Abstract
We investigated the safety and efficacy of a methotrexate, vinblastine, doxorubicin and cisplatin (M-VAC) combination regimen as second-line chemotherapy for patients with advanced or metastatic transitional cell carcinoma who failed first-line gemcitabine and cisplatin (GC) chemotherapy. Thirty patients who had progressed or relapsed after GC chemotherapy as first-line treatment were enrolled in this study. The major toxicities were neutropaenia and thrombocytopaenia. A grade 3 or 4 neutropaenia occurred in 19 (63.3%) and a grade 3 or 4 thrombocytopaenia developed in nine patients (30.0%). There were no life-threatening complications during the study. The overall response was 30%. A complete response was achieved in two patients (6.7%) and a partial response in seven (23.3%). The overall disease control rate was 50%. Seven out of 16 patients who had responded previously to GC responded to M-VAC, while 2 out of 14 who had not responded to GC responded to M-VAC. The median response duration was 3.9 months and the median progression-free survival was 5.3 months. The median overall survival was 10.9 months. M-VAC showed encouraging efficacy and reversible toxicities in patients who had progressed after GC chemotherapy and, especially, M-VAC appears to be a reasonable option as a sequential treatment regimen in patients who responded previously to GC chemotherapy.
Keywords: transitional cell carcinoma, chemotherapy, cisplatin, metastasis
Urothelial carcinoma is the second most common genitourinary malignancy. Since advanced urothelial carcinoma is sensitive to chemotherapeutic agents, systemic combination chemotherapy has been the standard treatment that can result in long-term survival in some patients with advanced or metastatic transitional cell carcinoma. The most commonly used regimens are the methotrexate, vinblastine, doxorubicin and cisplatin (M-VAC) combination or the gemcitabine and cisplatin (GC) combination regimen (Bamias et al, 2006; Garcia and Dreicer, 2006; Pectasides et al, 2007). The M-VAC regimen was introduced at the Memorial Sloan-Kettering hospital in the mid-1980s and it improved overall survival to a median slightly in excess of 12 months (Sternberg et al, 1988; Culine, 2002). Thereafter, a GC regimen was developed and, when it was compared with M-VAC, it showed comparable efficacy with reduced side effects (Moore et al, 1999; von der Maase et al, 1999, 2000; Kaufman et al, 2000). Recently published long-term results confirmed a similar 5-year survival and progression-free survival rates for GC and M-VAC (von der Maase et al, 2005). On the basis of these results, M-VAC or GC has become the standard first-line treatment for patients with advanced transitional cell carcinoma.
However, a dismal prognosis is predicted in patients whose disease progresses after first-line chemotherapy. Therefore, safe and effective second-line chemotherapy regimens are needed for patients who have a disease relapse after initial chemotherapy treatment. Currently, there is limited information on additional treatment of patients who fail first-line platinum-based chemotherapy. Taxanes, gemcitabine and ifosfamide have shown promising results after failure of first-line chemotherapy (Tu et al, 1995; McCaffrey et al, 1997; Witte et al, 1997; Sweeney et al, 1999; Krege et al, 2001; Meluch et al, 2001; Sternberg et al, 2001; Bellmunt et al, 2002; Vaughn et al, 2002). However, the response rates to these regimens have been variable and durable remissions have been limited.
Although GC is widely considered as the new standard for first-line treatment in patients with advanced bladder cancer, in several countries, there is no data on salvage chemotherapy after failure of GC. Moreover, the role of M-VAC as second-line treatment after failure of GC is unknown. We therefore investigated the safety and efficacy of the M-VAC regimen as salvage chemotherapy for the patients in whom first-line GC chemotherapy failed.
PATIENTS AND METHODS
Patient eligibility
Patients with histologically confirmed advanced or metastatic transitional cell carcinoma were eligible to participate in this study. All patients had evidence of disease progression or relapse after GC chemotherapy as first-line treatment. For GC chemotherapy, gemcitabine was given at a dose of 1000 mg m−2 on days 1, 8 and 15, and cisplatin was given at a dose of 70 mg m−2 on day 2. Adequate organ function was required and an Eastern Cooperative Oncology Group performance status of 0–2, an absolute granulocyte count ⩾1500 mm−3, platelet count ⩾100 000 mm−3, serum creatinine ⩽1.5 mg dl−1, creatinine clearance ⩾50 ml min−1 and a serum bilirubin ⩽2 mg dl−1 were required for the treatment. The exclusion criteria included the presence of brain metastases or persistent toxicity from previous GC chemotherapy. Informed consent was obtained before entry into the study. The study was conducted in accordance with the Declaration of Helsinki Principle and Good Clinical Practice and was approved by an independent ethics committee.
Treatment schedule
The patients received methotrexate 30 mg m−2 on days 1, 15 and 22; vinblastine 3 mg m−2 on days 2, 15 and 22; doxorubicin 30 mg m−2 on day 2; and cisplatin 70 mg m−2 on day 2. The cycles were repeated every 28 days. The patient response was evaluated every three cycles. An additional three cycles with same regimen were provided to patients with no progression at the response evaluation. A third-line chemotherapy regimen was initiated in patients who had progressed if the patients had a good performance status and adequate organ function.
Response and toxicity assessment
All patients were evaluated for their response to treatment after completing three cycles except for those cases with symptomatic progression. Early evaluations were performed in patients with clinical evidence of progressive disease (PD). Patient response was evaluated according to the Response Evaluation Criteria in Solid Tumors (Therasse et al, 2000). A complete response (CR) was defined as the disappearance of all clinical and radiological signs of target lesions. A partial response (PR) was a ⩾30% decrease in the overall sum of the diameter of the target lesions; a PD was a ⩾20% increase in the overall sum of the diameter of the target lesions; and stable disease was neither a sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. In cases with a PR or CR, a second assessment 4 weeks later was required for confirmation of the response. The toxicity was graded according to the National Cancer Institute's common toxicity criteria version 2.0. Patients who received at least one dose of M-VAC were assessed for toxicity.
Statistical considerations
The duration of response was defined as the time from the first objective status assessment of response to the initial date of a documented progression. The progression-free survival was defined as the time from study entry to the initial date of evidence of progression, death or loss to follow-up. The progression-free survival of patients alive without progression was recorded at the time of the last follow-up evaluation. The overall survival was defined as the interval between the date of study entry to death or the last follow-up evaluation. The progression-free survival and the overall survival were estimated using the Kaplan–Meier method. The analyses were performed using 5% as the level of significance.
RESULTS
Patient characteristics
Between May 2002 and December 2006, 30 patients were enrolled in this study. The baseline characteristics of the patients are shown in Table 1. The median age was 64 years (range, 38–79). There were 24 men and 6 women. Fourteen patients had primary tumours. Nineteen patients had visceral metastases and 11 patients had regional lymph-node metastases only.
Table 1. Characteristics of the study population.
| Characteristics | No. of patients |
|---|---|
| Total enrolled patients | 30 |
| Age | |
| Median (years) | 64 |
| Range (years) | 38–79 |
| Gender | |
| Males | 24 |
| Females | 6 |
| ECOG performance status | |
| 0 | 16 |
| 1 | 11 |
| 2 | 3 |
| Disease extents | |
| Visceral metastases | 19 |
| Regional lymph-node metastases only | 11 |
| Sites of diseases | |
| Primary | 14 |
| Local recurrence | 3 |
| Lymph nodes | 22 |
| Lung | 7 |
| Liver | 4 |
| Bone | 5 |
| Others | 2 |
For first-line GC chemotherapy, the median number of cycles was five (range, 2–9) and the response rate was 53.3%. There were no interrupted schedules or dose adjustments due to persistent toxicities. All patients received at least three cycles of GC except for one patient. In one patient, GC chemotherapy was discontinued during a second cycle because acute renal failure developed due to progression of pelvic lymph node metastases.
After disease progression or relapse was confirmed, the patients received the M-VAC regimen. The median treatment-free interval between GC and M-VAC was 2.5 months (range, 0.5–20.4) and the median number of cycles for the M-VAC regimen was three (range, 1–12).
Toxicity
All enrolled patients were evaluated for toxicity (Table 2). The treatment was generally well-tolerated. The major haematological toxicities were neutropaenia and thrombocytopaenia. A grade 3 or 4 neutropaenia occurred in 19 patients (63.3%); they received granulocyte colony-stimulating factor until their neutrophil counts were restored. A grade 3 or 4 thrombocytopaenia developed in nine patients (30.0%); there was no haemorrhagic event due to the thrombocytopaenia. Grade 3 or 4 anaemia developed less frequently and was identified in five patients (16.7%). The major nonhaematologic toxicities were alopecia and mucositis. Grade 2 alopecia developed in 14 patients (46.7%) and grade 3 or 4 mucositis in four (13.3%); most nonhaematologic toxicities were confined to grade 1 or 2 (Table 2). All toxicities were reversible and no life-threatening complications were observed during the study.
Table 2. Toxicities.
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Neutropaenia | 1 | 3.3 | 2 | 6.7 | 6 | 20.0 | 13 | 43.3 |
| Thrombocytopaenia | 5 | 16.7 | 1 | 3.3 | 3 | 10.0 | 6 | 20.0 |
| Anaemia | 9 | 30.0 | 3 | 10.0 | 4 | 13.3 | 1 | 3.3 |
| Mucositis | 1 | 3.3 | 3 | 10.0 | 4 | 13.3 | — | — |
| Alopecia | 13 | 43.3 | 14 | 46.7 | ||||
| Nausea/vomiting | 14 | 46.7 | 8 | 26.7 | — | — | — | — |
| Anorexia | 10 | 33.3 | 5 | 16.7 | 1 | 3.3 | — | — |
| Diarrhoea | 2 | 6.7 | 1 | 3.3 | — | — | — | — |
| Constipation | 3 | 10.0 | 1 | 3.3 | — | — | — | |
| Fatigue | 5 | 16.7 | 1 | 3.3 | — | — | — | — |
| Asthenia | 5 | 16.7 | 2 | 6.7 | — | — | — | — |
| Fever | 3 | 10.0 | 1 | 3.3 | — | — | — | — |
| Myalgia | 5 | 16.7 | 1 | 3.3 | — | — | — | — |
| Infection | — | — | — | — | 1 | 3.3 | — | — |
| Allergic reactions | 1 | 3.3 | 1 | 3.3 | — | — | — | — |
| Dizziness | 1 | 3.3 | — | — | — | — | — | — |
Omission of methotrexate and vinblastine on day 15 and/or 22 or delay of a subsequent cycle occurred in cases with persistent severe haematological toxicities despite appropriate management. Methotrexate and vinblastine on day 15 or 22 were omitted in 23 patients. The median for an omitted scheduled cycle was one per patient (range, 0–16). The schedules on day 15 or 22 were delayed in 11 patients. The median number of delayed schedules was zero (range, 0–3); all patients could receive scheduled treatments after 1 week. A subsequent cycle was delayed for 1 week in one patient.
Response rates and survival
The response to treatment was assessed in all registered patients. The overall response rate was 30%. A CR was achieved in two patients (6.7%) and a PR in seven (23.3%). Stable disease was observed in six patients (20.0%). The overall disease control rate was 50%. The characteristics of the responders are listed in Table 3. Seven out of 16 patients who had responded previously to GC responded to M-VAC, while 2 out of 14 who had not responded to GC responded to M-VAC. A response was observed in 4 out of 11 (36.4%) patients with only lymph node metastases and in 5 out of 19 (26.3%) patients with visceral metastases.
Table 3. Characteristics of responders to second-line M-VAC regimen.
| No. | Age | Site of disease | Response to GC | Treatment-free interval (month) | Response to M-VAC | RD (month) | PFS (month) | Current status | Survival (month) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Bone Supraclavicular LN | PR | 3.2 | PR | 6.6 | 8.6 | Death | 11.7 |
| 2 | 54 | Retroperitoneal LN | PR | 4.5 | PR | 3.3 | 6.4 | Death | 9.2 |
| 3 | 66 | Bladder Lung Retroperitoneal LN | PR | 5.7 | PR | 3.7 | 7.2 | Alive | 9.2+ |
| 4 | 72 | Lung Retroperitoneal LN | PR | 3 | PR | 3.9 | 7.6 | Death | 10.9 |
| 5 | 69 | Pelvic recurrence Lung Retroperitoneal LN | PR | 2 | PR | 3.8 | 7.1 | Death | 10.6 |
| 6 | 69 | Bladder Retroperitoneal LN | PD | 7 | CR | 1.5 | 4.4+ | Alive | 5.8+ |
| 7 | 64 | Retroperitoneal LN Carcinomatosis | CR | 1 | PR | 1.5 | 2.5+ | Alive | 5.3+ |
| 8 | 79 | Retroperitoneal LN | CR | 10.4 | CR | 7.2 | 10.6+ | Alive | 11.6+ |
| 9 | 52 | Bladder Scrotum | PD | 2.5 | PR | 3.9 | 6.7 | Death | 15.8 |
Abbreviations: CR=complete response; LN=lymph node; PD=progressive disease; PFS=progression-free survival; PR=partial response; RD=response duration.
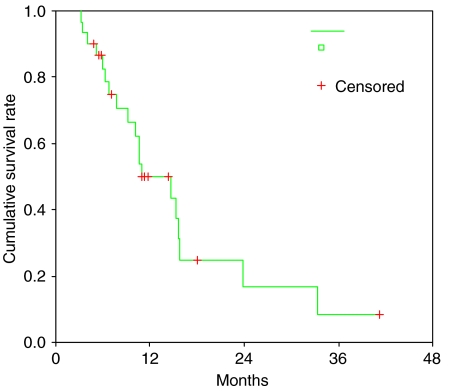
The median response duration was 3.9 months (95% CI: 3.7–4.1) and the median progression-free survival was 5.3 months (95% CI: 3.1–7.5). A majority of patients finally had disease progression; only three patients remained with no disease progression at the last follow-up evaluation. Twenty patients died and 10 patients were still alive at the last follow-up. The median overall survival was 10.9 months (95% CI: 5.5–16.3). The actuarial 1- and 2-year survival rates were 49.8 and 16.6%, respectively (Figure 1).
Figure 1.
Kaplan–Meier survival curves of overall survival.
DISCUSSION
M-VAC is the most effective regimen as first-line treatment for transitional cell carcinoma of the urothelial tract but the role of M-VAC as second-line chemotherapy has never been studied although a variety of other agents have been investigated. Since GC chemotherapy was introduced as first-line chemotherapy for urothelial carcinoma, the role of the M-VAC regimen after GC remains to be defined. The results from our study showed that M-VAC chemotherapy was effective for urothelial carcinoma progression following first-line GC chemotherapy. M-VAC appears to be effective in patients who become less sensitive to the synergistic effects of gemcitabine and cisplatin.
In our study, the M-VAC regimen had a response rate of 30% in patients with disease progression after GC failure. There may be several explanations for this result. Cisplatin is one of the most potent chemotherapeutic agents for advanced transitional cell carcinoma and the failure of GC treatment does not imply a cisplatin-refractory status. Cisplatin may still be active in patients who failed prior GC chemotherapy. In addition, patients who fail the GC protocol may be sensitive to other potent agents such as methotrexate, doxorubicin and vinblastine. Our favourable results suggest that M-VAC is another option in patients with advanced transitional cell carcinoma who failed first-line GC treatment. On the other hand, we found a trend for a correlation between the response to the first-line GC treatment and the response to second-line M-VAC. Seven out of 16 patients (43.8%) who had responded to the prior GC therapy responded to the second-line M-VAC chemotherapy, while only 2 out of 14 (14.3%) who did not respond to GC therapy responded to M-VAC. These findings suggest the possibility that the response to the second-line M-VAC can be predicted from the response to the first-line GC chemotherapy. The difference of the response rates to second-line M-VAC between those who responded to GC and those who did not respond to GC treatment was not statistically significant but the absence of significance might have been due to the small number of patients studied. Additional trials are needed to confirm whether the response to first-line GC treatment can be a significant predictor of the response to second-line M-VAC.
Most patients enrolled in this study had good renal function and performance status. Our favourable results might have been affected by these factors. It is reported (de Wit, 2003) that an estimated one-third of patients with advanced urothelial carcinoma are medically unfit for cisplatin-based chemotherapy , and in accordance with this, in clinical practice, there is frequently a significant deterioration of the performance status or renal function in patients with advanced urothelial carcinoma; this is most frequently observed in patients with first-line treatment failure and disease progression. This makes enrolment of patients into clinical studies or the administration of systemic chemotherapy outside of the context of a clinical trial difficult (Bamias et al, 2006; Garcia and Dreicer, 2006; Pectasides et al, 2007). Substitution of new agents for cisplatin in two-drug or three-drug combinations is required in these patients. Carboplatin can be a good alternative to cisplatin because it is less nephrotoxic (de Wit, 2003). Phase II trials of carboplatin, as a substitution for cisplatin in first-line chemotherapy regimens, demonstrated that carboplatin-based therapy was less effective than cisplatin-based therapy. However, there is few data on carboplatin as a substitution for cisplatin-based therapy in second-line chemotherapy (Petrioli et al, 1996; Bellmunt et al, 1997). Randomised trials with carboplatin-based regimens in patients who failed first-line platinum-based chemotherapy and development of new combinations consisting of safer and more effective agents is needed for the treatment of patients with depleted marrow reserves and impaired renal function after the failure of first-line chemotherapy.
Over the past 20 years, a relatively large number of agents have been evaluated for second-line chemotherapy. The agents studied included ifosfamide, taxanes, gemcitabine, oxaliplatin, vinflunine and pemetrexed. However, a review of the recent literature, on phase II trials in this population, confirms that there are very limited options for patients who have been previously treated with GC, M-VAC or CMV combinations (Culine et al, 2006). Taxanes are widely used as a second-line regimen in patients with cisplatin-refractory urothelial carcinoma. The taxanes (paclitaxel and docetaxel) have provided objective response rates of 10–13% with response durations of 3.0–7.4 months (McCaffrey et al, 1997; Vaughn et al, 2002). Combinations with other agents have shown better results. Paclitaxel combined with gemcitabine, ifosfamide, methotrexate or cisplatin showed a 15–60% response rate and taxanes combined with ifosfamide a 15–25% response rate (Tu et al, 1995; McCaffrey et al, 1997; Witte et al, 1997; Sweeney et al, 1999; Krege et al, 2001; Meluch et al, 2001; Sternberg et al, 2001; Bellmunt et al, 2002; Vaughn et al, 2002). Ifosfamide showed a 20% response rate in an ECOG study but an unacceptable frequency of grade 3–4 CNS and renal toxicities were noted (Witte et al, 1997). Toxicity remains the major limiting aspect of these regimens (Roth et al, 1994; Dreicer et al, 1996; Witte et al, 1997).
Vinflunine and pemetrexed as new single agents have been studied in phase II trials (Culine et al, 2006; Sweeney et al, 2006). Vinflunine showed an 18% response rate and a 67% disease control rate and pemetrexed, a new-generation antifolate, showed a favourable therapeutic index (an overall response rate of 27.7%). Incorporation of these new agents as second-line treatment combination regimens is expected in future studies.
Several regimens studied as second-line chemotherapy for urothelial carcinoma have variable response rates. This is likely due to the variability in drug activity as well as the different patient populations evaluated in different studies (Sweeney et al, 2006). In most studies, the regimens used for first-line chemotherapy were heterogeneous in a given study population (Tu et al, 1995). Most studies on second-line chemotherapy include patients who received M-VAC-, CMV-, GC- or taxane-based regimens, as initial chemotherapy, or patients who did not receive any chemotherapy (Tu et al, 1995; McCaffrey et al, 1997; Witte et al, 1997; Sweeney et al, 1999; Krege et al, 2001; Meluch et al, 2001; Bellmunt et al, 2002; Vaughn et al, 2002; Culine et al, 2006). These confounding factors make it difficult to interpret the true efficacy of a second-line chemotherapy regimen. In our study, all patients received the same regimen as first-line chemotherapy.
In conclusion, this is the first report on the efficacy and toxicity of M-VAC as second-line chemotherapy in patients who failed first-line GC. The main limitation of this study was the small number of cases evaluated. M-VAC showed encouraging efficacy and reversible toxicities in patients who had disease progression after GC chemotherapy. Therefore, M-VAC appears to be a reasonable option for patients who initially responded to first-line GC chemotherapy. There are few randomised trials on second-line chemotherapy for urothelial carcinoma; therefore, large randomised controlled studies are required to confirm the findings reported here.
References
- Bamias A, Tiliakos I, Karali MD, Dimopoulos MA (2006) Systemic chemotherapy in inoperable or metastatic bladder cancer. Ann Oncol 17: 553–561 [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Cos J, Clèries R, Pérez M, Ribas A, Eres N, Murio JE, Margarit C, Baselga J (2002) Feasibility trial of methotrexate–paclitaxel as a second line therapy in advanced urothelial cancer. Cancer Invest 20: 673–685 [DOI] [PubMed] [Google Scholar]
- Bellmunt J, Ribas A, Eres N, Albanell J, Almanza C, Bermejo B, Sole LA, Baselga J (1997) Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 80: 1966–1972 [DOI] [PubMed] [Google Scholar]
- Culine S (2002) The present and future of combination chemotherapy in bladder cancer. Semin Oncol 29: 32–39 [DOI] [PubMed] [Google Scholar]
- Culine S, Theodore C, De Santis M, Bui B, Demkow T, Lorenz J, Rolland F, Delgado FM, Longerey B, James N (2006) A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer 94: 1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit R, European Organization for Research and Treatment (2003) Overview of bladder cancer trials in the European Organization for Research and Treatment. Cancer 97: 2120–2126 [DOI] [PubMed] [Google Scholar]
- Dreicer R, Gustin DM, See WA, Williams RD (1996) Paclitaxel in advanced urothelial carcinoma: its role in patients with renal insufficiency and as salvage therapy. J Urol 156: 1606–1608 [DOI] [PubMed] [Google Scholar]
- Garcia JA, Dreicer R (2006) Systemic chemotherapy for advanced bladder cancer: update and controversies. J Clin Oncol 24: 5545–5551 [DOI] [PubMed] [Google Scholar]
- Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, Kuzel T, Nicol S, Oh W, Stadler W (2000) Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol 18: 1921–1927 [DOI] [PubMed] [Google Scholar]
- Krege S, Rembrink V, Börgermann C, Otto T, Rübben H (2001) Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol 165: 67–71 [DOI] [PubMed] [Google Scholar]
- McCaffrey JA, Hilton S, Mazumdar M, Sadan S, Kelly WK, Scher HI, Bajorin DF (1997) Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15: 1853–1857 [DOI] [PubMed] [Google Scholar]
- Meluch AA, Greco FA, Burris III HA, O'Rourke T, Ortega G, Steis RG, Morrissey LH, Johnson V, Hainsworth JD (2001) Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie pearl cancer research network. J Clin Oncol 19: 3018–3024 [DOI] [PubMed] [Google Scholar]
- Moore MJ, Winquist EW, Murray N, Tannock IF, Huan S, Bennett K, Walsh W, Seymour L (1999) Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 17: 2876–2881 [DOI] [PubMed] [Google Scholar]
- Pectasides D, Pectasides E, Economopoulos T (2007) Systemic therapy in metastatic or recurrent endometrial cancer. Cancer Treat Rev 33: 177–190 [DOI] [PubMed] [Google Scholar]
- Petrioli R, Frediani B, Manganelli A, Barbanti G, De Capua B, De Lauretis A, Salvestrini F, Mondillo S, Francini G (1996) Comparison between a cisplatin-containing regimen and a carboplatin containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer 77: 344–351 [DOI] [PubMed] [Google Scholar]
- Roth BJ, Dreicer R, Einhorn LH, Neuberg D, Johnson DH, Smith JL, Hudes GR, Schultz SM, Loehrer PJ (1994) Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 12: 2264–2270 [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Yagoda A, Scher HI, Watson RC, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N, Weiselberg LR (1988) M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol 139: 461–469 [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Calabrò F, Pizzocaro G, Marini L, Schnetzer S, Sella A (2001) Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 92: 2993–2998 [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, Obasaju CK, Wang Y, Nicol SJ, Kaufman DS (2006) Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 24: 3451–3457 [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Williams SD, Finch DE, Bihrle R, Foster RS, Collins M, Fox S, Roth BJ (1999) A Phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer 86: 514–518 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) : New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Tu SM, Hossan E, Amato R, Kilbourn R, Logothetis CJ (1995) Paclitaxel, cisplatin and methotrexate combination chemotherapy is active in the treatment of refractory urothelial malignancies. J Urol 154: 1719–1722 [PubMed] [Google Scholar]
- Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB (2002) Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20: 937–940 [DOI] [PubMed] [Google Scholar]
- von der Maase H, Andersen L, Crinò L, Weinknecht S, Dogliotti L (1999) Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trial. Ann Oncol 10: 1461–1465 [DOI] [PubMed] [Google Scholar]
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18: 3068–3077 [DOI] [PubMed] [Google Scholar]
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23: 4602–4608 [DOI] [PubMed] [Google Scholar]
- Witte RS, Elson P, Bono B, Knop R, Richardson RR, Dreicer R, Loehrer PJ (1997) Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol 15: 589–593 [DOI] [PubMed] [Google Scholar]