Abstract
Human ether-a-gogo related gene (HERG) K+ channels are key elements in the control of cell excitability in both the cardiovascular and the central nervous systems. For this reason, the possible modulation by reactive oxygen species (ROS) of HERG and other cloned K+ channels expressed in Xenopus oocytes has been explored in the present study. Exposure of Xenopus oocytes to an extracellular solution containing FeSO4 (25–100 μM) and ascorbic acid (50–200 μM) (Fe/Asc) increased both malondialdehyde content and 2′,7′-dichlorofluorescin fluorescence, two indexes of ROS production. Oocyte perfusion with Fe/Asc caused a 50% increase of the outward K+ currents carried by HERG channels, whereas inward currents were not modified. This ROS-induced increase in HERG outward K+ currents was due to a depolarizing shift of the voltage-dependence of channel inactivation, with no change in channel activation. No effect of Fe/Asc was observed on the expressed K+ currents carried by other K+ channels such as bEAG, rDRK1, and mIRK1. Fe/Asc-induced stimulation of HERG outward currents was completely prevented by perfusion of the oocytes with a ROS scavenger mixture (containing 1,000 units/ml catalase, 200 ng/ml superoxide dismutase, and 2 mM mannitol). Furthermore, the scavenger mixture also was able to reduce HERG outward currents in resting conditions by 30%, an effect mimicked by catalase alone. In conclusion, the present results seem to suggest that changes in ROS production can specifically influence K+ currents carried by the HERG channels.
Reactive oxygen species (ROS) have been proposed as crucial regulators of cellular responses in both physiological and pathological states (1), such as cardiovascular (2) and neurodegenerative disorders (3), senescence (4), and programmed cell death (5). In the heart, ROS participate in the tissue damage observed under ischemia-reperfusion conditions (2). Similarly, in the brain, where a large proportion of body oxygen is consumed, glutamate-induced neurotoxicity has been associated with the production of ROS, because free radical scavengers or inhibitors of ROS-generating processes can prevent glutamate-induced neuronal degeneration (6, 7). The cellular mediators of the ROS-induced damage still remain elusive, although their modulatory action occurring at the level of specific ion channels has been proposed as one of the possible mechanisms (8).
In excitable tissues, K+ channels play a crucial role in the modulation of the resting membrane potential, the modulation of the firing frequency, and the shaping of the action potential (9). By these means, K+ channels exert an important control of fundamental functions such as neurotransmitter release, cardiac frequency, and vascular tone. This heterogeneity of cellular functions is accomplished through the expression of several types of K+ channels exhibiting specific biophysical and pharmacological properties such as selectivity, rectification, activation threshold, voltage-dependence of activation and inactivation, and blockade by drugs and toxins (10). The crucial role played by K+ channels in the control of cell excitability has gained considerable attention after discovering that the long Q-T syndrome, a human genetic abnormality of the cardiac action potential repolarization, was caused by mutations in the human ether-a-gogo related gene (HERG) encoding for a K+ channel (11). When the HERG product was expressed in Xenopus oocytes (12) it was found that this K+ channel underlies the cardiac repolarizing K+ current Ikr. The main features of Ikr are its modulation by the extracellular concentration of K+ ions, a peculiar inward rectification mechanism (13, 14), and its blockade by class III antiarrhythmics (15–17) and by second-generation H1 receptor antagonists (18). More recently, HERG K+ channels have been implicated both in the changes of the resting membrane potential associated with the cell cycle and in the control of neuritogenesis and differentiation in neuronal cells (19, 20). The relevance of HERG K+ channels in neuronal function has been further reinforced by recent evidence showing that the seizure locus in Drosophila encodes for the fly homolog of HERG. Mutations in the seizure locus cause a temperature-sensitive paralytic phenotype associated with hyperreactivity of the flight motor pathway (21, 22).
Because a growing body of evidence suggests that oxidative damage might profoundly influence cell excitability in both physiological and pathological states, the possible modulation by ROS of HERG and other cloned K+ channels expressed in Xenopus oocytes has been explored in the present study. To this aim, ROS formation was monitored via the evaluation of lipid peroxidation (malondialdehyde production) (23) and of the fluorescence intensity of 2′,7′-dichlorofluorescin (DCF), which has been used successfully to assess ROS formation in living cells (24). The activity of cloned K+ channels was investigated by the two-microelectrode voltage-clamp technique upon their expression in Xenopus oocytes.
MATERIALS AND METHODS
Xenopus Oocytes Isolation.
Ovarian lobes were surgically removed from adult female Xenopus frogs (Rettili di Schneider, Varese, Italy) and placed in 100-mm Petri dishes containing a Ca2+-free solution of the following composition: 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM Hepes, 2.5 mM piruvic acid, 100 units/ml penicillin, and 100 μg/ml streptomycin, pH 7.5 with NaOH. After four extensive washes, the oocytes (stage V-VI) were dissociated by collagenase treatment (type IA, 45–80 min at a concentration of 2 mg/ml). Dissociated oocytes then were placed in a Ca2+-containing solution of the following composition: 100 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes, 2.5 mM piruvic acid, 100 units/ml penicillin, and 100 μg/ml streptomycin, pH 7.5 with NaOH, in a 19°C incubator and used for the experiments on the next day.
Determination of Lipid Peroxidation and Oxygen Free Radicals Production in Xenopus Oocytes.
Lipid peroxidation was determined by assaying the intracellular malondialdehyde (MDA) production by means of the thiobarbituric acid test (23). Free radical production was measured by incubation of the oocytes in the presence of the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (24). This compound, which freely crosses the membranes, is hydrolyzed by cellular esterases to 2′,7′-dichlorofluorescein, a nonfluorescent molecule that can be reduced to 2′,7′-DCF by ROS. Xenopus oocytes, isolated as previously described, were incubated with 200 μM DCFH-DA for 3–4 hr in ND88 (see below) at 19°C. At the end of this period they were washed twice with ND88, divided in groups of three, and incubated in the appropriate solutions according to the experiment to be performed. At the end of the experiment the reaction was stopped by adding 1 mM EDTA and 0.001% butylated hydroxytoluene, and the cells were lysed with a glass micropotter. DCF fluorescence in the cell homogenate was measured using a Perkin–Elmer LS5B spectrophotofluorimeter (excitation 495 nm, emission 530 nm).
Molecular Biology and Oocyte Injection.
The cloning of HERG (25), EAG (26), DRK1 (27), and IRK1 (28) already has been described. EAG cDNA, cloned from bovine brain tissue (bEAG), was subcloned into a modified pSP64 vector. cDNAs were linearized with the following restriction enzymes: HindIII for bEAG, NotI for rDRK1 and mIRK1, and EcoRI for HERG. cRNAs were in vitro-transcribed from linearized cDNAs by means of commercially available kits (mCAP, Stratagene), using T7 RNA polymerase for bEAG, rDRK1 and mIRK1 and SP6 RNA polymerase for HERG. RNAs were stored in a stock solution (250 ng/μl) at −20°C in 0.1 M KCl. One day after isolation, Xenopus oocytes were microinjected with 76 nl of the respective cRNA stock solution or appropriate dilutions.
Electrophysiology.
Two to 10 days after the cRNA microinjection, K+ currents expressed were measured by the two-microelectrode voltage-clamp technique using a commercially available amplifier (Warner OC-725A, Warner Instruments, Hamden, CT). Current and voltage electrodes were filled with 3 M KCl, 10 mM Hepes (pH 7.4; ≈1 MΩ resistance). The bath solution contained 88 mM NaCl, 10 mM KCl, 2.6 mM MgCl2, 0.18 mM CaCl2, and 5 mM Hepes, pH 7.5 (ND88). This solution was perfused in the recording chamber at a rate of about 0.2 ml/min. Data were stored on the hard disk of a 486 IBM compatible computer for off-line analysis. The pclamp (version 6.0.2, Axon Instruments, Foster City, CA) software was used for data acquisition and analysis. Currents were recorded at room temperature. During the experiments with 100 μM FeSO4 and 200 μM ascorbate, those batches of oocytes that showed signs of membrane deterioration (an increase in the holding current at −90 mV > −200 nA) were excluded from the electrophysiological analysis. In the experiments with 25 μM FeSO4 and 50 μM ascorbate no increase of the holding current was observed in oocytes isolated from at least eight different batches.
Drugs and Statistics.
All of the chemicals used were purchased from Sigma. FeSO4 and ascorbate stock solutions (10 mM and 25 mM, respectively) were prepared daily and stored in light-protected tubes to avoid spontaneous oxidation. Statistical significance between the data was obtained by means of the Student’s t test or the ANOVA test followed by the Tukey test. When appropriate, data are expressed as the mean ± SEM. In the figures asterisks denote values statistically different from the controls (P < 0.05).
RESULTS
ROS Production in Xenopus Oocytes.
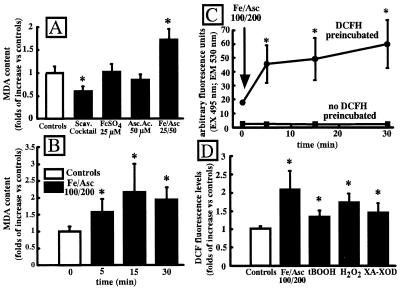
Incubation of Xenopus oocytes for 15 min in the presence of a ROS-scavenging mixture (see below) composed of 200 ng/ml superoxide dismutase (SOD), 1,000 units/ml catalase (CAT), and 2 mM mannitol (MAN) inhibited MDA levels. On the other hand, when oocytes were exposed to 25 μM FeSO4 and 50 μM ascorbate (Fe/Asc), an experimental condition that, by keeping iron in a reduced form, is able to increase the synthesis of more reactive ROS species (1), an increase in MDA levels was observed (Fig. 1A). No effect was observed upon oocyte exposure to 25 μM FeSO4 or 50 μM ascorbate separately (Fig. 1A). In Fig. 1B, a time-course of the MDA increase induced by Fe/Asc (100 μM FeSO4 and 200 μM ascorbate) is shown. Fe/Asc induced an increase of MDA content already at the earliest time point (5 min), peaking at 15 min, with no further increase after this time. In agreement with the results obtained with MDA measurements, the Fe/Asc induced approximately a 2-fold increase in DCF fluorescence emission, which already was evident after 5 min of incubation (Fig. 1C). To compare the effects of different conditions that are known to induce an oxidative stress in various cellular systems, we tested four different oxidating conditions in the same oocyte batches: 100 μM FeSO4 and 200 μM ascorbate, 1.5 mM tert-butyl hydroperoxide, 100 μM hydrogen peroxide, and 0.5 mM Xantine-20 units/ml Xantine oxidase. All of these experimental conditions significantly increased DCF fluorescence in Xenopus oocytes, although the largest increase was observed in the presence of the Fe/Asc (Fig. 1D).
Figure 1.
ROS production in Xenopus oocytes. (A) Effect of a ROS-scavenger mixture (200 ng/ml SOD, 1,000 units/ml CAT, and 2 mM MAN), 25 μM FeSO4, 50 μM ascorbate, and 25 μM FeSO4 + 50 μM ascorbate on MDA levels. (B) Time-course of the MDA increase in Xenopus oocytes induced by extracellular incubation with 100 μM FeSO4 and 200 μM ascorbate. (C) Time-course of DCF fluorescence increase in Xenopus oocytes induced by extracellular incubation with 100 μM FeSO4 and 200 μM ascorbate. Also reported is the cell autofluorescence in Xenopus oocytes that were not preincubated with 2′,7′-dichlorofluorescein diacetate (DHCF-DA). (D) Effect of the incubation of the oocytes in the presence of various oxidating conditions on DCF fluorescence. The incubation conditions were (for 15 min): 100 μM FeSO4 and 200 μM ascorbate (Fe/Asc), 1.5 mM tert-butyl-hydroperoxide (tBOOH), 100 μM hydrogen peroxyde (H2O2), 0.5 mM xantine, and 20 units/ml xantine oxidase (XA-XOD). Each experimental point is the mean ± SEM of 4–16 determinations performed in triplicate.
Effect of Fe/Asc on Different K+ Channels Expressed in Xenopus Oocytes.
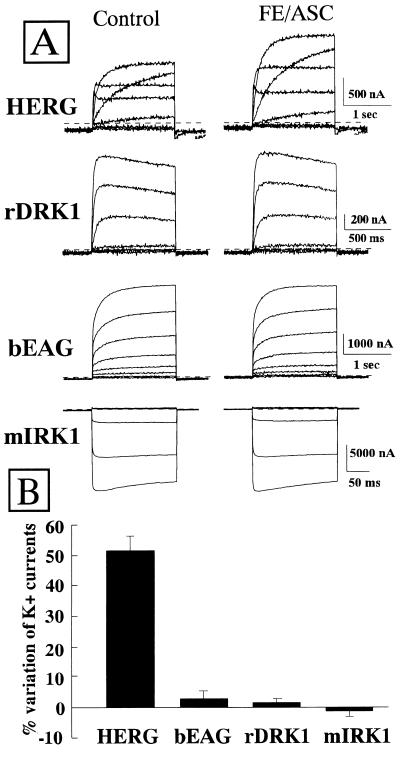
The K+ channels studied in the present investigation were HERG (25), bEAG (26), rDRK1 (27), and mIRK1 (28), members of four of the main K+ channels subfamilies. bEAG and rDRK1 have the biophysical properties of depolarization-activated delayed outward rectifier currents and share a proposed transmembrane topology with six transmembrane domains. HERG, on the other hand, encodes for a six-putative transmembrane domains channel, which is activated by depolarization but displays a pronounced inward rectification of the current/voltage relationship (12–14). Finally, mIRK1 is a classical inward rectifier K+ channel with a two-transmembrane domain proposed structure.
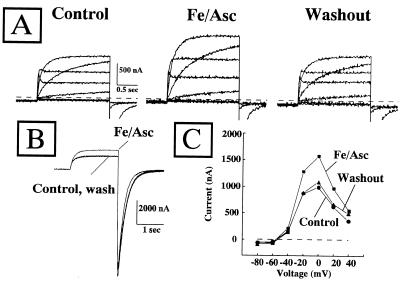
Perfusion of HERG-expressing oocytes with Fe/Asc (100 μM and 200 μM, respectively) potentiated by approximately 50% the outward K+ currents flowing through the expressed channels (Fig. 2 A and B). By contrast, the K+ currents carried by bEAG, rDRK1, and mIRK1 were not modified by the presence of Fe/Asc (Fig. 2 A and B). The effect of Fe/Asc on the increase of HERG outward currents was reversible upon the removal of the ROS-generating system (Fig. 3A). In addition, the time-course of the Fe/Asc-induced stimulation of HERG outward currents showed that the K+ currents slowly rose and reached a steady value after approximately 5 min and that a continuous superfusion with Fe/Asc for over 30 min did not produce any decline in the HERG outward K+ currents (data not shown). Despite increasing the outward currents, Fe/Asc superfusion did not modify the peak of the inward currents carried by HERG K+ channels (Fig. 3B). Fig. 3B also shows that the kinetics of channel deactivation were faster in the presence of Fe/Asc (the τ at −90 mV was 172.4 ± 4.1 ms vs. 141 ± 3.4 ms in control and Fe/Asc, respectively; P < 0.05). The current to voltage relationship (I/V) for the effect of Fe/Asc on HERG K+ currents shows that the outward currents are similarly enhanced at any potential (between −40 and +40 mV), and that this potentiation was reversible upon Fe/Asc washout (Fig. 3C).
Figure 2.
Effect of 100 μM FeSO4 and 200 μM ascorbate on cloned K+ currents expressed in Xenopus oocytes. (A) Effect of Fe/Asc on HERG, bEAG, rDRK1, and mIRK1 K+ channels. K+ currents were recorded in the same oocyte in control condition and after Fe/Asc exposure (100 μM FeSO4 and 200 μM ascorbate). Holding potential was −90 mV. Test potentials were from −80 mV to +40 mV in 20-mV steps for HERG, bEAG and rDRK1, and from −120 mV to 0 mV in 20-mV steps for mIRK1. The inward current component elicited upon HERG repolarization to −100 mV has been blanked for clarity. (B) Modulation of different cloned K+ channels by 100 μM FeSO4 and 200 μM ascorbate. The percent variation of the K+ currents at voltages that fully activated the conductances (0 mV for HERG, +40 mV for bEAG and rDRK1, and −120 mV for mIRK1) are reported. Each experimental point is the mean ± SEM of 3–8 separate experiments.
Figure 3.
The effect of Fe/Asc is reversible upon washout and specific for the outward current component. (A) Reversibility of Fe/Asc effect on HERG K+ channels. The same HERG-expressing oocyte was voltage-clamped, and the depolarization-activated currents were recorded in control condition, after Fe/Asc exposure (100 μM FeSO4 and 200 μM ascorbate) and upon subsequent washout. Holding potential was −90 mV, and test potentials were from −80 mV to +40 mV in 20-mV steps. The inward current component elicited upon repolarization to −100 mV has been blanked for clarity. (B) Lack of effect of Fe/Asc perfusion on HERG peak inward currents elicited upon membrane repolarization. A single current trace, recorded in the same oocyte in control conditions, after Fe/Asc exposure and upon subsequent washout, is shown. Holding potential was −90 mV. Depolarizing step was 0 mV, and return potential was −100 mV. (C) I/V relationship of Fe/Asc-induced potentiation of HERG outward K+ currents. The current elicited by 1.5 sec-depolarizing pulses from −80 mV to +40 mV is plotted against the membrane voltages. The same HERG-expressing cell was studied in control conditions, after Fe/Asc exposure (100 μM FeSO4 and 200 μM ascorbate) and upon subsequent washout.
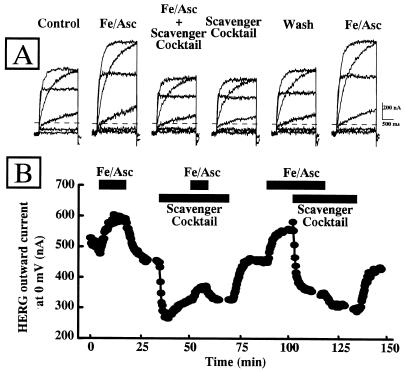
Effect of ROS Scavengers on Resting and Fe/Asc-Induced Enhancement of HERG Outward K+ Currents.
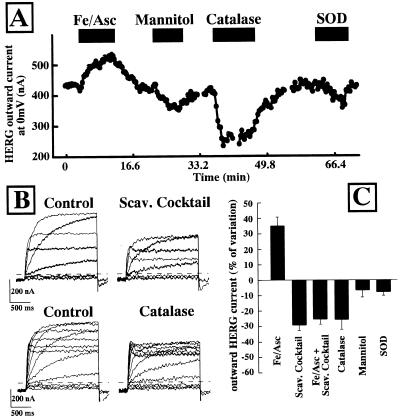
If the Fe/Asc-induced increase of the HERG outward K+ currents was due to an increase in ROS production, ROS scavengers should prevent this effect. For this purpose, a ROS-scavenging mixture identical to that used in Fig. 1A for MDA measurements and composed of 200 ng/ml SOD, 1,000 units/ml CAT, and 2 mM MAN has been used. This mixture previously has been shown to revert the electrophysiological and contractile consequences at the cardiac level of the reperfusion after ischemia (29, 30). Fig. 4A shows the same HERG-expressing oocyte subsequently exposed to control solution, Fe/Asc (25 μM and 50 μM, respectively), Fe/Asc + ROS scavenger mixture (200 ng/ml SOD, 1,000 units/ml CAT, and 2 mM MAN), ROS scavenger mixture alone, control solution again, and finally back to Fe/Asc. Fe/Asc induced an enhancement of HERG outward current that was prevented by the simultaneous presence of the cocktail. Under this experimental condition (Fe/Asc + scavenger cocktail), HERG outward currents reached levels lower than control values. When Fe/Asc was removed but the ROS-scavenging solution was still present HERG outward K+ currents were still inhibited when compared with controls. The subsequent removal of the scavenger mixture caused the recovery of HERG outward K+ currents back to control values. Furthermore, a subsequent re-exposure of the same oocyte to Fe/Asc stimulated HERG outward K+ currents by the same extent as observed during the first exposure. Fig. 4B shows the time-course of HERG outward K+ currents in another oocyte exposed to the ROS-increasing (Fe/Asc) and ROS-neutralizing (scavenger cocktail) conditions. The presence of the ROS-scavenging solution not only prevented the Fe/Asc-induced increase of HERG outward currents, but also reduced basal HERG activity.
Figure 4.
Effect of a ROS-scavenging mixture on Fe/Asc-induced enhancement of HERG outward K+ currents. (A) Representative current traces. K+ currents elicited in the same HERG-expressing oocyte were recorded in control condition (ND88) and after subsequent exposure to Fe/Asc (25 μM FeSO4 and 50 μM ascorbate), Fe/Asc + ROS scavenger mixture (200 ng/ml SOD, 1,000 units/ml CAT, and 2 mM MAN), ROS scavenger cocktail, control (ND88 again), and Fe/Asc again. After about 5 min after each experimental condition, the HERG outward K+ currents were activated by depolarizing pulses (from −80 to + 20 mV in 20-mV steps; holding potential: −90 mV; return potential: −100 mV). (B) Time-course of the effect of ROS-scavenging solution on the Fe/Asc-induced stimulation of HERG outward K+ currents. The outward HERG K+ current, measured at the end of repetitive depolarizing pulses to 0 mV elicited every 20 sec, is plotted versus time during the exposure to the indicated experimental conditions.
The results described in Figs. 1 and 4, showing that the scavenger mixture inhibited resting ROS production and reduced resting HERG outward K+ currents, prompted us to investigate the modulation of the HERG outward currents induced by each of the individual components present in the ROS-scavenging mixture solution in resting conditions. Therefore, experiments were performed in the presence of 2 mM MAN, 1,000 units/ml CAT, and 200 ng/ml SOD separately (Fig. 5A). The results obtained indicate that although each of the three ROS scavengers inhibited the HERG K+ channels (P < 0.05), the effect was more prominent with CAT. Furthermore, CAT alone was able to inhibit HERG outward K+ currents by the same degree as the three scavengers together (Fig. 5 B and C).
Figure 5.
Effect of the antioxidants CAT, MAN, and SOD on the outward currents of HERG K+ channels in resting conditions. (A) Time-course. The outward HERG K+ current, measured as in Fig. 3B, is plotted versus time during the following experimental conditions (as indicated): Fe/Asc (25 μM FeSO4 and 50 μM ascorbate), 2 mM MAN, 1,000 units/ml CAT, and 200 ng/ml SOD. (B) Representative current traces of the effects of the ROS-scavenging mixture and CAT on resting HERG outward currents. HERG outward K+ currents were activated by depolarizing pulses (from −80 to +40 mV in 20-mV steps, holding potential: −90 mV, return potential: −100 mV for the scavenger mixture experiment; and from −80 to +30 mV in 10-mV steps, holding potential: −90 mV, return potential: −100 mV for the CAT experiment). After recording the control traces, the cells were perfused as indicated either with the ROS-scavenging mixture (200 ng/ml SOD, 1,000 units/ml CAT, 2 mM MAN), or with 1,000 units/ml CAT alone, and the same voltage steps were repeated after 5-min perfusion in both experimental conditions. (C) Effect of the different antioxidants on HERG outward K+ currents. On the abscissa is reported the percent of variation induced by each experimental condition on HERG outward current measured at the end of a depolarizing pulse to 0 mV. Each point is the mean ± SEM of 4–9 determinations in different cells.
Biophysical Mechanism of the ROS-Induced Stimulation of HERG Outward K+ Currents.
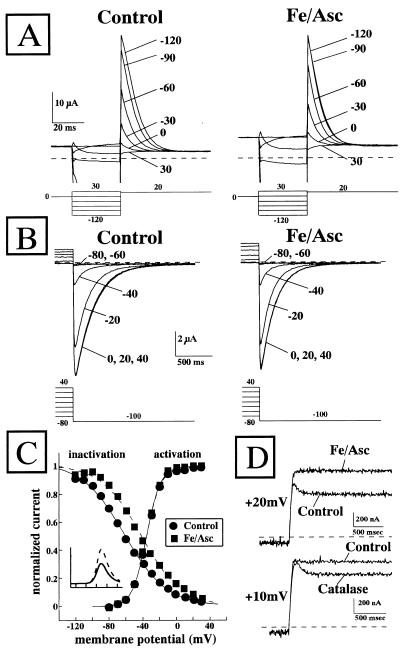
HERG K+ channels are characterized by a peculiar inward rectification mechanism, which seems to arise from a rapid and voltage-dependent inactivation process, reducing the conductance at positive potentials (12–14). For this reason, we tested the effect of the perfusion with Fe/Asc on the activation and inactivation parameters of HERG K+ channels. To study the inactivation process, we took advantage of the kinetic difference existing between the fast recovery from inactivation and the slower channel deactivation occurring at hyperpolarized potentials. We therefore used a voltage protocol in which the channels first were opened and inactivated by a depolarizing pulse at 0 mV, then were recovered from inactivation by means of 25-ms conditioning pulses from −120 mV to +30 mV, and finally depolarized again to +20 mV. Despite the fact that the duration of the conditioning pulses might not have been long enough to be considered steady-state, longer conditioning pulses could not be used due to the occurrence of substantial channel deactivation, particularly at negative potentials (13). Using this protocol, a fast inactivating outward current is observed (Fig. 6A). The effect of Fe/Asc (25 μM and 50 μM, respectively) on the voltage-dependence of this fast-inactivating K+ current was studied (Fig. 6A). Perfusion with Fe/Asc caused a +12.4 ± 2.6 mV (n = 5) shift of the HERG voltage-dependence of inactivation (Fig. 6C). On the other hand, the activation properties of the HERG K+ channels were unaffected by Fe/Asc perfusion (Fig. 6 B and C). Although the 12-mV depolarizing shift of the steady-state inactivation curve induced by Fe/Asc perfusion could be partially accounted for by the faster channel deactivation during the 25-ms conditioning pulses, this hypothesis seems unlikely because the peak current elicited upon subsequent depolarization to +20 mV is identical in both controls and Fe/Asc conditions. In Fig. 6C, the steady-state activation and inactivation curves for both controls and Fe/Asc-treated groups are reported together. The product of these two curves should give the steady-state I/V relationships for each experimental condition. Fig. 6C also shows that the positive shift of the steady-state inactivation curve of HERG channels induced by Fe/Asc perfusion, in the absence of any modification of the steady-state activation curve, reproduced the increase in the outward K+ currents (Fig. 6C, Inset). Fig. 6D shows that at rather positive potentials (≥ +20 mV), HERG outward currents showed a clear fast inactivating component. Fe/Asc treatment removed this fast inactivation, a result that is consistent with the positive shift of the steady-state inactivation curve. Interestingly, the ROS scavenger catalase (Fig. 6D, Lower), as well as the whole ROS scavenger mixture solution (data not shown), induced changes in HERG inactivation kinetics opposite to those observed with Fe/Asc perfusion.
Figure 6.
Modulation of HERG channel activation and inactivation by Fe/Asc. (A) Fe/Asc modulates the inactivation process of HERG K+ channels. The same HERG-expressing oocyte was recorded in control condition and after 5-min exposure to Fe/Asc (25 μM FeSO4 and 50 μM ascorbate). The voltage protocol is indicated at the bottom. (B) Effect of Fe/Asc on HERG K+ channels activation. The same HERG-expressing oocyte was recorded in control condition and after 5-min exposure to Fe/Asc (25 μM FeSO4 and 50 μM ascorbate). The voltage protocol is indicated at the bottom. (C) Steady-state inactivation and activation curves of HERG K+ channels in control condition and upon Fe/Asc exposure. The experimental data were fitted to the following form of the Boltzmann equation: gKv = max/(1+exp(V½−V)/k), where V is the test potential, V½ is the half-activation potential, and k (or kT/ze) is the slope of the conductance to voltage relationship. The values for V½ for the inactivation curve were −61.6 ± 4.1 mV (n = 5) and −49.1 ± 3.5 mV (n = 5), P < 0.05, in controls and Fe/Asc conditions, respectively. The values for V½ for the activation curve were −35.3 ± 4 mV (n = 4) and −35.3 ± 3.4 mV (n = 4), P > 0.05, in controls and Fe/Asc conditions, respectively. The k values (about 2.4 for the inactivation curve and 8.4 for the activation curve) were not different between controls and Fe/Asc groups (data not shown). Solid line, controls; dotted line, Fe/Asc (25 μM FeSO4 and 50 μM ascorbate). (Inset) Solid line in controls and dotted line in the Fe/Asc-treated group) indicate the product of the fitted steady-state activation and inactivation curves. (D) Modulation of HERG inactivation by Fe/Asc and CAT. One single trace of HERG outward current is shown in control and after 5-min exposure to Fe/Asc (25 μM FeSO4 and 50 μM ascorbate, +20 mV, Upper) or CAT (1,000 units/ml, +10 mV, Lower).
DISCUSSION
The results of the present study suggest that the outward K+ currents carried by the HERG channels expressed in Xenopus oocytes are selectively modulated by changes in ROS production. In fact, an enhancement of ROS production induced by the perfusion with Fe/Asc caused an increase in HERG outward K+ currents. On the other hand, the decrease in ROS levels achieved by oocyte perfusion with ROS scavengers can inhibit the resting outward K+ currents and prevent their increase induced by Fe/Asc.
The ROS-induced modulation of HERG K+ channel function is highly specific, because other cloned K+ channels such as bEAG, rDRK1, and mIRK1 were insensitive to Fe/Asc perfusion. Furthermore, the effect of Fe/Asc on HERG K+ channels appears to be mediated by ROS, rather than being a direct action of iron. In fact, the modulation of the gating of the HERG channels by the trivalent cations lanthanum and gadolinium causes a decrease of the outward currents and a marked rightward shift of the voltage-dependence of channel activation (12), changes that are opposite to those observed with Fe/Asc perfusion in the present experiments.
The observation that the ROS-scavenging cocktail, which decreased resting ROS production, and CAT alone both inhibited HERG outward K+ currents also in the absence of ROS-enhancing stimuli, suggests that the endogenous production of ROS in Xenopus oocytes may exert a permissive role in the depolarization-induced opening of HERG K+ channels.
Despite the enormous complexity of the reactions involved in ROS production and neutralization (1, 31), it should be underlined that the Fe/Asc oxidative stimulus used in the present study mainly acts via the conversion of H2O2 into the more reactive oxygen species such as the hydroxyl radical ⋅OH via the Fenton reaction (1). This hypothesis seems to be suggested by the observation that ROS production was effectively increased in Xenopus oocytes only after exposure to solutions containing both Fe and Asc, whereas Fe and Asc alone were ineffective. In addition, CAT, the ROS scavenger most active on the HERG channel function, neutralizes the action of H2O2 by converting it to H2O. Both of these results suggest that endogenously produced H2O2 may be one of the major mediators involved in HERG modulation. Furthermore, although CAT is exerting its modulation of HERG K+ channels when added in the extracellular space, this does not mean that ROS are exerting their influence on an extracellular domain of the channel protein, because many ROS will equilibrate freely across the cell membrane.
The understanding of the mechanism by which ROS exert their modulatory influence on HERG function requires further insights into HERG channel gating. In fact, HERG K+ channels display a peculiar biophysical behavior because they are activated by depolarization but exhibit marked inward rectification at positive potentials. This inward rectification is the consequence of a rapid inactivation process (13, 14, 32). The selective modulation exerted by the Fe/Asc on the HERG outward K+ current, with no modification of the inward component, is suggestive of an interference of ROS with this intrinsic inactivation mechanism. In fact, conditions able to increase ROS production caused a 12 mV rightward shift of the steady-state voltage-dependence of channel inactivation. This effect increased the availability of the channels to be opened in response to depolarization. At negative potentials the channels are fully recovered from inactivation, and therefore the modulation by Fe/Asc of the inactivation process cannot be exerted, possibly explaining the lack of Fe/Asc-induced modulation of the inward current component of HERG K+ channels.
Several studies have attempted to correlate the function of native K+ channels with ROS production (33, 34). In some of these studies, K+ currents were increased by different oxidative stresses, although the specific K+ current modulated by ROS remains undetermined. In other reports, ROS have been shown to modulate several cloned K+ channels expressed in Xenopus oocytes (8, 35, 36). In these studies, however, ROS production never has been correlated with the electrophysiological changes, and the action of ROS-scavenging systems has not been investigated. In the present study, the modulation of HERG K+ channels by ROS, occurring in both resting and stimulated conditions, might represent a physiological mechanism by which ROS can influence cell excitability. In addition, ROS-induced HERG modulation could represent an important functional mechanism relating the changes in the levels of O2 and ROS in heart tissue with the electrophysiological modifications occurring during ischemia-reperfusion phenomena (37). In fact, it seems possible to speculate that the burst of ROS production that follows the reperfusion of the tissue after an ischemic period can increase the outward currents mediated by HERG. This would tend to repolarize the membrane potential and shorten the action potential duration. However, species-specific differences as well as regional differences in the effects of ischemia-reperfusion conditions on the action potential in the cardiac tissue have been extensively documented (29, 37). Furthermore, K+ channels are not the only targets for ROS, because several ion channels and transporters have been shown to be sensitive to the modulation by oxidative stress (2). Nevertheless, the pathophysiological relevance of the proposed mechanism seem to be confirmed by the consistent shortening of the action potential duration induced by oxidative damage recorded in Purkinje fibers (38, 39) and ventricular myocytes after longer times of exposure to ischemia-reperfusion conditions (40, 41). Finally, the present experimental model of ROS induction by Fe/Asc perfusion used to modulate K+ channels might assume a pathophysiological relevance if one considers that a 10-fold increase in iron levels occurs in the coronary flow from rat hearts undergoing global ischemia followed by reperfusion (42), and that the acidic conditions promoted by ischemia favor the liberation of cellular stores of Fe2+, which promotes the Fenton reaction to yield an increased production of toxic hydroxyl radicals (3, 43).
Acknowledgments
We are indebted to Dr. M.T. Keating (Salt Lake City) for HERG cDNA; Dr. A.M. Brown (Cleveland) for mIRK1 cDNA; Dr. A.M.J. VanDongen (Durham, NC) for rDRK1 cDNA; Dr. A. Baumann (Juelich, Germany) for bEAG cDNA; and Dr. E. Wanke (Milan, Italy) for helpful discussions. The study was supported by the following grants: Telethon 748 to M.T., National Research Council 95.02452.CT04 to M.T. and 95.02857.CT04 to L.A., Ministero dell’Universitá e della Ricerca Scientifica e Tecnologica 60% and 40% to L.A., and a grant from the Regione Campania to L.A. This work was carried out under the auspices of the National Program of Research on Drugs (Phase II) arranged by the Ministero dell’ Universitá e della Ricerca Scientifica e Tecnologies.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HERG, human ether-a-gogo related gene; ROS, reactive oxygen species; Fe/Asc, extracellular solution containing FeSO4 (25–100 μM) and ascorbic acid (50–200 μM); MDA, malondialdehyde; DCF, dichlorofluorescin; SOD, superoxide dismutase; CAT, catalase; MAN, mannitol.
References
- 1.Yu B P. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko M, Matsumoto Y, Hayashi H, Kobayashi A, Yamazaki N. Mol Cell Biochem. 1994;139:91–100. doi: 10.1007/BF00944207. [DOI] [PubMed] [Google Scholar]
- 3.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 4.Sohal R S, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korsmeyer S J, Yin X-M, Oltvai Z N, Veis-Novak D J, Linette G P. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- 6.Monyer H, Hartley D M, Choi D W. Neuron. 1990;5:121–126. doi: 10.1016/0896-6273(90)90302-v. [DOI] [PubMed] [Google Scholar]
- 7.Chan P H, Kinouchi H, Epstein C J, Carlson E, Chen S F, Imaizumi S, Yang G Y. Prog Brain Res. 1993;96:97–104. doi: 10.1016/s0079-6123(08)63260-4. [DOI] [PubMed] [Google Scholar]
- 8.Ruppesberger J P, Stocker M, Pongs O, Heinemann S H, Frank R, Koenen M. Nature (London) 1991;35:711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- 9.Strong M, Chandy K G, Gutman G A. Mol Biol Evol. 1993;10:221–242. doi: 10.1093/oxfordjournals.molbev.a039986. [DOI] [PubMed] [Google Scholar]
- 10.Taglialatela M, Brown A M. Kidney Int. 1995;48:918–922. doi: 10.1038/ki.1995.372. [DOI] [PubMed] [Google Scholar]
- 11.Curran M E, Splawski I, Timothy K W, Vincent G M, Green E D, Keating M T. Cell. 1995;80:795–804. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 12.Sanguinetti M C, Jiang C, Curran M E, Keating M T. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith P L, Baukrowitz T, Yellen G. Nature (London) 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 14.Schonherr R, Heinemann S H. J Physiol (London) 1996;493:635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spector P S, Curran M E, Keating M T, Sanguinetti M C. Circ Res. 1996;78:499–503. doi: 10.1161/01.res.78.3.499. [DOI] [PubMed] [Google Scholar]
- 16.Trudeau M C, Warmke J W, Ganetzky B, Robertson G A. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 17.Snyders D J, Chaudhary A. Mol Pharmacol. 1996;49:949–955. [PubMed] [Google Scholar]
- 18.Roy M-L, Dumaine R, Brown A M. Circulation. 1996;94:817–823. doi: 10.1161/01.cir.94.4.817. [DOI] [PubMed] [Google Scholar]
- 19.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. J Physiol (London) 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faravelli L, Arcangeli A, Olivotto M, Wanke E. J Physiol (London) 1996;469:13–23. doi: 10.1113/jphysiol.1996.sp021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titus S A, Warmke J, Ganetzky B. J Neurosci. 1997;17:875–881. doi: 10.1523/JNEUROSCI.17-03-00875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Reynolds E R, Déak P, Hall L. J Neurosci. 1997;17:882–890. doi: 10.1523/JNEUROSCI.17-03-00882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esterbauer H, Cheeseman K H. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 24.Bass D A, Parce J W, Dechatet L R, Szejda P, Seeds M C, Thomas M. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 25.Warmke J W, Ganetzky B. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warmke J W, Drysdale R, Ganetzky B. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 27.Frech G C, VanDongen A M, Schuster G, Brown A M, Joho R H. Nature (London) 1989;340:642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- 28.Kubo Y, Baldwin T J, Jan Y N, Jan L Y. Nature (London) 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 29.Aiello E A, Jabr R I, Cole W C. Circ Res. 1995;77:153–162. doi: 10.1161/01.res.77.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Woodward B, Zakaria M N N. J Mol Cell Cardiol. 1985;17:485–493. doi: 10.1016/s0022-2828(85)80053-5. [DOI] [PubMed] [Google Scholar]
- 31.Aust S D, Morehouse L A, Thomas C E. J Free Radicals Biol Med. 1985;1:3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 32.Spector P S, Curran M E, Zou A, Keating M T, Sanguinetti M C. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo S S, Saad A H, Koong A C, Hahn G M, Giacca A J. Proc Natl Acad Sci USA. 1993;90:908–912. doi: 10.1073/pnas.90.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam M S, Berggren P-O, Larsson O. FEBS Lett. 1993;319:128–132. doi: 10.1016/0014-5793(93)80051-u. [DOI] [PubMed] [Google Scholar]
- 35.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Proc Natl Acad Sci USA. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busch A E, Waldegger S, Herzer T, Raber G, Gulbins E, Takumi T, Moriyoshi K, Nakanishi S, Lang F. J Biol Chem. 1995;270:3638–3641. doi: 10.1074/jbc.270.8.3638. [DOI] [PubMed] [Google Scholar]
- 37.Whalley D W, Wendt D J, Grant A O. In: Cardiac Arrhythmias: Mechanisms, Diagnosis, and Management. Podrid P J, Kowey P R, editors; Podrid P J, Kowey P R, editors. Baltimore: Williams & Wilkins; 1994. pp. 109–130. [Google Scholar]
- 38.Nakaya H, Tohse N, Kanno M. Am J Physiol. 1987;253:H1089–H1097. doi: 10.1152/ajpheart.1987.253.5.H1089. [DOI] [PubMed] [Google Scholar]
- 39.Tsushima R G, Moffat M P. J Cardiovasc Pharmacol. 1990;16:50–58. doi: 10.1097/00005344-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Jabr R, Cole W C. Circ Res. 1993;72:1229–1244. doi: 10.1161/01.res.72.6.1229. [DOI] [PubMed] [Google Scholar]
- 41.Cerbai E, Ambrosio G, Porciatti F, Chiariello M, Giotti A, Mugelli A. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- 42.Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossy N. Proc Natl Acad Sci USA. 1993;90:1102–1106. doi: 10.1073/pnas.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halliwell B, Gutteridge J M C. In: Free Radicals in Biology and Medicine. 2nd Ed. Halliwell B, Gutteridge J M C, editors; Halliwell B, Gutteridge J M C, editors. Oxford: Clarendon; 1989. pp. 1–81. [Google Scholar]