDNA (Sigma, St. Louis, MO); blocking BSA (Pierce, Rockford, IL). Synthetic peptides Ac-PEGWN, Ac-RLTPR, Ac-DLLDR and Ac-DGA-DLLDR (purity > 98%,), where the residues required for IgM recognition are underlined, were prepared at Mirus Corporation (Madison, WI).
2.2 Animals
Male Balb/c mice, 6−8 wks old, were from Jackson (Bar Harbor, MN). Rag-1 mice (Jackson) were bred in our SPF facility. All animal work was conducted according to protocols approved by the Animal Care and Use Committee of The University of Wisconsin.
2.3 Sera
Undiluted FBS (HyClone, Logan, UT) was heated at 56°C for 1 h (FBSHI) or supplemented prior to use with 5 mM EDTA (FBSEDTA) to eliminate its residual complement activity. FBS contained ∼ 50 times less IgG (∼200 μg/ml) than adult bovine serum. With the typical IgG:IgM:IgA ratio for FBS being ∼ 3:1:1 (Ellis et al., 1978), this serum also was expected to contain small amounts of IgM and IgA. The FBSHI precipitate (FBS-P) and supernatant (FBS-S) fractions were prepared by precipitation with 45% ammonium sulfate and dialyzed against TBS. Extensively dialyzed FBSHI was prepared by dialysis against four changes of TBS over 48 h. Complement-grade Rag-1 mouse serum was prepared by clotting blood on ice for 1 h. Rag-1 serum used as a binding medium was prepared by clotting blood at room temperature for 2−4 h. Prior to use, this serum was supplemented with 5 mM EDTA. All FBS and Rag-1 sera were stored at −20°C and −80°C, respectively.
2.4 Phage
A peptide library with the general structure DGA(X)5, where DGA is a “spacer” and (X)5 is a random sequence, was displayed at the C-terminus of the T7 phage protein 10B as described previously (Sokoloff et al., 2000; Sokoloff et al., 2004). Phage was stored in TBS/1 mM MgCl2/30% glycerol at −80°C.
2.5 Polyspecific IgM Abs
Hybridoma clones secreting IgM41, IgM58 and IgM60 were prepared from the splenocytes of naïve 6−7 week old Balb/c mice using the myeloma line NS1 as the fusion partner at Harlan Inc. (Madison, WI). Primary polyspecific clones (HAT medium) were detected by testing culture supernatants for reactivity with common screening Ags (Section 2.1) by ELISA (Section 2.7) and then subjected to several additional rounds of cloning using the limited dilution method (http://www.hbps.com) and expanded in ascites. IgM was isolated by chromatography on a mannan-binding protein column using an ImmunoPure®IgM Purification Kit (Pierce, Rockford, IL). This procedure is conducted at neutral pH and does not involve the use of chaotropic or acidic agents (Nevens et al., 1992). Isolated IgM was dialyzed against TBS and stored at 0.4−1.5 mg/ml at −80°C in aliquots. Unfractionated IgM was prepared by growing hybridomas in DMEM supplemented with 10% FBSHI, 20 mM Hepes, 50 μM 2-ME, 1 mM Na-pyruvate, and 25 μg/ml gentamycin. Culture supernatants were filtered and stored at −80° in aliquots. The IgM concentration was measured by capture ELISA using MOPC 104E as a standard.
All three IgM's contained κ-chains, as determined by ELISA using IgMλ (MOPC −104E) and IgMκ (TEPC 183) as standards. The VH genes encoding for IgM41, IgM58 and IgM60 were sequenced ((Seidl et al., 1997); GenBank accession no. DQ340230, DQ340231, and DQ340232, respectively) and found to be identical to the germline genes VH1/H13−3, VH5/7183.9, and VH1/J558.5 (GenBank accession no. X02459), AF290971), and AF303836, respectively). The VH germline structure suggested that all three IgM's were typical representatives of polyspecific natural Abs (Casali and Schettino, 1996).
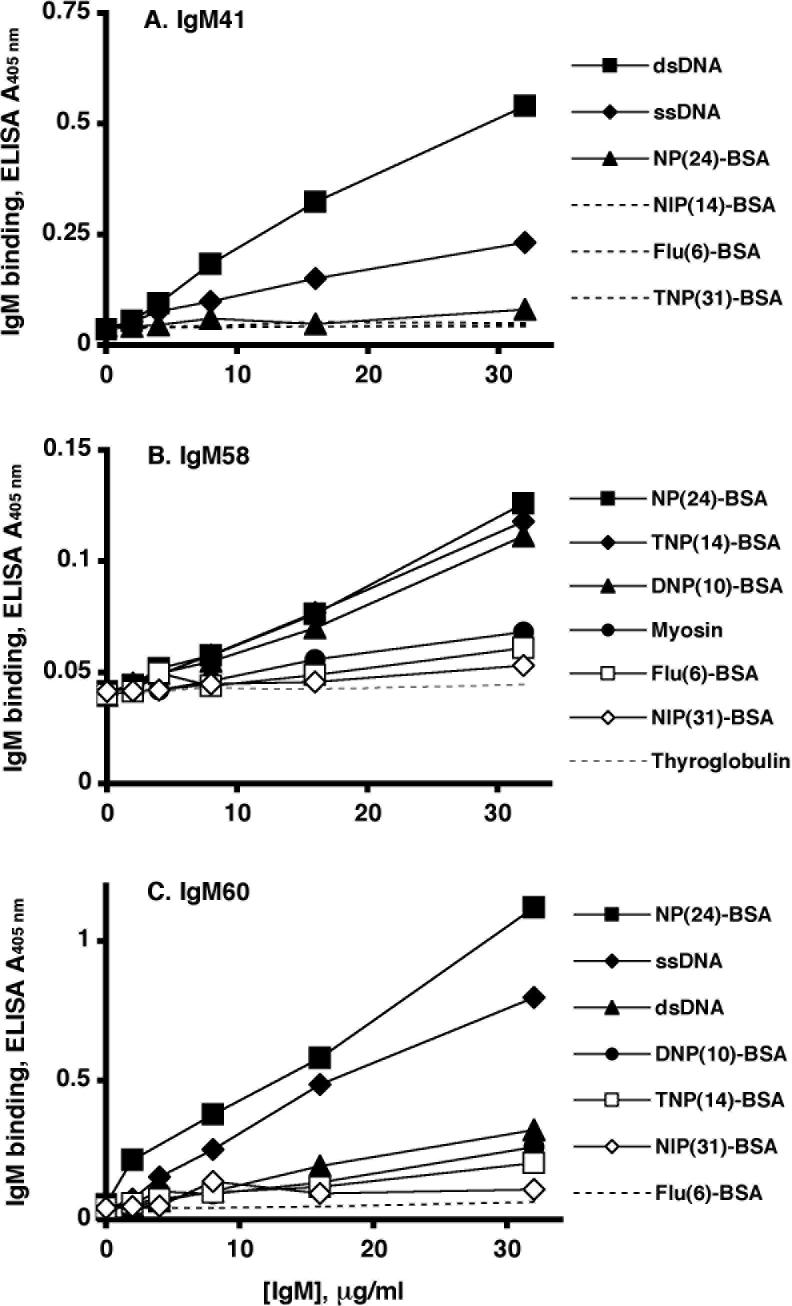
IgM41, IgM58 and IgM60 were chosen for this study due to different specificities that they expressed in reactions with common screening Ags. IgM41 and IgM60 demonstrated distinct preferences for hapten conjugates while similarly binding to ssDNA and dsDNA and not binding to myosin or thyroglobulin (Fig. 1, A and C). IgM58 reacted with myosin, thyroglobulin and various BSA-hapten conjugates while showing little reactivity with DNA (Fig. 1B).
Fig. 1.
Binding of polyspecific IgM to screening Ags and phage-displayed peptides in ELISA. (A-C) Solid lines with symbols - IgM binding to common screening Ags. Dashed lines with symbols - IgM binding to cognate phage-displayed peptides. Dashed lines without symbols - IgM binding to ssDNA and dsDNA. (D). Activation of complement by IgM (2 μg/ml) bound to peptides carrying consensus determinants. All incubations were done in triplicate. Data are shown as mean ± SD and are representative of at least three independent experiments.
2.6 Peptide selections
IgM denaturation on polystyrene (Schwab and Bosshard, 1992) was prevented by using ELISA plates coated with Protein G (Pierce, Rockford, IL). A high density of displayed peptides, 415 copies per phage particle, allowed selection of high-avidity IgM-phage complexes that were mediated by low-affinity IgM-peptide interactions (Perkins et al., 1991; Sokoloff et al., 2001; Sokoloff et al., 2000). Non-specific IgM binding was minimized using displayed peptides that were just five amino acid residues long. The peptide length was sufficient, however, for specific IgM-peptide interactions (Sokoloff et al., 2001; Sokoloff et al., 2004).
Plates were coated overnight with goat IgG specific for the IgM Fc5μ region (10 μg/ml; 50 μl/well) and then washed with TBS and coated with polyspecific IgM (2−50 μg/ml) in TBS/1% BSA/0.05% (TBS-BT) or DMEM/10% FBS. IgM-coated plates were washed and incubated with T7 peptide library (50 μl; 2 × 1011 pfu/ml) in TBS-BT for 2 h. TBS-BT closely resembled typical binding media used in polyspecificity studies (Dighiero et al., 1983; Haspel et al., 1983). Unbound phage was washed out and bound phage was extracted with TBS/1% SDS for 30 min and amplified (Sokoloff et al., 2000). Two more selection rounds were conducted in the same manner. The structure of selected peptides was determined by DNA sequencing (Sokoloff et al., 2004). Consensus peptide residues are shown underlined throughout the text. Phage-displayed peptides are shown as T7-XXXXX, to distinguish them from synthetic peptides (Ac-XXXXX).
The selection system was tested with the monoclonal IgM produced by MOPC 104E (IgM104). Due to its high specificity for dextran 1→3, IgM104 was likely to react mostly with structurally related peptide mimotopes (Schepers et al., 1978). The percentage of the input phage that bound to IgM104 increased from 0.007% in the 1st selection round to 1.6 and 13.2% in the 2nd and 3rd rounds, respectively. Seven of ten selected peptides displayed the consensus sequence T7-A/G/N/K)LWKS (Table 1; IgM104, a), and the remaining peptides contained different elements of this sequence (Table I; IgM104, b). The absence of unrelated selected peptides suggested that the selection system could be used with polyspecific IgM Abs, which could potentially react with multiple unrelated mimotopes (Manivel et al., 2002).
Table 1.
Peptides selected for binding to IgM104 and polyspecific IgM in TBS-BT.
| IgM104 |
IgM41 |
IgM58 |
IgM60 |
|---|---|---|---|
 |
 |
 |
 |
Consensus residues are shown in bold. Homologous sequences are outlined.
aW-containing peptides without D/E residues.
2.7 IgM and C3 ELISA's
All steps were performed at 22−24°C unless indicated otherwise. ELISA plates were coated overnight with phage (4 × 1010 pfu/ml), BSA-hapten conjugates (10 μg/ml) or purified proteins (10 μg/ml) in TBS (50 μl/well). The efficiency of phage immobilization, assessed with anti-T7 Ab, was similar for different clones (Sokoloff et al., 2004). IgM binding was detected using an ELISA described previously (Sokoloff et al., 2004). IgM binding to DNA was assessed in a similar fashion, using poly-D-Lysine plates (Sigma, St. Louis, MO) coated with DNA (50 μg/ml) for 2 h and blocked with TBS/1% BSA (TBS-B). To measure the deposition of complement C3, IgM-phage complexes were incubated with 1.25% complement-grade Rag-1 mouse serum in TBS-BT/1 mM MgCl2/1 mM CaCl2 at 37°C for 1 h. Plates were washed with TBS, incubated for 1 h with C3 antiserum in TBS-BT and then washed again and incubated for 1 h with AP-conjugated rabbit Ab directed against goat IgG. All remaining steps were as above.
2.8 Confocal microscopy
Balb/c mice were euthanized and their upper and lower limb muscles were immediately removed and embedded in OCT compound (Sakura, CA). Five-micron muscle sections were prepared and air-dried at room temperature. Sections were washed twice in PBS and incubated with polyspecific IgM (2 μg/ml) in TBS-B or 98% FBSHI in a moist chamber at room temperature for 1 h. Next, sections were washed three times with PBS, fixed with 4% formaldehyde for 15 min, washed twice with PBS, and blocked using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Blocked sections were rinsed with PBS and incubated with biotinylated anti-IgM Ab in TBS-B for 30 min. Sections were then washed twice with PBS and incubated with Cy3-streptavidin (Molecular Probes, Eugene, OR) in TBS-B for 20 min. Nuclei and actin were counterstained with ToPro-3 and Alexa-488-Phalloidin (Molecular Probes, Eugene, OR), respectively. Stained sections were washed twice with PBS and examined in a Carl Zeiss LSM 510 confocal microscope.
3. Results
3.1 The majority of peptides selected for binding to polyspecific IgM share common structural determinants
The phage yields observed in selection experiments with polyspecific IgM41 and IgM58 were comparable to those observed with IgM104 (Section 2.6). IgM41 bound 0.03, 5.6, and 11.9% of the input phage in the 1st, 2nd, and 3rd rounds of selection, respectively. The corresponding values for IgM58 were 0.08, 3.8, and 15.2%. IgM60 bound phage more efficiently, with the yields of 0.5, 25.8, and 33.9%.
Seventeen of twenty peptides selected with IgM41 featured a rare W residue (W-peptides) in a −2 through −5 position, and twelve of these peptides contained one or more D/E residues a maximum of one residue away from the W residue. The combination of W and D/E residues was termed a consensus W-determinant (Table 1; IgM41, bold). The W-peptides formed a few homology groups, which are shown outlined (Table 1; IgM41). Three W-peptides, T7-LWNS, T7-PEWQ and T7-SWT, were truncated by random stop-codons. Three peptides contained no W residues (Table 1; IgM41, e).
Thirteen of twenty peptides selected with IgM58 contained the dipeptide TP (TP-determinant), which was located one or two residues away from the C-terminus. The TP-peptides also formed a few homology groups (Table 1; IgM58). Two peptides from this selection contained only one consensus residue (Table 1; IgM58, d). The remaining five peptides were devoid of consensus residues (Table 1; IgM58, e).
Seventeen of twenty peptides selected with IgM60 contained an L residue in the − 4 position (L-determinant). Eight of these peptides had a G residue and five peptides, either N or Q residue in the − 5 position (Table 1, IgM60). Two peptides from this selection were devoid of L-determinants and one peptide was truncated by a stop-codon (Table 1; IgM60, d).
3.2 Polyspecific IgM Abs in solution predominantly bind to cognate selected peptides carrying consensus determinants
The binding specificity of free IgM was significantly higher than that of immobilized IgM. Thus, only five of twenty peptides selected with immobilized IgM41 strongly bound free IgM41 in ELISA. Notably, all five peptides contained W-determinants (Table 2; IgM41, “+”). Only fifteen of twenty peptides selected with immobilized IgM58 bound free IgM58, with thirteen reactive peptides containing the consensus TP-determinant (Table 2; IgM58, “+”). Unlike IgM41, IgM58 reacted with two peptides devoid of the consensus determinant (Table 2; IgM58, outlined). IgM60 bound fifteen of nineteen cognate peptides, with fourteen peptides containing the L-determinant (Table 2, IgM60). Similarly to IgM58, IgM60 reacted with a peptide devoid of the consensus determinant (Table 2; IgM60, outlined). The binding avidity shown by all three Abs in reactions with cognate peptides was comparable to their avidity in reactions with screening Ags (Fig. 1, dashed lines with symbols).
Table 2.
Binding () of polyspecific IgM in solution to cognate selected peptides.
| IgM41 |
IgM58 |
IgM60 |
|---|---|---|
 |
 |
 |
Consensus residues are shown in bold. IgM-binding peptides devoid of consensus determinants are outlined.
The IgM complexes with peptides carrying consensus determinants invariably activated complement (Fig. 1D). Hence, these complexes formed in a physiologically meaningful manner (Perkins et al., 1991; Thornton et al., 1994). This was consistent with our observations that complement inactivated a T7 phage display library in serum and blood in an IgM-dependent and peptide-specific fashion (Sokoloff et al., 2001; Sokoloff et al., 2000).
3.3 Cognate selected peptides compete with common screening Ags for IgM binding
The relationship between the IgM sites binding selected peptides and those binding screening Ags was analyzed in competition experiments with free synthetic peptides that were used as a substitute for phage-displayed peptides to minimize steric hindrance effects. The utility of synthetic peptides for this purpose was confirmed by examining their competition with phage-displayed peptides for binding cognate IgM. IgM41 binding to the peptide T7-PEGWT was completely inhibited by the homologous synthetic peptide Ac-PEGWN (Fig. 2A, closed symbols). No inhibition of IgM41 binding was observed when the same displayed peptide was paired with the unrelated synthetic peptides Ac-RLTPR and Ac-DGA-DLLDR that reacted with IgM58 and IgM60, respectively (Fig. 2A, open symbols). Likewise, IgM58 binding to the cognate peptide T7-RLTPR was inhibited by the homologous synthetic peptide Ac-RLTPR but not by the unrelated peptides Ac-PEGWN and Ac-DGA-DLLDR (Fig. 2B). The synthetic peptide Ac-DLLDR just weakly inhibited IgM60 binding to the peptide T7-DLLDR. Additional selection experiments showed that the L-determinant was recognized more efficiently when it was flanked by the spacer sequence DGA (data not shown). The new synthetic peptide Ac-DGA-DLLDR significantly inhibited IgM60 binding to the peptide T7-DLLDR (Fig. 2C, closed symbols), while the unrelated peptides Ac-PEGWN and Ac-RLTPR were ineffective (Fig. 2C, open symbols).
Fig. 2.
IgM binding competition between synthetic and phage-displayed peptides (A-C) and between synthetic peptides and screening Ags (D-F). Polyspecific IgM (2 μg/ml) was incubated with immobilized Ags in the presence of synthetic peptides in TBS-BT and bound IgM was detected by ELISA. Dashed lines - IgM binding to phage-displayed peptides devoid of consensus determinants. Data are representative of at least three independent experiments.
The peptide Ac-PEGWN just weakly inhibited IgM41 binding to NP(24)-BSA and had no effect on its binding to NIP(31)-BSA or Flu(6)-BSA (Fig. 2D). In contrast, the peptide Ac-RLTPR completely inhibited IgM58 binding to thyroglobulin and myosin and moderately, to TNP(14)-BSA (Fig. 2E, closed symbols). This peptide also completely inhibited IgM58 binding to the cognate peptides T7-RYIGK and T7-ARTRL, which were devoid of the consensus TP determinant (Fig. 2E, dashed lines). Similarly, the peptide Ac-DGA-DLLDR significantly reduced IgM60 binding to TNP(14)-BSA, DNP(10)-BSA and PC(15)-BSA (Fig. 2F, closed symbols). This peptide also completely inhibited IgM60 binding to the peptide PERVF, which was devoid of the consensus L-determinant (Fig. 2F, dashed line). The IgM binding competition observed between synthetic peptides and screening Ags suggested that these two types of Ags reacted with the same or overlapping IgM sites. However, it could not be ruled out that the synthetic peptides bound to distinct sites and “locked” IgM molecules in conformational states that did not support polyspecificity (Foote and Milstein, 1994; James et al., 2003).
3.4 Undiluted serum does not inhibit IgM binding to cognate selected peptides containing consensus determinants
The reactivity of polyspecific IgM Abs with cognate selected peptides in serum was first examined by conducting selection experiments in 90% FBSHI. IgM from culture supernatants was used in these selections to prevent IgM-serum interactions that could be caused by IgM purification. The peptides selected in 90% FBSHI contained exactly the same consensus determinants as those selected in TBS-BT (Table 3; compare with Table 1). Thus, the IgM reactivity with peptides carrying consensus determinants was evidently medium-independent and was not associated with the use of a particular IgM isolation procedure.
Table 3.
Peptides selected for binding to polyspecific IgM in 90% FBSHI.
| IgM41 |
IgM58 |
IgM60 |
|---|---|---|
 |
 |
 |
Consensus residues are shown in bold. Homologous sequences are outlined.
In keeping with the selection results, IgM binding to peptides displaying consensus determinants was unaffected or even slightly stimulated by 95−97% FBSEDTA and FBSHI (Fig. 3, A-C, closed bars). This suggested that the IgM Abs did not significantly react with FBS macromolecules despite their polyspecificity in regard to screening Ags. The possibility that the Abs retained their full binding capacity with respect to phage-displayed peptides while reacting with FBS macromolecules seemed unlikely. In the experiments modeling IgM-FBS interactions, the addition of NP(24)-BSA, NIP(31)-BSA or Flu(6)-BSA (1 mg/ml) to TBS-BT reduced the efficiency of IgM41 binding to the cognate peptide T7-PEGWT by 50−75%. IgM60 binding to the cognate peptide T7-DLLDR was similarly inhibited by Flu(6)-BSA and TNP(14)-BSA. NP(24)-BSA and NIP(31)-BSA completely inhibited IgM60 binding at concentrations below 0.1 mg/ml. The efficiency of IgM58 binding to the cognate peptide T7-QFTPM was inhibited by 30−40% by TNP(14)-BSA. Finally, the binding of all three Abs was abolished by Fab Abs (∼10 μg/ml) directed against the mouse Fab region (data not shown).
Fig. 3.
IgM binding to phage-displayed peptides and screening Ags in 95−97% FBSEDTA. Polyspecific IgM was diluted with FBSEDTA to a final concentration of 2 μg/ml and immediately incubated with immobilized Ags. Bound IgM was detected by ELISA. All incubations were done in triplicate. Data are shown as mean ± SD and are representative of at least three independent experiments.
3.5 Undiluted serum inhibits IgM binding to cognate selected peptides devoid of consensus determinants
IgM58 binding to the cognate peptides T7-ARTRL and T7-RYIGK, both of which were lacking the consensus TP-determinant, was strongly inhibited by FBSEDTA (Fig. 3B, asterisk-labeled bars). FBSEDTA similarly suppressed IgM60 binding to the peptide T7-PERVF, which was devoid of the consensus L-determinant (Fig. 3C, asterisk-labeled bar). FBSEDTA and FBSHI inhibited IgM binding to these peptides with similar efficiency (Fig. 4, dashed lines). Thus, neither FBS heat inactivation nor chelation of FBS metal cations by EDTA contributed to the inhibition process.
Fig. 4.
IgM binding to phage-displayed peptides and screening Ags at different concentrations of FBSEDTA, FBSHI or Rag-1 serum. Polyspecific IgM (2 μg/ml) was incubated with immobilized Ags in serum that was serially diluted with TBS-BT and bound IgM was detected by ELISA. Dashed lines - IgM binding to cognate peptides devoid of consensus determinants. Data are representative of at least three independent experiments.
No IgM binding inhibition was observed when the peptides whose IgM-binding activity was inhibited by FBS were preincubated with 100% FBSHI for 30 min and then washed with TBS and allowed to react with IgM in TBS-BT. This ruled out detachment or irreversible blocking of immobilized phage as a cause of IgM binding inhibition (data not shown). The IgM binding inhibition also could be trivially explained by the blocking of peptides by low-level natural Abs present in FBS. However, IgM binding was inhibited even stronger by immunoglobulin-free Rag-1 mouse serum (Fig. 4). Like FBS, Rag-1 serum did not inhibit IgM binding to cognate peptides that carried consensus determinants (Fig. 3, “Rag-1”-labeled bars).
The inhibition of IgM binding to peptides devoid of consensus determinants could be due to differential blocking of these peptides by serum macromolecules or due to IgM conformational changes induced by the serum environment. In the former case, the peptides RYIGK and PERVF could represent examples of broadly reactive epitopes. Both of these peptides featured distinctive sequences, RYI and RVF, which contained one positively charged and two hydrophobic residues, one of which had an aromatic side chain. No similar sequences were found among twenty-four selected peptides that did not react with free IgM (Table 2).
3.6 Undiluted serum inhibits IgM binding to conventional screening Ags
FBSHI, FBSEDTA and Rag-1 serum invariably inhibited IgM binding to screening Ags (Fig. 3 and Fig. 4). An insignificant decrease in IgM binding was observed when screening Ags were preincubated for 30 min with 100% FBSHI and then washed with TBS and incubated with IgM in TBS-BT. This ruled out detachment or irreversible blocking of screening Ags in the presence of serum (data not shown).
Increasing the concentration of IgM reduced the efficiency of the IgM binding inhibition by FBS in an Ag-dependent manner. There was a noticeable increase in the amount of IgM41 bound to ssDNA and dsDNA, but not to other Ags (Fig. 5A). IgM58 showed no significant binding at any concentration (Fig. 5B). An increase in the amount of bound IgM60 was observed with NP(24)-BSA and ssDNA (Fig. 5C). Even at the highest IgM concentration, 32 μg/ml, the binding activity of all three IgM Abs in FBS was by far lower than that in TBS-BT (compare Fig. 5 and Fig. 1).
Fig. 5.
IgM binding to screening Ags in 91−99% FBSHI at different IgM concentrations. Polyspecific IgM was diluted with FBSHI and immediately incubated with immobilized Ags. Bound IgM was detected by ELISA. All incubations were done in triplicate. Data are shown as mean ± SD and are representative of at least three independent experiments.
The inhibition of IgM binding to screening Ags could be attributed to blocking of these Ags by serum macromolecules. Alternatively, IgM could undergo serum-induced conformational changes that restrict polyspecificity. As judged from the shape of binding isotherms, the conspicuous absence of IgM binding inhibition observed with the peptides carrying consensus determinants was not due to their higher binding avidity (Fig. 1, dashed lines with symbols).
3.7 Different serum factors contribute to the loss of IgM polyspecificity
The FBSHI fraction precipitated by 45% ammonium sulfate (FBS-P) inhibited IgM41 binding to screening Ags nearly as effectively as non-fractionated FBSHI (Fig. 6, A and C). The FBS supernatant fraction (FBS-S) was ∼ 2-fold less inhibitory (Fig. 6B). IgM60 binding to screening Ags was inhibited by FBS-P and FBS-S with approximately the same efficiency as observed for IgM41 (Fig. 6, G-I). IgM58 binding to all tested Ags, except TNP(15)-BSA, was inhibited by FBS-P ∼2- and 5−10-fold more effectively than by FBSHI and FBS-S, respectively (Fig. 6, D-F). Thus, the FBS inhibitory activity seemed to be at least partly associated with serum macromolecules.
Fig. 6.
IgM binding to screening Ags in fractionated and in extensively dialyzed FBS. Polyspecific IgM (2 μg/ml) was incubated with immobilized Ags in FBS-P, or FBS-S or control non-fractionated FBS and bound IgM was detected by ELISA (A-I). IgM incubations in FBSHI dialyzed for 48 h against four changes of was conducted in the same fashion (J-L). All incubations were done in triplicate. Data are shown as mean ± SD and are representative of two independent experiments.
Involvement of low-molecular-weight FBS components in inhibiting IgM binding was suggested by the experiments with extensively dialyzed FBS. This FBS inhibited IgM41 binding to screening Ags somewhat less effectively than the moderately dialyzed FBS described above (Fig. 6, J and C, respectively). IgM58 binding was inhibited by these sera with approximately the same efficiency (Fig. 6, K and F, respectively). However, the extensively dialyzed FBS did not inhibit IgM60 binding to NP(24)-BSA or TNP(15)-BSA and only weakly inhibited its binding to NP(31)-BSA and Flu(6)-BSA (Fig. 6, L and I). This suggested that the blocking of IgM60 polyspecificity required the presence of low-molecular-weight FBS components that could be removed by extensive dialysis. Alternatively, IgM60 could be more sensitive than IgM41 and IgM60 to changes in the redox state of FBS macromolecules, which might occur during extensive dialysis (McIntyre, 2004).
The contribution of low-molecular-weight serum components to the loss of IgM polyspecificity was also suggested by the results of IgM binding experiments conducted with DMEM as a binding medium. TBS/0.05% Tween-20 (TBS-T), which supported polyspecific IgM binding as efficiently as TBS-BT (data not shown), was used as a DMEM counterpart. The use of DMEM significantly reduced the expression of polyspecificity by all three Abs (Fig. 7).
Fig. 7.
IgM binding to screening Ags in DMEM. Polyspecific IgM (2 μg/ml) was incubated with immobilized Ags in DMEM and bound IgM was detected by ELISA. All incubations were done in triplicate. Data are shown as mean ± SD and are representative of at least three independent experiments.
3.8 Undiluted serum blocks natural IgM polyspecificity in reactions with muscle tissue Ags
To address the concern that the loss of IgM polyspecificity in serum was a specific phenomenon, restricted to certain displayed peptides and highly purified screening Ags, we conducted IgM binding experiments using mouse muscle tissue as a source of naturally occurring endogenous Ags (Zhang et al., 2006). All three Abs produced extensive staining when incubated with unfixed skeletal muscle sections in TBS-B, with Tween-20 omitted to avoid Ag extraction (Fig. 8; a, e, and i). The use of undiluted FBSHI instead of TBS-B led to dramatic changes in IgM reactivity. The staining produced by IgM58 and IgM60 nearly completely disappeared (Fig. 8; g and k, respectively). The weak residual signal near the sarcolemma was virtually indistinguishable from the background staining attributed to endogenous IgM (Fig. 8, o).
Fig. 8.

IgM binding to muscle tissue sections in TBS-B and 98% FBSHI. Polyspecific IgM was diluted with TBS-B or FBSHI to a final concentration of 2 μg/ml and immediately incubated with non-fixed muscle tissue sections. After incubation, sections were fixed and bound IgM was stained and detected by confocal microscopy. Unless indicated otherwise, images are shown as projections of nine stacked optical sections with a thickness of 0.4 μm each. In each image pair, one image is stained for IgM only (red) and the other one is stained for IgM (red), actin (green), and nuclei (blue). a and b - IgM41 in TBS-BT; c and d – IgM41 in FBSHI; e and f - IgM58 in TBS-BT; g and h – IgM58 in FBSHI; i and j - IgM60 in TBS-BT; k and l - IgM60 in FBSHI; m and n – IgM41 in FBSHI, individual optical sections (0.4 μm); o and p - control samples without IgM.
The effect of FBSHI on IgM41 was more interesting. While the bulk of the IgM41 staining disappeared, the remaining staining was apparently specific for myofilaments (Fig. 8; c and m). In the individual optical sections (0.4 μm), the IgM staining pattern (red) was continuous with the staining pattern of Phalloidin that bound to actin (green; Fig. 8, n). The general myofiber morphology, assessed by staining sections for actin (green) and nuclei (blue) in addition to IgM, was similarly preserved in all samples (Fig. 8).
These experiments with tissue sections showed that the loss of IgM polyspecificity in serum was a general phenomenon, observed with both highly purified and naturally occurring Ags. This conclusion was supported by our preliminary data obtained from testing seven more natural IgM Abs derived from Balb/c mice. Five new Abs stained muscle sections in TBS-B, with three of them losing reactivity and two others showing narrow specificity in FBS (data not shown).
4. Discussion
This study demonstrates that natural IgM Abs acquire narrow binding specificity when transferred from buffer into undiluted serum. It appears that different Abs may lose polyspecificity via different mechanisms. The molecular mechanisms that mediate the loss of IgM polyspecificity are not known. We speculate that there could be three general mechanisms contributing to this process. First, natural IgM Abs could react with serum “cofactors”, which would reduce their conformational plasticity and, thereby, polyspecificity (Foote and Milstein, 1994; James et al., 2003; Tissot et al., 2002). Second, cysteine-rich IgM molecules could undergo conformational changes in response to variations in the medium redox potential (McIntyre, 2004). Third, serum macromolecules reacting with immobilized Ags could create a “crowded” environment with restricted IgM penetration (Ellis, 2001; Sebestyen et al., 2006), which would be similar to the exclusion of large proteins from a glycosylated cell surface (Owen and Campbell, 1998). The SDS-PAGE of FBS proteins binding to immobilized NP-BSA, NIP-BSA, Flu-BSA and thyroglobulin, with BSA as a negative control, has shown enrichment for α2-macroglobulin (α2-M; data not shown). Alpha2-M is a bulky protein (∼17 nm) that binds a variety of ligands (Armstrong and Quigley, 1999) and seems to be well suited for inhibiting IgM binding through a steric repulsion mechanism. Further studies, exploring the ability of α2-M to affect natural IgM binding, are under way.
The serum-induced loss of polyspecificity suggests that the natural IgM Abs showing broad polyspecificity in vitro may express narrow specificity in vivo. This might explain why many endogenous Ags are comparably reactive with autologous natural Abs in vitro while just few of them effectively elicit autoimmune responses in vivo (Shan et al., 1994). The loss of IgM polyspecificity in serum is consistent with our observations that the IgM present in normal undiluted serum contains a large variety of peptide-specific binding activities but no significant polyspecific activity directed against the same peptides (Sokoloff et al., 2001; Sokoloff et al., 2000; Sokoloff et al., 2004).
5. Concluding remarks
The results from this study broaden the notion that polyspecificity may be caused by exposure of conformationally labile natural Abs to mild denaturing conditions by demonstrating that such conditions apparently include the absence of normal serum components in the binding medium (Bouvet et al., 2001; Dimitrov et al., 2007; McIntyre, 2004). The IgM binding data obtained with muscle tissue sections suggest a simple new strategy for finding Ags that are potentially recognized by natural Abs in vivo, which is based on the use of tissue sections and semi-physiological binding conditions.
Acknowledgements
The fruitful discussion of this work by Dr. J. A. Wolff is greatly appreciated. This study was partly supported by NIH grant no. DK53314-02.
Abbreviations
- Flu
fluorescein
- FBSHI
heat-inactivated FBS
- FBSEDTA
FBS containing 5 mM EDTA
- FBS-P
FBS fraction precipitated with 45% ammonium sulfate
- FBS-S
FBS supernatant after precipitation with 45% ammonium sulfate
- NP
4-hydroxy-3-nitrophenylacetyl
- NIP
4-hydroxy-3-iodo-5-nitrophenylacetyl
- PC
phosphorylcholine
- ssDNA
single-stranded DNA
- dsDNA
double-stranded DNA
- TBS
Tris-buffered saline (pH 7.4)
- TBS-BT
TBS/1% BSA/0.05% Tween 20
- TBS-B
TBS/1% BSA
- TBS-T
TBS/0.05% Tween 20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong PB, Quigley JP. Alpha2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev Comp Immunol. 1999;23:375–390. doi: 10.1016/s0145-305x(99)00018-x. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- Bouvet JP, Stahl D, et al. Induction of natural autoantibody activity following treatment of human immunoglobulin with dissociating agents. J. Autoimmun. 2001;16:163–172. doi: 10.1006/jaut.2000.0472. [DOI] [PubMed] [Google Scholar]
- Casali P, Schettino EW. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- Chen C, Stenzel-Poore MP, et al. Natural auto- and polyreactive antibodies differing from antigen-induced antibodies in the H chain CDR3. J. Immunol. 1991;147:2359–2367. [PubMed] [Google Scholar]
- Diaw L, Magnac C, et al. Structural and affinity studies of IgM polyreactive natural autoantibodies. J. Immunol. 1997;158:968–976. [PubMed] [Google Scholar]
- Dighiero G, Lymberi P, et al. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J. Immunol. 1983;131:2267–2272. [PubMed] [Google Scholar]
- Dimitrov JD, Ivanovska ND, et al. Ferrous ions and reactive oxygen species increase antigen-binding and anti-inflammatory activities of immunoglobulin G. J. Biol. Chem. 2006;281:439–446. doi: 10.1074/jbc.M509190200. [DOI] [PubMed] [Google Scholar]
- Dimitrov JD, Lacroix-Desmazes S, et al. Transition towards antigen-binding promiscuity of a monospecific antibody. Mol. Immunol. 2007;44:1854–1863. doi: 10.1016/j.molimm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Cook HT, et al. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J. Exp. Med. 2000;191:1253–1258. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein MR, O'Keefe TL, et al. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Nat. Acad. Sci. USA. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- Ellis WA, Logan EF, et al. Serum immunoglobulins in aborted and non-aborted bovine foetuses. Clinical and Experimental Immunology. 1978;33:136–141. [PMC free article] [PubMed] [Google Scholar]
- Foote J, Milstein C. Conformational isomerism and the diversity of antibodies. Proc. Nat. Acad. Sci. USA. 1994;91:10370–10374. doi: 10.1073/pnas.91.22.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel MV, Onodera T, et al. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983;304:73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- James LC, Roversi P, et al. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- Manivel V, Bayiroglu F, et al. The primary antibody repertoire represents a linked network of degenerate antigen specificities. J. Immunol. 2002;169:888–897. doi: 10.4049/jimmunol.169.2.888. [DOI] [PubMed] [Google Scholar]
- McIntyre JA. The appearance and disappearance of antiphospholipid autoantibodies subsequent to oxidation--reduction reactions. Thromb. Res. 2004;114:579–587. doi: 10.1016/j.thromres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- McMahon M, J.O'Kennedy R. Polyreactivity as an acquired artefact, rather than a physiologic property, of antibodies: evidence that monoreactive antibodies may gain the ability to bind to multiple antigens after exposure to low pH. J. Immunol. Meth. 2000;241:1–10. doi: 10.1016/s0022-1759(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Nevens JR, Mallia AK, et al. Affinity chromatographic purification of immunoglobulin M antibodies utilizing immobilized mannan binding protein. J. Chromat. A. 1992;597:247–256. doi: 10.1016/0021-9673(92)80117-d. [DOI] [PubMed] [Google Scholar]
- Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ochsenbein AF, Fehr T, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- Owen CA, Campbell EJ. Angiotensin II generation at the cell surface of activated neutrophils: novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J. Immunol. 1998;160:1436–1443. [PubMed] [Google Scholar]
- Perkins SJ, Nealis AS, et al. Solution structure of human and mouse immunoglobulin M by synchrotron X-ray scattering and molecular graphics modelling. A possible mechanism for complement activation. J. Mol. Biol. 1991;221:1345–1366. doi: 10.1016/0022-2836(91)90937-2. [DOI] [PubMed] [Google Scholar]
- Schepers G, Blatt Y, et al. Binding site of a dextran-specific homogeneous IgM: thermodynamic and spectroscopic mapping by dansylated oligosaccharides. Biochemistry. 1978;17:2239–2245. doi: 10.1021/bi00604a035. [DOI] [PubMed] [Google Scholar]
- Schwab C, Bosshard HR. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J. Immunol. Meth. 1992;147:125–134. doi: 10.1016/s0022-1759(12)80037-8. [DOI] [PubMed] [Google Scholar]
- Sebestyen MG, Budker VG, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J. Gene Med. 2006;8:852–873. doi: 10.1002/jgm.921. [DOI] [PubMed] [Google Scholar]
- Seidl KJ, MacKenzie JD, et al. Frequent occurrence of identical heavy and light chain Ig rearrangements. Inter. Immunol. 1997;9:689–702. doi: 10.1093/intimm/9.5.689. [DOI] [PubMed] [Google Scholar]
- Shan H, Shlomchik MJ, et al. The mechanism of autoantibody production in an autoimmune MRL/lpr mouse. J. Immunol. 1994;153:5104–5120. [PubMed] [Google Scholar]
- Sokoloff AV, Bock I, et al. Specific recognition of protein carboxy-terminal sequences by natural IgM antibodies in normal serum. Mol. Ther. 2001;3:821–830. doi: 10.1006/mthe.2001.0340. [DOI] [PubMed] [Google Scholar]
- Sokoloff AV, Bock I, et al. The interactions of peptides with the innate immune system studied with use of T7 phage peptide display. Mol. Ther. 2000;2:131–139. doi: 10.1006/mthe.2000.0110. [DOI] [PubMed] [Google Scholar]
- Sokoloff AV, Puckett M, et al. Sequence-specific binding of normal serum IgM to exposed protein C-termini. Immunology. 2004;112:237–249. doi: 10.1111/j.1365-2567.2004.01868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BP, Vetvicka V, et al. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J. Immunol. 1994;152:1727–1737. [PubMed] [Google Scholar]
- Tissot JD, Sanchez JC, et al. IgM are associated to Sp alpha (CD5 antigen-like). Electrophoresis. 2002;23:1203–1206. doi: 10.1002/1522-2683(200204)23:7/8<1203::AID-ELPS1203>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Alicot EM, et al. Identification of the target self-antigens in reperfusion injury. J. Exp. Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]