Abstract
Quality of life (QOL) was studied in gastric cancer patients treated on a randomised, controlled trial comparing D1 (level 1) with D3 (levels 1, 2 and 3) lymphadenectomy. A total of 221 patients were randomly assigned to D1 (n=110) and D3 (n=111) surgery. Quality-of-life assessments included functional outcomes (a 14-item survey about treatment-specific symptoms) and health perception (Spitzer QOL Index) was performed before and after surgery at disease-free status. Patients suffered from irrelative events such as loss of partners was excluded thereafter. Main analyses were done by intention-to-treat. Thus, 214 D1 (106/110=96.4%) and D3 (108/111=97.3%) R0 patients were assessed. Longitudinal analysis showed that functional outcomes decreased at 6 months after surgery and increased over time thereafter, while health perceptions increased over time in general. On the basis of linear mixed model analyses, patients having total gastrectomy, advanced cancer and hemipancreaticosplenectomy, but not complications had poorer QOL than those without. D1 and D3 patients showed no significant difference in QOL. The results suggest that changes of QOL were largely due to scope of gastric resection, disease status and distal pancreaticosplenectomy, rather than the extent of lymph node dissection. This indicates that nodal dissection can be performed for a potentially curable gastric cancer.
Keywords: quality of life, nodal dissection, gastric cancer, trial
Gastric cancer remains the second most common cause of cancer-related deaths worldwide, and a major clinical challenge because of its poor prognosis and limited treatment options. The efficacy of nodal dissection is controversial (Cuschieri et al, 1999; Hartgrink et al, 2004). However, we have conducted a single institutional trial and demonstrated that D3 surgery has survival benefit (Wu et al, 2006b) but acceptable morbidity (Wu et al, 2004).
Quality of life (QOL) is looked upon as a multidimensional entity comprising physical, psychological, social and medical parameters. Cross-sectional studies (Koster et al, 1987; Kusche et al, 1987; Buhl et al, 1990; Korenaga et al, 1992; Jentschura et al, 1997; Wu et al, 1997a; Zieren et al, 1998; Svedlund et al, 1999; Thybush-Bernhardt et al, 1999) of QOL after gastric cancer surgery provided important background for design of this study, delineating a number of specific hypotheses and research questions related to surgery and survivorship. This report examines QOL of gastric cancer patients receiving either D1 or D3 surgery.
MATERIALS AND METHODS
Gastric cancer surgical trial summary
From October 1993 to August 1999, 221 patients were randomised at laparotomy to compare D1 (level 1) and D3 (level 1, 2 and 3) lymphadenectomy surgery for gastric cancer (Wu et al, 2004, 2006b). The primary surgical treatment report contains the full details. D3 surgery had more complications than D1 surgery, but both had no deaths (Wu et al, 2004, 2006a). No patients received preoperative or postoperative adjuvant radiotherapy or chemotherapy. D3 surgery offers a survival benefit when done by well-trained, experienced surgeons (Wu et al, 2006b).
Eligibility for the QOL study
The QOL study, a companion protocol, was opened simultaneously with surgical trial. Patients were eligible if they were registered to the National Health Research Institutes and had fitted the requirement of operation criteria and randomisation. The exclusion criteria included patients not curatively resected and not treated according to protocol. All patients were at disease-free status when the survey was carried out, because tumour recurrence was the decisive exclusion factor. The Ethics Committee approved the study protocol and an informed consent was obtained from each patient.
Instruments, assessment strategy and procedures
The Spitzer QOL Index (Spitzer et al, 1981), a global health assessment with valid questionnaire, includes five items rated on a three-point scale: activity, daily living, health, support of family and friends, and outlook. Functional outcome was assessed using a 14-item survey designed to assess treatment-specific symptoms, largely gastrointestinal function. Low scores reflect more symptoms (Korenaga et al, 1992; Wu et al, 1997a). Its validity was checked by the Cronbach’s α-scores.
The assessment strategy focused on QOL at a disease-free status not to be confounded by irrelevant events such as loss of partners and bony fractures after falling. We hypothesised that patients receiving D3 resection would have more symptoms and greater fatigue than patients receiving D1 surgery, with treatment arm differences most prominent at the 6-month assessment, and possibly continuing up to 1 year after random assignment. Questionnaires were administered at registration, 6 months, 1 year and annually thereafter until recurrence of tumour, if any. A research nurse (Hsueh-Pin Yu) briefly described and explained the procedure for responding to each question. Each interview took approximately 20 min.
Statistical considerations and analytic plan
All analyses used the intention-to-treat philosophy, whereby patients were grouped according to randomly assigned surgery treatment arms and under the assumption of a missing-at-random mechanism. The lymph node dissection effects on the functional outcome, and Spitzer QOL Index were examined by the linear mixed models (Diggle et al, 2002), adjusting for fixed effects for each time point, treatment, baseline covariate, age, sex, marital status, scope of gastric resection and gross appearance of tumour, and with random effects for intercept and each time point. Quadratic times as well as interaction terms were considered in the models to adjust for nonlinear temporal trend. A general unstructured covariance was assumed, that is, no specific forms of the random-effects covariance matrix were assumed. The lymph node dissection effects were assessed based on P-values of the fitted coefficients, adjusting for significant covariates in the model. The time trends of the functional outcome and Spitzer Index were assessed by significance tests of adjacent time periods in the mixed models, assisted by the longitudinal plots classified by the D1 and D3 treatment groups. These models and analyses were implemented by SAS PROC Mixed procedure.
RESULTS
Patient characteristics
In all, 214 R0 patients (mean age 63.9±11.1 years) were recruited. Comparison of the QOL study sample with the final surgical trial sample demonstrated no statistical difference between D1 (106/110=96.4%) and D3 (108/111=97.3%) patients in the QOL study (P=0.772, χ2 test). Male patients were older (65.43±10.3 vs 58.8±12.2) and underwent more distal pancreaticosplenectomy procedures. Female patients as compared with male patients were more likely to be married (100 vs 90.8%). The two groups did not differ significantly according to age, gender, marital status, type of gastric resection, reconstructive procedures (Table 1), comorbidity and location of tumour. But D3 patients had more cholecystectomy, distal hemipancreaticosplenectomy procedures (Table 1) and complications. During follow-up, five patients encountered irrelevant events; two spouse suicides (fifth year), three bony fractures (at second, second and sixth years, respectively). Four patients immigrated to Mainland China (at second, second, third and third years, respectively) and therefore could not be assessed at regular time points thereafter. Quality of life was not assessed at scheduled across assessment points after surgery in 3.2% of the subjects due to prolonged absence overseas. The Cronbach’s α-scores for treatment-specific symptoms were 0.67.
Table 1. Patient and operation characteristics.
| D1 (n=106) | D3 (n=108) | P | |
|---|---|---|---|
| Age (years) | |||
| Mean (95%) | 62.8 (60.6–64.9) | 65.0 (62.9–67.0) | 0.149 |
| Sex | |||
| Men | 80 | 83 | |
| Women | 26 | 25 | 0.813 |
| Marital status | |||
| Yes | 100 | 99 | |
| No | 6 | 9 | 0.444 |
| Type of gastric resection | |||
| Total gastrectomy | 29 | 22 | |
| Distal subtotal gastrectomy | 77 | 86 | 0.230 |
| Combined resection | |||
| Cholecystectomy | 14 | 38 | <0.001 |
| Distal pancreaticosplenectomy | 1 | 13 | 0.001 |
| Segmental resection of colon | 3 | 0 | 0.120 |
| Splenectomy | 3 | 1 | 0.015 |
| Partial liver resection | 2 | 1 | 0.620 |
| Oophorectomy (for ovarian cyst) | 1 | 0 | 0.495 |
| Reconstructive procedures | |||
| Billroth I | 6 | 8 | |
| Billroth II | 71 | 78 | |
| Roux-en-Y | 29 | 22 | 0.459 |
Longitudinal analysis of QOL
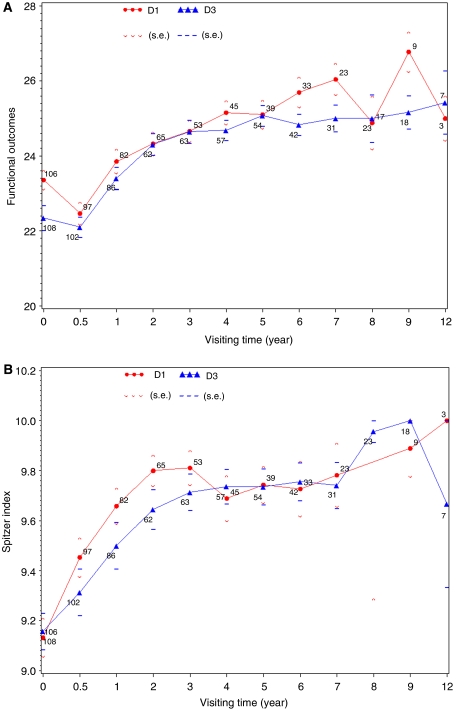
Nodal dissection effect showed transient discrepancy of food volume at baseline, diarrhoea and heartburn at first and second years after surgery (Table 2). The linear mixed model analysis for functional outcome showed that there were no significant differences in scores between two surgical treatment groups (P=0.5338, 0.6423 and 0.3941 for nodal dissection effect and its interaction effects with time and time2, respectively), after adjusting for significant covariate effects (Table 3). These significant covariate effects included time trend, baseline score, age, gender, gross appearance of tumour, scope of gastric resection and hemipancreaticosplenectomy. Surgical complications, depth of cancer invasion and marital status did not affect functional outcomes, and therefore were dropped from the model. These results coincide with univariate analyses (which were not shown here) based on linear mixed effects models when a single covariate effect is considered, adjusted for time trends and baseline scores. The overall time trends (Figure 1) indicated that the functional outcomes were significantly lower at the 6th month after surgical treatment than at the baseline (P<0.0001), and improved from the sixth month to the first year after surgery (P<0.0001), continuously improving up to the second year after surgery (P=0.006). Thereafter, the differences in functional outcomes between adjacent years were not statistically significant. The time trends of functional outcomes had to be adjusted by significant interaction effects with time, including scope of gastric resection, gross appearance of tumour, age and baseline score.
Table 2. Effect of nodal dissection on quality of life.
|
Time after operation
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Baseline
|
Half year
|
First year
|
Second year
|
Third year
|
Fourth year
|
Fifth year
|
|||||||||||||||
| Disease-specific symptom | D1 (n=106) | D3 (n=108) | P-value | D1 (n=97) | D3 (n=102) | P-value | D1 (n=82) | D3 (n=86) | P-value | D1 (n=65) | D3 (n=62) | P-value | D1 (n=53) | D3 (n=63) | P-value | D1 (n=45) | D3 (n=57) | P-value | D1 (n=39) | D3 (n=54) | P-value |
| Treatment-specific symptoms | |||||||||||||||||||||
| Appetite | 1.03 (0.58) | 0.91 (0.59) | 0.13 | 1.15 (0.46) | 1.15 (0.55) | 0.92 | 1.35 (0.55) | 1.34 (0.52) | 0.84 | 1.45 (0.53) | 1.44 (0.53) | 0.91 | 1.49 (0.50) | 1.48 (0.50) | 0.88 | 1.64 (0.48) | 1.51 (0.50) | 0.17 | 1.49 (0.51) | 1.54 (0.50) | 0.64 |
| Consistency of food | 1.82 (0.41) | 1.82 (0.41) | 0.95 | 1.79 (0.41) | 1.83 (0.37) | 0.48 | 1.93 (0.26) | 1.92 (0.31) | 0.85 | 1.94 (0.24) | 1.97 (0.18) | 0.44 | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 1.98 (0.13) | 0.38 | 1.95 (0.22) | 2.00 (0.00) | 0.09 |
| Volume of food | 0.80 (0.42) | 0.68 (0.47) | 0.04* | 0.48 (0.58) | 0.40 (0.58) | 0.32 | 0.87 (0.60) | 0.80 (0.61) | 0.50 | 0.95 (0.60) | 0.94 (0.62) | 0.87 | 1.00 (0.44) | 1.02 (0.49) | 0.86 | 1.07 (0.45) | 1.09 (0.39) | 0.80 | 1.26 (0.44) | 1.26 (0.44) | 0.98 |
| Frequency of eating | 1.96 (0.19) | 1.94 (0.27) | 0.58 | 1.12 (0.53) | 1.21 (0.55) | 0.28 | 1.44 (0.55) | 1.51 (0.53) | 0.38 | 1.69 (0.50) | 1.66 (0.48) | 0.72 | 1.91 (0.30) | 1.84 (0.37) | 0.31 | 1.96 (0.21) | 1.96 (0.19) | 0.81 | 1.97 (0.16) | 1.98 (0.14) | 0.82 |
| Eating time | 1.96 (0.19) | 1.95 (0.21) | 0.76 | 1.99 (0.10) | 1.97 (0.17) | 0.34 | 2.00 (0.00) | 2.00 (0.00) | — | 1.98 (0.12) | 1.98 (0.13) | 0.97 | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 2.00 (0.00) | — |
| Postprandial abdominal fullness | 1.19 (0.52) | 1.17 (0.56) | 0.82 | 1.41 (0.54) | 1.34 (0.47) | 0.29 | 1.50 (0.50) | 1.48 (0.50) | 0.76 | 1.54 (0.50) | 1.52 (0.50) | 0.80 | 1.47 (0.50) | 1.56 (0.50) | 0.37 | 1.49 (0.51) | 1.35 (0.48) | 0.16 | 1.54 (0.51) | 1.46 (0.50) | 0.48 |
| Heartburn | 1.71 (0.50) | 1.62 (0.59) | 0.24 | 1.95 (0.22) | 1.97 (0.17) | 0.43 | 1.95 (0.27) | 1.94 (0.24) | 0.81 | 1.88 (0.38) | 1.98 (0.13) | 0.04* | 1.92 (0.27) | 1.97 (0.18) | 0.29 | 1.98 (0.15) | 1.93 (0.26) | 0.27 | 1.95 (0.22) | 1.91 (0.29) | 0.46 |
| Diarrhoea | 1.81 (0.44) | 1.73 (0.54) | 0.24 | 1.82 (0.38) | 1.72 (0.47) | 0.08 | 1.88 (0.36) | 1.69 (0.51) | 0.01* | 1.80 (0.40) | 1.74 (0.44) | 0.44 | 1.81 (0.39) | 1.76 (0.50) | 0.56 | 1.84 (0.37) | 1.74 (0.44) | 0.19 | 1.79 (0.47) | 1.76 (0.47) | 0.72 |
| Constipation | 1.78 (0.41) | 1.71 (0.53) | 0.30 | 1.85 (0.42) | 1.82 (0.43) | 0.70 | 1.82 (0.42) | 1.85 (0.39) | 0.61 | 1.88 (0.38) | 1.77 (0.46) | 0.17 | 1.87 (0.34) | 1.71 (0.49) | 0.06 | 1.62 (0.53) | 1.72 (0.45) | 0.32 | 1.64 (0.54) | 1.69 (0.47) | 0.67 |
| Insomnia | 1.88 (0.41) | 1.90 (0.34) | 0.73 | 1.86 (0.43) | 1.89 (0.31) | 0.51 | 1.84 (0.48) | 1.81 (0.45) | 0.70 | 1.85 (0.44) | 1.92 (0.27) | 0.27 | 1.83 (0.43) | 1.84 (0.41) | 0.89 | 1.82 (0.44) | 1.91 (0.29) | 0.22 | 1.79 (0.41) | 1.87 (0.34) | 0.33 |
| Body weight | 1.95 (0.74) | 1.81 (0.73) | 0.14 | 1.56 (1.00) | 1.38 (0.98) | 0.21 | 1.60 (1.04) | 1.50 (1.06) | 0.55 | 1.62 (1.00) | 1.69 (0.92) | 0.65 | 1.64 (0.90) | 1.75 (0.93) | 0.54 | 1.89 (0.91) | 1.77 (0.91) | 0.52 | 1.87 (0.98) | 1.85 (0.96) | 0.92 |
| Swallowing problem | 1.96 (0.19) | 1.98 (0.14) | 0.42 | 1.97 (0.23) | 1.98 (0.14) | 0.68 | 2.00 (0.00) | 1.99 (0.11) | 0.33 | 1.98 (0.12) | 1.98 (0.13) | 0.97 | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 2.00 (0.00) | — |
| Vomiting | 1.81 (0.44) | 1.70 (0.57) | 0.10 | 1.97 (0.17) | 1.93 (0.26) | 0.22 | 1.96 (0.19) | 1.97 (0.18) | 0.95 | 1.98 (0.12) | 1.98 (0.13) | 0.97 | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 1.98 (0.13) | 0.38 | 2.00 (0.00) | 2.00 (0.00) | — |
| Dizziness | 1.69 (0.54) | 1.70 (0.52) | 0.83 | 1.54 (0.52) | 1.61 (0.49) | 0.28 | 1.72 (0.55) | 1.62 (0.49) | 0.20 | 1.78 (0.41) | 1.73 (0.45) | 0.44 | 1.72 (0.45) | 1.73 (0.45) | 0.88 | 1.84 (0.37) | 1.74 (0.44) | 0.19 | 1.85 (0.37) | 1.74 (0.44) | 0.23 |
| Spitzer Index | |||||||||||||||||||||
| Activity | 1.93 (0.25) | 1.95 (0.21) | 0.53 | 1.97 (0.17) | 1.94 (0.24) | 0.35 | 1.99 (0.11) | 1.95 (0.21) | 0.19 | 2.00 (0.00) | 1.98 (0.13) | 0.31 | 1.98 (0.14) | 1.98 (0.13) | 0.90 | 2.00 (0.00) | 1.98 (0.13) | 0.38 | 2.00 (0.00) | 2.02 (0.14) | 0.40 |
| Daily living | 1.99 (0.10) | 1.99 (0.10) | 0.99 | 2.00 (0.00) | 1.98 (0.14) | 0.17 | 2.00 (0.00) | 2.00 (0.00) | — | 1.98 (0.12) | 1.98 (0.13) | 0.97 | 1.98 (0.14) | 2.00 (0.00) | 0.28 | 2.00 (0.00) | 2.00 (0.00) | — | 2.00 (0.00) | 2.00 (0.00) | — |
| Health | 1.70 (0.46) | 1.68 (0.47) | 0.73 | 1.72 (0.45) | 1.68 (0.47) | 0.49 | 1.84 (0.37) | 1.79 (0.41) | 0.40 | 1.89 (0.31) | 1.85 (0.36) | 0.53 | 1.94 (0.23) | 1.86 (0.35) | 0.13 | 1.87 (0.34) | 1.88 (0.33) | 0.88 | 1.87 (0.34) | 1.87 (0.34) | 0.98 |
| Support | 1.93 (0.25) | 1.93 (0.26) | 0.82 | 1.99 (0.10) | 1.95 (0.22) | 0.11 | 1.99 (0.11) | 1.95 (0.21) | 0.19 | 2.00 (0.00) | 1.95 (0.22) | 0.07 | 1.98 (0.14) | 1.98 (0.13) | 0.90 | 1.93 (0.25) | 1.96 (0.19) | 0.47 | 1.95 (0.22) | 1.96 (0.19) | 0.74 |
| Outlook | 1.58 (0.50) | 1.61 (0.49) | 0.60 | 1.77 (0.42) | 1.76 (0.43) | 0.89 | 1.84 (0.37) | 1.80 (0.40) | 0.51 | 1.92 (0.27) | 1.87 (0.34) | 0.34 | 1.92 (0.27) | 1.89 (0.32) | 0.52 | 1.89 (0.32) | 1.91 (0.29) | 0.70 | 1.92 (0.27) | 1.91 (0.29) | 0.79 |
*Statistically significant.
Table 3. Estimates of regression coefficients by linear mixed model for functional outcomes and health perception (Spitzer Index) scores.
| Estimate | s.e. | P>F | |
|---|---|---|---|
| Effect on functional outcomes | |||
| Intercept | 13.4639 | 1.6958 | <0.0001 |
| Time | 5.0898 | 0.9718 | <0.0001 |
| Time2 | −0.4664 | 0.1229 | 0.0002 |
| Node dissection (D3) | −0.2386 | 0.3832 | 0.5338 |
| Node dissection*time | 0.0825 | 0.1776 | 0.6423 |
| Node dissection*time2 | −0.0190 | 0.0223 | 0.3941 |
| Scope of gastrectomy (subtotal) | 2.0668 | 0.4825 | <0.0001 |
| Scope of gastrectomy*time | −0.5924 | 0.2276 | 0.0095 |
| Scope of gastrectomy*time2 | 0.0616 | 0.0295 | 0.0368 |
| Gender (male) | 1.6467 | 0.3255 | <0.0001 |
| Gross appearance (Borrmann III and IV) | −0.7142 | 0.3381 | 0.0352 |
| Gross appearance*time | 0.1696 | 0.0594 | 0.0044 |
| Age*time | −0.0175 | 0.0066 | 0.0079 |
| Age*time2 | 0.0011 | 0.0009 | 0.2284 |
| Hemipancreaticosplenectomy (yes) | −1.4653 | 0.6907 | 0.0344 |
| Baseline | 0.2834 | 0.0670 | <0.0001 |
| Baseline*time | −0.1189 | 0.0333 | 0.0004 |
| Baseline*time2 | 0.0124 | 0.0041 | 0.0021 |
| Effect on health perception (Spitzer Index) scores | |||
| Intercept | 8.0906 | 0.3663 | <0.0001 |
| Time | 0.1879 | 0.0475 | 0.0001 |
| Time2 | −0.0254 | 0.0065 | 0.0002 |
| Node dissection (D3) | −0.0547 | 0.0951 | 0.5652 |
| Node dissection*time | −0.0662 | 0.0633 | 0.2956 |
| Node dissection*time2 | 0.0150 | 0.0085 | 0.0784 |
| Scope of gastrectomy (subtotal) | 0.1074 | 0.0655 | 0.1016 |
| Gender (male) | 0.2281 | 0.0585 | 0.0001 |
| Gross appearance (Borrmann III and IV) | −0.1361 | 0.0479 | 0.0047 |
| Age | −0.0056 | 0.0023 | 0.0137 |
| Marital status (married) | 0.2642 | 0.0929 | 0.0047 |
| Hemipancreaticosplenectomy (yes) | −0.6687 | 0.1638 | <0.0001 |
| Hemipancreaticosplenectomy*time | 0.1346 | 0.0598 | 0.0250 |
| Baseline | 0.1396 | 0.0321 | <0.0001 |
Figure 1.
(A) Average total evaluation score for functional outcome (treatment-specific symptoms), adjusted for time trend and baseline score by treatment arms. Mean scores are presented with s.e. (bars). High scores reflect better quality of life. (B) Average total evaluation score for general health perception (Spitzer Index) adjusted for time trend and baseline score by treatment arms. Mean scores are presented with s.e. (bars). High scores reflect better perception of health status.
For the Sptizer Index scores, the linear mixed model analysis revealed similar results that there were no significant differences in scores between two surgical treatment groups (P=0.5652, 0.2956 and 0.0784 for node dissection effect and its interaction effects with time and time2, respectively), after adjusting for significant covariate effects (Table 3). The significant covariate effects are similar to those on the functional outcomes. An interesting finding is that the marital status is a significant effect on the Sptizer Index scores, but not on the functional outcomes. The overall health perceptions (Spitzer Index) (Figure 1) were significantly lower at the baseline than at the 6th month after surgical treatment (P<0.0001), and improved from the 6th month to the first year after surgery (P=0.0022). Thereafter, there were no significant differences in health perceptions between adjacent years. Random effects are included in the statistical analysis, but not reported in Table 3.
Further analysis based on overall averaged scores showed that patients who underwent a distal pancreatic resection had poorer appetite (P=0.001 based on t-test), decreased consistency (P<0.0001) and volume of food (P=0.0003) and frequency of eating (P<0.0001), lost of more body weight (P=0.012), could not readily engage in full-time work or study (P<0.0001), lacked energy (P<0.0001) and reported less psychosocial support (P=0.021) than those without pancreatic resection.
DISCUSSION
The results of the longitudinal QOL measurements indicate that D1 and D3 gastric cancer patients have similar health perception and functional outcomes after surgery.
When we designed QOL study the Functional Assessment of Cancer Therapy–General (FACT-G) (Cella et al, 1993) and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) (Aaronson et al, 1993) were not available, and no Chinese version was available. The Spitzer QOL Index is a valid but suboptional questionnaire due to the limited number of items and ceiling effect. However, it is easily applied and can be summed into a single score while retaining its domain-specific properties. It has been used worldwide in various cancers (Greimel et al, 2002; Erridge et al, 2005) including gastric cancer (Kusche et al, 1987; Buhl et al, 1990). Our treatment-specific instrument, which was modified from Korenaga's study for Japanese gastric cancer patients, is a short questionnaire, which has proved to be reliable in implementation (Wu et al, 1997a, 2000) and in current study (Cronbach's α-scores 0.67). Taken all questionnaires together, data acquisition for 19 items assisted by a research nurse had shown its validity throughout the entire study.
Patients had anxiety, depression, loss of appetite and body weight at diagnosed of gastric cancer, but returned to normal gradually after surgery. Similar to other observations (Jentschura et al, 1997; Wu et al, 1997a; Davies et al, 1998), patients after a total gastrectomy tended to suffer poorer tolerance of normal food, need for more frequent eating and loss of more body weight than those after a subtotal gastrectomy. And younger patients had better QOL than older (Koster et al, 1987; Wu et al, 1997a). We noted that gender plays an important role in facing gastric cancer. Male patients usually have more morbidity after gastric cancer surgery (Viste et al, 1988). It is noteworthy that female patients were younger and mostly married, but male patients tended to feel better and calmer, suffer less insomnia and have a more positive outlook. This may not be simply explained by different culture roles attributes for men and women, since Koster et al (1987) also recorded the same phenomenon in German gastric cancer patients.
Lymph node dissection along the upper border of the pancreas is essential for radical gastric cancer surgery. Resection of distal pancreas to facilitate lymph node dissection had a high morbidity and mortality. (Bonenkamp et al, 1995; Cuschieri et al, 1996; Wu et al, 2006a) Our data revealed that diarrhoea, except at first year, was similarly experienced in both groups. This study further disclosed its worse functional outcome and health perception, but no negative effect of QOL among D3 patients was drawn as the number of affected patients (1% in D1 vs 12% in D3 patients) was small. However, a technique developed by Maruyama et al (1995) to preserve the pancreas is feasible and safe, and should be considered in gastric cancer surgery.
Although the morbidity rate was higher in D3 patients than in D1 patients, our analysis indicates that lymph node dissection did not adversely influence QOL. The possible explanations are that the anastomotic leaks were minor in extent and abscess was detected early. These patients had no fever and did not need intensive care. They consumed regular diet while treating by a closed continuous irrigation system for abscess, and leaks were managed nonoperatively with nutritional support (Wu et al, 2004) Nevertheless, one should be very cautious in interpreting these results because the postoperative morbidity rate was very low, as compared with other reported studies (Bonenkamp et al, 1995; Cuschieri et al, 1996). Our previous study and current trial showed that operative morbidity did not influence survival (Wu et al, 1997b, 2006b). The findings we observed are apparently at variance with those of a previous study (Siewert et al, 1998). A similar disparity also exists in QOL.
Acknowledgments
We thank Yurh-Ling Ho, Hsueh-pin Yu and Hui-Tzu Yu, of the data center at the National Health Research Institutes, for assistance with data collection and processing.
This work is supported by the Institute of Cancer Research, National Health Research Institutes, Taiwan.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Felchtner H, Fleishman DB, De Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe P, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 [DOI] [PubMed] [Google Scholar]
- Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JTM, van EIK P, Obertop H, Gouma DJ, Taat CW, van Lanschot J, Meyer S, de Graaf PW, von Meyenfeldt MF, Tilanus H, van de Velde CJH (1995) Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 345: 745–748 [DOI] [PubMed] [Google Scholar]
- Buhl K, Schlag P, Herfarth C (1990) Quality of life and functional results following different types of resection for gastric carcinoma. Eur J Surg Oncol 16: 404–409 [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11: 570–579 [DOI] [PubMed] [Google Scholar]
- Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook p, for The Surgical Cooperative Group (1996) Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomized controlled surgical trial. Lancet 347: 995–999 [DOI] [PubMed] [Google Scholar]
- Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P, for The Surgical Cooperative Group (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer 79: 1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I (1998) Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg 22: 1048–1055 [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang K-Y, Zeger S (2002) Analysis of Longitudinal Data 2nd edn. Oxford University Press, Oxford [Google Scholar]
- Erridge SC, Gaze MN, Price A, Kelly CG, Kerr GR, Cull A, MacDougall RH, Howard GCW, Cowie VJ, Gregor A (2005) Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 17: 61–67 [DOI] [PubMed] [Google Scholar]
- Greimel E, Thiel I, Peintinger F, Cegnar I, Pongratz E (2002) Prospective assessment of quality of life of female cancer patients. Gynecol Oncol 85: 140–147 [DOI] [PubMed] [Google Scholar]
- Hartgrink HH, van de Velde CJH, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JHJM, Meeijer S, Plukker JTM, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJB, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M (2004) Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22: 2069–2077 [DOI] [PubMed] [Google Scholar]
- Jentschura D, Winkler M, Strohmeier N, Rumstadt B, Hagmuller E (1997) Quality-of-life after curative surgery for gastric cancer: a comparison between total gastrectomy and subtotal gastric resection. Hepatogastroenbterology 44: 1137–1142 [PubMed] [Google Scholar]
- Korenaga D, Orita H, Okuyama T, Moriguchi S, Maehara Y, Sugimachi K (1992) Quality of life after gastrectomy in patients with carcinoma of stomach. Br J Surg 79: 248–250 [DOI] [PubMed] [Google Scholar]
- Koster R, Gerbbenslerben B, Stutzer H, Salzberger B, Ahrens P, Rohde H (1987) Quality of life in gastric cancer. Karnofsky's scale and Spitzer's index in comparison at the time of surgery in a cohort of 1081 patients. Scand J Gastroenterol 22: 102–106 [Google Scholar]
- Kusche J, Vestweber K-H, Troidl H (1987) Quality of life after total gastrectomy for stomach cancer. Results of three types of quality of life evaluative methods. Scand J Gastroenterol 22: 96–101 [Google Scholar]
- Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Okajima K (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19: 532–536 [DOI] [PubMed] [Google Scholar]
- Siewert JR, Bottcher K, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, Battista RN, Catchlove BR (1981) Measuring the quality of life of cancer patients. A concise QL-index for use by physicians. J Chronic Dis 34: 585–597 [DOI] [PubMed] [Google Scholar]
- Svedlund J, Sullivan M, Liedman B, Lundell L (1999) Long-term consequences of gastrectomy for patients’ quality of life: the impact of reconstructive techniques. Am J Gastroenterol 94: 438–445 [DOI] [PubMed] [Google Scholar]
- Thybush-Bernhardt A, Schmidt C, Kuchler T, Schmid A, Henne-Bruns D, Kremer B (1999) Quality of life following radical surgical treatment of gastric carcinoma. World J Surg 23: 503–508 [DOI] [PubMed] [Google Scholar]
- Viste A, Haugstvedt T, Eide GE, Soreide O, The Norwegian Stomach Cancer Trial Members (1988) Postoperative complications and mortality after surgery for gastric cancer. Ann Surg 207: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Chang IS, Lo SS, Hsieh MC, Shia LT, Wang-Peng J (2006a) Complications following D3 gastrectomy: post hoc analysis of a randomized trial. World J Surg 30: 12–16 [DOI] [PubMed] [Google Scholar]
- Wu CW, Hsieh MC, Lo SS, Lui WY, P’eng FK (1997a) Quality of life of patients with gastric adenocarcinoma after curative gastrectomy. World J Surg 21: 777–782 [DOI] [PubMed] [Google Scholar]
- Wu CW, Hsieh MC, Lo SS, Tsay SH, Li AF, Lui WY, P’eng FK (1997b) Prognostic indicators in patients with gastric cancer after resection for patients with carcinoma of the stomach. Dig Dis Sci 42: 1265–1269 [DOI] [PubMed] [Google Scholar]
- Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen CH, Li AF, Shia LT, Lui WY, Whang-Peng J (2006b) Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 7: 309–315 [DOI] [PubMed] [Google Scholar]
- Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Wang-Peng J (2004) Randomized clinical study of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 91: 283–287 [DOI] [PubMed] [Google Scholar]
- Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P’eng FK (2000) Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg 24: 465–472 [DOI] [PubMed] [Google Scholar]
- Zieren HU, Zippel K, Zieren J, Muller JM (1998) Quality of life after surgical treatment of gastric cancer. Eur J Surg 164: 119–125 [DOI] [PubMed] [Google Scholar]