Abstract
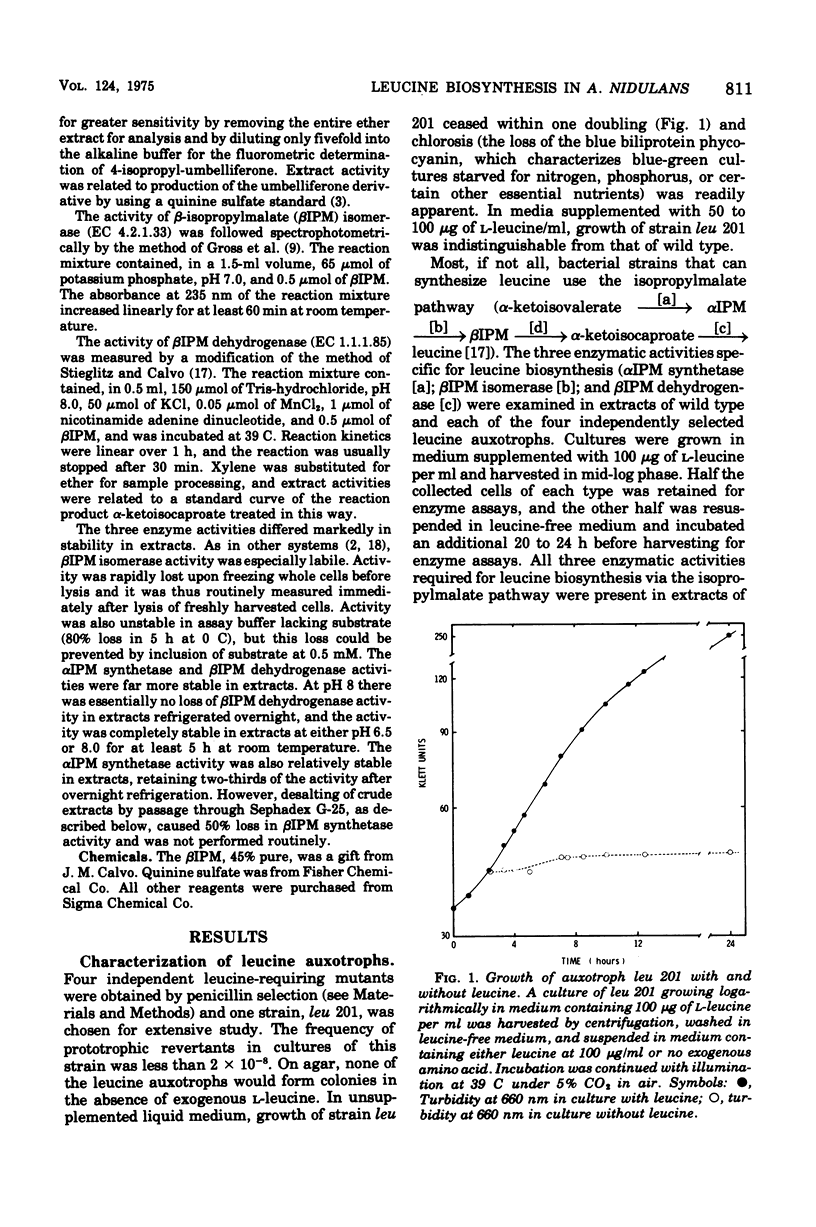
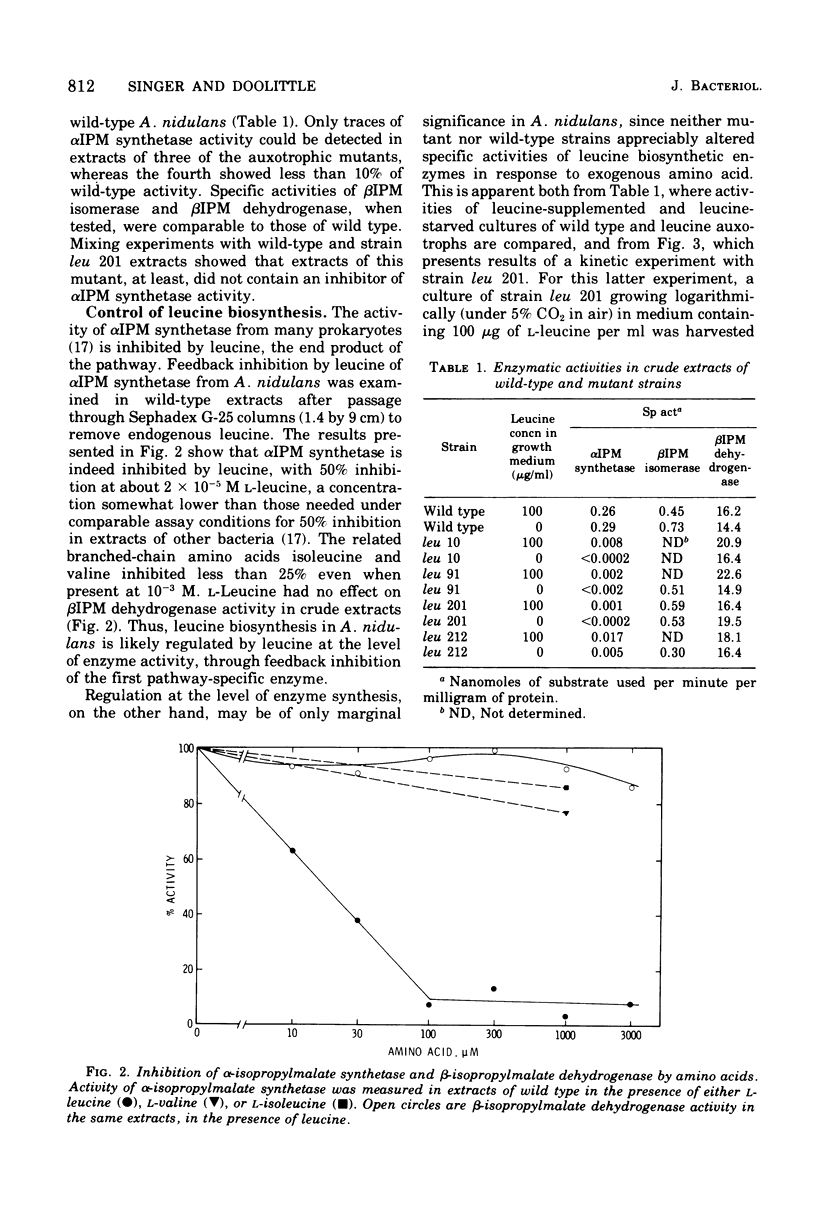
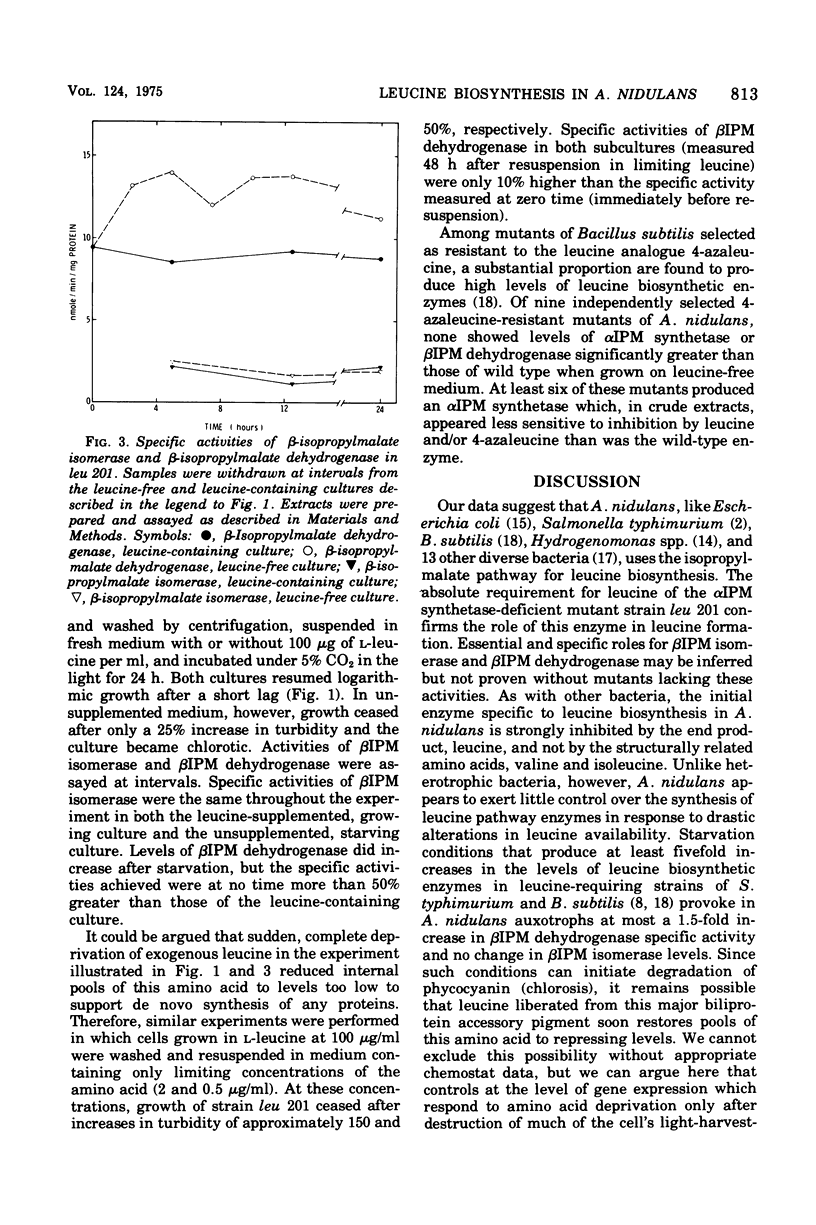
Leucine-requiring auxotrophs of the unicellular blue-green bacterium Anacystis nidulans have been isolated. Extracts of these mutants were deficient in alpha-isopropylmalate synthetase (EC 4.1.3.12). In wild-type cells, this enzyme was subject to feedback inhibition by leucine. However, formation of the enzymes of leucine biosynthesis was little affected by exogenous leucine in either wild-type or mutant strains. Cultures of the latter subjected to extreme leucine deprivation showed no change in specific activity of beta-isopropylmalate isomerase (EC 4.2.1.33) and at most a 50% increase in the specific activity of beta-isopropylmalate dehydrogenase (EC 1.1.1.85). These results are compared with others bearing on the evolution of the control of amino acid biosynthesis in blue-green bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Bartholomew J. C., Stieglitz B. I. Fluorometric assay of enzymatic reactions involving acetyl Coenzyme A in aldol condensations. Anal Biochem. 1969 Apr 4;28(1):164–181. doi: 10.1016/0003-2697(69)90168-7. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. Postmaturational cleavage of 23s ribosomal ribonucleic acid and its metabolic control in the blue-green alga Anacystis nidulans. J Bacteriol. 1973 Mar;113(3):1256–1263. doi: 10.1128/jb.113.3.1256-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Singer R. A. Mutational analysis of dark endogenous metabolism in the blue-green bacterium Anacystis nidulans. J Bacteriol. 1974 Sep;119(3):677–683. doi: 10.1128/jb.119.3.677-683.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Kaney A. R., Jhabvala P. Absence of repression in a phenylalanine auxotroph of Synechococcus cedrorum. J Gen Microbiol. 1975 Apr;87(2):370–372. doi: 10.1099/00221287-87-2-370. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz B. I., Calvo J. M. Distribution of the isopropylmalate pathway to leucine among diverse bacteria. J Bacteriol. 1974 Jun;118(3):935–941. doi: 10.1128/jb.118.3.935-941.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Jr, Zahler S. A. Regulation of leucine biosynthesis in Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):727–735. doi: 10.1128/jb.116.2.727-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


