Abstract
Purpose
To evaluate the prevalence and risk factors for vision loss in patients with clinical or immunologic AIDS without infectious retinitis.
Design
A prospective multicentered cohort study of patients with AIDS.
Methods
1,351 patients (2,671 eyes) at 19 clinical trials centers diagnosed with AIDS but without major ocular complications of HIV. Standardized measurements of visual acuity, automated perimetry, and contrast sensitivity were analyzed and correlated with measurements of patients’ health and medical data relating to HIV infection. We evaluated correlations between vision function testing and HIV-related risk factors and medical testing.
Results
There were significant (p<0.05) associations between measures of decreasing vision function and indices of increasing disease severity including Karnofsky score and hemoglobin. A significant relationship was seen between low contrast sensitivity and decreasing levels of CD4+ T-cell count. Three percent of eyes had a visual acuity worse than 20/40 Snellen equivalents, which was significantly associated with a history of opportunistic infections and low Karnofsky score.
When compared to external groups with normal vision, 39% of eyes had abnormal mean deviation on automated perimetry, 33% had abnormal pattern standard deviation, and 12% of eyes had low contrast sensitivity.
Conclusions
This study confirms that visual dysfunction is common in patients with AIDS but without retinitis. The most prevalent visual dysfunction is loss of visual field; nearly 40% of patients have some abnormal visual field. There is an association between general disease severity and less access to care and vision loss. The pathophysiology of this vision loss is unknown but is consistent with retinovascular disease or optic nerve disease.
Introduction
Vision loss in patients with Human Immunodeficiency Virus (HIV) disease is most devastating when it is due to opportunistic infections. Such infections include Cytomegalovirus (CMV) retinitis, herpes viral retinitis (necrotizing herpetic retinopathy), and other less common infections including toxoplasmosis, syphilis, and cryptococcosis. Several clinical studies have demonstrated visual dysfunction in HIV-positive patients without any infectious retinitis and with normal fundus. These visual dysfunctions include visual field loss as measured by changes in standard and short-wavelength sensitive perimetry,1 color and contrast sensitivity,2 3 4 electrophysiological parameters1 and topographic patterns of peripheral visual field loss.5 6 Such vision loss was initially shown in the pre-Highly Active Antiretroviral Therapy (HAART) era. Since the inception of HAART, many patients on these therapies have demonstrated an increase in CD4 T-lymphocyte count and a corresponding drop in viral load.7 There is a dramatic increase in longevity, but the long-term impact on vision loss is unknown.
In pilot studies, members of our group have explored visual dysfunction using standard achromatic perimetry (SAP) and frequency doubling technology (FDT) perimetry.4 8 9 10 11 12 This study expands on preliminary studies of contrast sensitivity4 to other measures of visual function using a larger population. In the era of HAART, a significant number of HIV-positive patients continue to demonstrate a singular pattern of visual function loss: that is peripheral visual field loss with preservation of the papillomacular bundle5 6 and that SAP loss occurs despite HAART therapy. Several studies have suggested that HIV associated vision loss is likely to be due, at least in part, to retinal dysfunction and not due to a generalized loss of cognitive function. These studies are consistent with the concept that the retinovascular disease including nerve fiber layer infarcts13 14 seen in HIV patients causes cumulative damage to the inner retina with resulting loss of neuronal elements. This is consistent with studies that have shown that a loss of optic nerve axons is demonstrable in HIV patients without CMV retinitis15 and that in vivo this loss can be documented using optical techniques such as confocal scanning laser topography16 and most recently by optical coherence tomography.16
The Studies of Ocular Complications of AIDS (SOCA) research group is conducting the Longitudinal Study of Ocular Complication of AIDS (LSOCA), which is a multicentered clinical observational study following large numbers of patients with AIDS longitudinally at multiple centers. Although the study design did not include experimental or uncommon visual function testing such as FDT, short wavelength perimetry, multifocal or pattern electroretinogram (ERG), it does include standardized Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity, Humphrey achromatic visual fields, Pelli-Robson contrast sensitivity measurements as well as measurements of quality of life.17 It has been previously suggested that vision loss in HIV-positive patients significantly correlates with several subscales of the National Eye Institute Visual Function Questionnaire (NEI VFQ) (Plummer DJ, Marcotte T, Sample PA, Heaton R, Grant I, Freeman WR. Relationship of visual field and neuropsychological disturbances due to HIV infection. Invest Ophthalmol Vis Sci (suppl.) 1996;37:373).18 19 20 21 We wished to evaluate the prevalence of vision loss in a large cohort of HIV-positive patients without infectious retinitis and to determine the factors associated with vision loss in a cross sectional analysis at study enrollment. The SOCA research group data affords a unique opportunity to investigate these issues.
Methods
LSOCA is a prospective, observational study designed to collect data on the incidence, prevalence, and complications due to AIDS-related ocular morbidities during the era of HAART. Patients must be diagnosed with AIDS as defined by the 1993 CDC diagnostic criteria for AIDS.21 Enrollment started in September 1998 and will continue to approximately 2,000 patients, with about 25% having a major ocular complication (MOC) at baseline. Details of the design, methods, and baseline results have been published elsewhere.21
At baseline and each follow-up visit, patients were given an ophthalmic eye examination which included dilated indirect ophthalmoscopy, medical history interview, treatment history interview, best-corrected visual acuity exam, contrast sensitivity exam, and a quality of life questionnaire. Laboratory tests also obtained at baseline and each follow-up visit included hematology, lymphocyte subset analysis, CMV and HIV viral load determinations. Patients with an MOC were seen every 3 months whereas patients without an MOC were seen every 6 months. Humphrey automated perimetry was performed at baseline and annually thereafter. Fundus photographs were obtained at baseline for all patients and at each follow-up visit for MOC patients.
Contrast sensitivity tests were performed by certified examiners during the visual acuity exam using the method of Pelli-Robson.22 23 Patients sat or stood 1 meter from the Pelli-Robson contrast sensitivity chart adding +0.75 diopter to best corrected vision. Patients read the chart until at least 2 letters in a triple were missed. Since contrast sensitivity is linear on a log scale, analyses use log10 contrast sensitivity score calculated as the sum of the total number of letters read correctly, subtracting 3 and multiplying by 0.05.23 Abnormal contrast sensitivity was calculated using the expected distribution from 106 10-year-old children with normal vision.24 Established norms of contrast sensitivity in adults allowed for letters confused as C or O to be considered as correct24 unlike the protocol used in both LSOCA and the study of 10-year-olds with normal vision. This would tend to make the contrast sensitivity distribution artifactually higher (2.5 percentile at 1.55 in adults vs. 1.50 using the 10-year olds). An analysis using the 1.55 cutpoint yielded qualitatively similar results.
Automated perimetry was performed using the Humphrey field analyzer (HFA) model 600 or 700 using the 24-2 program for full threshold testing according to the Humphrey Field Analyzer User’s Guide.25 Patient reliability was assessed and the point-by-point field data were summarized into two global indices, mean deviation and pattern standard deviation. Data were excluded if fixation losses > 20% or the false positive rate > 33% or the false negative rate > 33% as recommended by the manufacturer. 25
The visual field data for the healthy normal eyes came from a 14-year longitudinal study of visual function in glaucoma, the Diagnostic Innovations in Glaucoma Study (DIGS) at the Hamilton Glaucoma Center, University of California, San Diego. Control participants with normal vision for this study were recruited from the community, staff, and spouses or friends of patients. One eye from each of 123 participants with normal vision similarly aged to the patient participants was included. These data were used because the SOCA database did not include the printouts or electronic disks, which tell how each individual compares to the HFA normative database. To determine normal ranges for automated visual fields, the lower 2.5 percentile from the expected distribution of DIGS controls with normal vision (−2.63 dB) was used to define the cutoff for abnormal mean deviation. The upper 2.5 percentile from the expected distribution of DIGS controls with normal vision (2.57 dB) was used to define the cutoff for abnormal pattern standard deviation.
Because the distance at visual acuity lanes varied among the clinics, visual acuity measurements were standardized as the number of ETDRS letters read at 10 feet. Standardized ETDRS letters of 85, 70, and 50 are equal to 20/20, 20/40, and 20/100 Snellen equivalents, respectively. The expected distribution from 72 similarly aged (mean±SD = 35±15 years; range = 16 to 67 years) individuals with normal vision was calculated for comparison to the LSOCA patients.26
This cross-sectional analysis included data from the enrollment visit. Because of the learning effect of automated perimetry, data were also analyzed at the next visit one year later. Results were similar and therefore these data shown are only for the enrollment visit. Data obtained and keyed into the LSOCA database as of 31 May 2005 are included. Multiple logistic regression was used for binary outcomes. Eyes were the unit of analysis and generalized estimating equations were used to account for the correlation between eyes of the same patient. Eyes with a major ocular complication (typically CMV retinitis), glaucoma, or cataract were excluded from all analyses. Continuous covariates were categorized using clinically meaningful cutpoints where applicable or quartiles if there were no established cutpoints, and were modeled ordinally. Covariates were modeled both unadjusted (data not shown) and adjusted starting with the entire set of covariates and using regression with backward selection with removal if p>0.05. In the adjusted analysis, covariates with missing values were imputed with the most frequent category. P-values are two-sided and were not adjusted for multiple outcomes or multiple looks. Both SAS 8.0 (SAS Institute, Cam, North Carolina) and Stata 9.0 (State Corp College Station, Texas) were used to analyze the data.
Results
Data include 2,671 eyes with at least one non-missing outcome from 1,351 patients. Patient-specific characteristics are summarized in Table 1. Eighty-one percent of the patients are male, 52% are non-white, and the median age is 42 years. Half the patients have at least some college and 16% have no insurance. Measures of patients’ health include median hemoglobin of 13.7 g/dL and median Karnofsky score of 90. HIV-related characteristics include 56% of men having sex with men (MSM) only as their HIV risk factor, a median time since diagnosis of AIDS of 4.1 years, 78% having one or more opportunistic infections, and 80% currently taking HAART. Immunologic characteristics include a median CD4+ T-cell count of 180 cells/μL, median nadir CD4+ T-cell count of 42 cells/μL, and a median CD8+ T-cell count of 751 cells/μL. The median HIV viral load is 1,158 copies/mL and only 2% have detectable (> 400 copies/mL) CMV viral load. Measures of visual function in the better eye include medians of 91 letters (Snellen equivalent of 20/15+1) for visual acuity, −1.74 dB for mean deviation, 2.32 dB for pattern standard deviation, and 1.65 log units for contrast sensitivity.
Table 1.
Characteristics of patients with AIDS but with no major ocular complication, cataract or glaucoma
| %/median (quartiles) | N | |
|---|---|---|
| Demographics | ||
| Gender | ||
| % male | 80.8 | 1,338 |
| Race | 1,340 | |
| % white, non-Hispanic | 48.4 | |
| % black, non-Hispanic | 33.9 | |
| % Hispanic | 14.6 | |
| % other | 3.2 | |
| Age (yrs) - median (quartiles) | 42 (37, 47) | 1,329 |
| Education | 1,337 | |
| % < Grade 12 | 16.5 | |
| % Grade 12/high school graduate | 23.0 | |
| % Some college | 31.3 | |
| % College degree | 19.2 | |
| Insurance status | 1,339 | |
| % Uninsured | 16.2 | |
| % Medicaid | 12.4 | |
| % Medicare | 37.4 | |
| % Private* | 34.0 | |
| Cohort | 1,351 | |
| % enrolled 1 Sep 98 – 31 Dec 99 | 21.7 | |
| % enrolled 1 Jan 00 – 31 Dec 01 | 40.5 | |
| % enrolled 1 Jan 02 – 31 Mar 03 | 25.6 | |
| % enrolled 1 Apr 03 – 31 May 05 | 12.2 | |
| Health | ||
| Hemoglobin (g/dL) - median (quartiles) | 13.7 (12.4, 14.8) | 1,337 |
| Karnofsky - median (quartiles) | 90 (80, 90) | 1,335 |
| HIV-related | ||
| HIV risk factor | 1,340 | |
| % Men having sex with men only | 56.0 | |
| % IV drug user only | 9.6 | |
| % MSM and IDU | 4.0 | |
| % Heterosexual/other | 30.5 | |
| Time since AIDS diagnosis (yrs) - median (quartiles) | 4.1 (1.7, 6.9) | 1,308 |
| Opportunistic infections | ||
| % one or more | 78.0 | 1,340 |
| HAART status | ||
| % currently on HAART | 79.9 | 1,344 |
| Immunology and virology | ||
| CD4+ T cell count (cells/μL) - median (quartiles) | 180 (71, 328) | 1,330 |
| Nadir CD4+ T cell count (cells/μL) - median (quartiles) | 42 (13, 106) | 1,317 |
| CD8+ T cell count (cells/μL) - median (quartiles) | 751 (480, 1124) | 1,322 |
| HIV viral load (copies/mL) - median (quartiles) | 1518 (200, 56957) | 1,267 |
| CMV viral load (copies/mL) | ||
| % detectable (> 400) | 1.9 | 1,136 |
| Visual function in better eye | ||
| Visual acuity (standardized ETDRS letters) - median (quartiles) | 91 (87, 94)† | 1,347 |
| Mean deviation (dB) - median (quartiles) | −1.74 (−3.64, −0.38) | 1,336 |
| Pattern standard deviation (dB) - median (quartiles) | 2.32 (1.85, 3.48) | 1,336 |
| Contrast sensitivity (log cs) - median (quartiles) | 1.65 (1.65, 1.70) | 1,330 |
Includes Veteran’s Administration and Champus.
Snellen equivalent of 20/15+1 (20/20+2, 20/10-1).
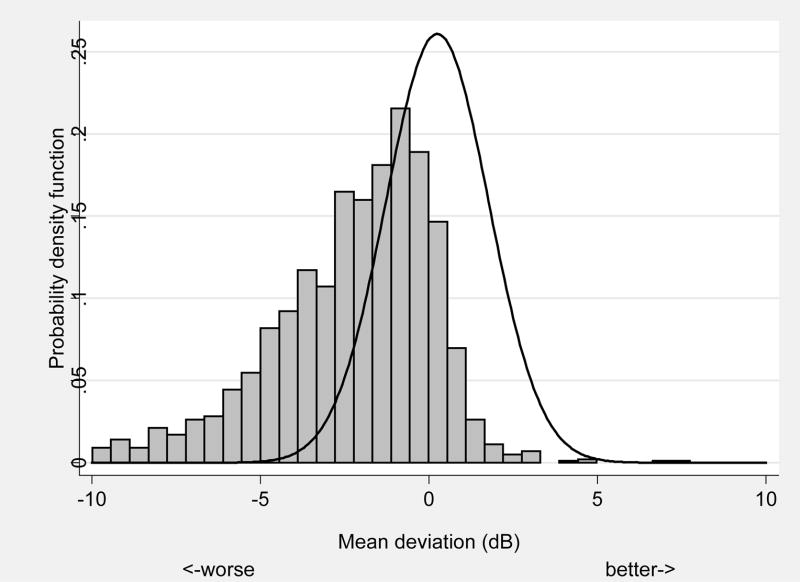
The results of significant associations between demographic, health, virologic and immunologic measures with abnormal mean deviation from automated perimetry are shown in Table 2. Approximately 39% of eyes had abnormal mean deviation defined as less than the 2.5 percentile from the expected distribution of DIGS controls with normal vision (Figure 1). Risk factors for low mean deviation include race (higher for blacks, Hispanics, and other race vs. whites; p=0.001), HIV risk factor (higher odds for IDU only, IDU and MSM, and other HIV risk factor vs. MSM only; p=0.0003), decreasing hemoglobin (p=0.005), and decreasing Karnofsky score (p<0.0001).
Table 2.
Vision function in HIV individuals without retinitis: significant* associations between demographic, health, virologic, and immunologic measures with abnormal mean deviation†
| No. eyes | Odds ratio of Abnormal MD (95% CI) | P | |
|---|---|---|---|
| Race | 0.001 | ||
| White, not Hispanic | 951 | 1.0 (reference) | |
| Black, not Hispanic | 546 | 1.4 (1.0 – 2.0) | |
| Hispanic | 277 | 1.9 (1.4 – 2.8) | |
| Other | 56 | 2.1 (1.0 – 4.5) | |
| HIV risk factor | 0.0003 | ||
| Men having sex with men (MSM) only | 1089 | 1.0 (reference) | |
| IV drug user (IDU) only | 156 | 2.2 (1.4 – 3.6) | |
| MSM and IDU | 67 | 1.5 (0.8 – 2.7) | |
| Other | 518 | 1.7 (1.3 – 2.3) | |
| Hgb (g/dL) | 0.005 | ||
| < 12.5 | 433 | 1.6 (1.1 – 2.4) | |
| 12.5 – 13.9 | 535 | 1.2 (0.9 – 1.7) | |
| 14.0 – 14.9 | 423 | 0.9 (0.6 – 1.3) | |
| 15.0+ | 439 | 1.0 (reference) | |
| Karnofsky score | <0.0001 | ||
| < 80 | 271 | 2.6 (1.6 – 4.0) | |
| 80 | 550 | 2.1 (1.4 – 3.1) | |
| 90 | 713 | 1.2 (0.8 – 1.8) | |
| 100 | 296 | 1.0 (reference) |
MD = mean deviation.
Based on backward stepwise logistic regression of 18 covariates (cohort, sex, race, age, education, insurance status, hemoglobin level, Karnofsky score, HIV risk factor, time since AIDS diagnosis, no. of OIs, HAART status, HIV viral load, CD4 count, nadir CD4 count, CD8 count, CMV viral load, and right vs. left eye) with probability of removal p>0.05.
Defined as less than 2.5 percentile derived from 123 similarly aged subjects with normal vision. Event rate is 39.0% (713/1830).
FIGURE 1.
Frequency distribution of mean deviation in Longitudinal Study of Ocular Complication of AIDS patients (n=1,336) (vertical bars) vs. expected distribution from similarly aged subjects with normal vision (n=123) (solid line).
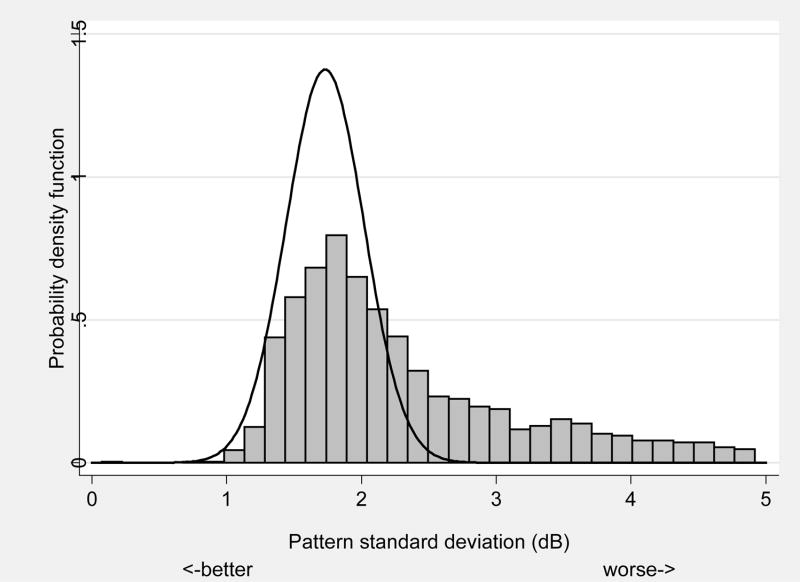
The results of significant associations between demographic, health, virologic and immunologic measures with abnormal pattern standard deviation from automated perimetry are shown in Table 3. Approximately 33% of eyes had abnormal pattern standard deviation defined as greater than the 2.5 percentile from the expected distribution of DIGS controls with normal vision (Figure 2). Risk factors for abnormal pattern standard deviation include increasing age (p=0.03), type of insurance (higher odds for uninsured, Medicare, and Medicaid vs. private; p=0.01), decreasing hemoglobin (p=0.03), and decreasing Karnofsky score (p=0.02).
Table 3.
Vision function in HIV individuals without retinitis: significant* associations between demographic, health, virologic, and immunologic measures with abnormal pattern standard deviation†
| No. eyes | Odds ratio of Abnormal PSD (95% CI) | P | |
|---|---|---|---|
| Age (yrs) | 0.03 | ||
| < 35 | 299 | 1.0 (reference) | |
| 35 – 42 | 654 | 1.0 (0.7 – 1.4) | |
| 43 – 49 | 575 | 1.1 (0.8 – 1.6) | |
| 50+ | 302 | 1.6 (1.1 – 2.5) | |
| Insurance type | 0.01 | ||
| Uninsured | 298 | 1.4 (1.0 – 2.1) | |
| Medicaid | 216 | 1.2 (0.8 – 1.8) | |
| Medicare | 639 | 1.6 (1.2 – 2.1) | |
| Private/VA/Champus | 677 | 1.0 (reference) | |
| Hgb (g/dL) | 0.03 | ||
| < 12.5 | 433 | 1.7 (1.2 – 2.4) | |
| 12.5 – 13.9 | 535 | 1.3 (0.9 – 1.8) | |
| 14.0 – 14.9 | 423 | 1.3 (0.9 – 1.8) | |
| 15.0+ | 439 | 1.0 (reference) | |
| Karnofsky score | 0.02 | ||
| < 80 | 271 | 1.6 (1.0 – 2.5) | |
| 80 | 550 | 1.5 (1.0 – 2.2) | |
| 90 | 713 | 1.0 (0.7 – 1.5) | |
| 100 | 296 | 1.0 (reference) |
PSD = pattern standard deviation.
Based on backward stepwise logistic regression of 18 covariates (cohort, sex, race, age, education, insurance status, hemoglobin level, Karnofsky score, HIV risk factor, time since AIDS diagnosis, no. of OIs, HAART status, HIV viral load, CD4 count, nadir CD4 count, CD8 count, CMV viral load, and right vs. left eye) with probability of removal p>0.05.
Defined as greater than 97.5 percentile derived from 123 similarly aged subjects with normal vision. Event rate is 33.4% (612/1830).
FIGURE 2.
Frequency distribution of pattern standard deviation in Longitudinal Study of Ocular Complication of AIDS patients (n=1,336) (vertical bars) vs. expected distribution from similarly aged subjects with normal vision (n=123) (solid line).
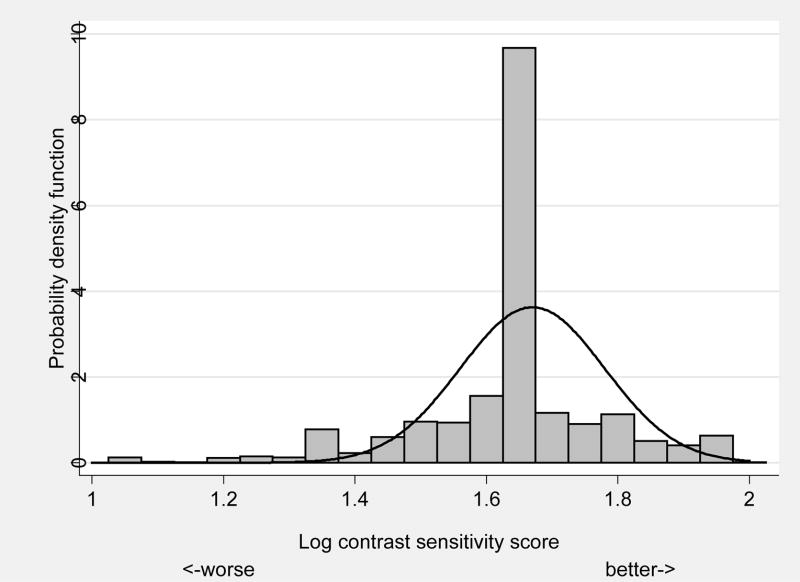
The results of significant associations between demographic, health, virologic and immunologic measures with abnormal contrast sensitivity are shown in Table 4. Approximately 12% of eyes had low contrast sensitivity defined as less than the 2.5 percentile from a distribution of 10-year-old children with normal vision (Figure 3). Risk factors for low contrast sensitivity include HIV risk factor (higher odds for IDU only, IDU and MSM, and other HIV risk factor vs. MSM only; p=0.006), decreasing education (p=0.001), and decreasing CD4+ T-cell count (p=0.02).
Table 4.
Vision function in HIV individuals without retinitis: significant* associations between demographic, health, virologic, and immunologic measures with abnormal contrast sensitivity†
| No. eyes | Odds ratio of Abnormal CS (95% CI) | P | |
|---|---|---|---|
| HIV risk factor | 0.004 | ||
| MSM only | 1469 | 1.0 (reference) | |
| IDU only | 251 | 2.0 (1.2 – 3.3) | |
| MSM and IDU | 103 | 1.6 (0.7 – 3.5) | |
| Other | 801 | 1.9 (1.3 – 2.8) | |
| Education | 0.001 | ||
| < Grade 12 | 427 | 2.3 (1.3 – 3.8) | |
| Grade 12/HS grad | 601 | 1.9 (1.2 – 3.1) | |
| Some college | 839 | 1.5 (0.9 – 2.4) | |
| College grad | 757 | 1.0 (reference) | |
| CD4+ T cell count (cells/μL) | 0.02 | ||
| < 50 | 522 | 1.5 (1.0 – 2.4) | |
| 50–99 | 306 | 1.5 (0.9 – 2.6) | |
| 100–199 | 570 | 1.0 (0.6 – 1.6) | |
| 200–349 | 642 | 1.0 (0.6 – 1.6) | |
| 350+ | 584 | 1.0 (reference) |
CS = contrast sensitivity.
Based on backward stepwise logistic regression of 18 covariates (cohort, sex, race, age, education, insurance status, hemoglobin level, Karnofsky score, HIV risk factor, time since AIDS diagnosis, no. of OIs, HAART status, HIV viral load, CD4 count, nadir CD4 count, CD8 count, CMV viral load, and right vs. left eye) with probability of removal p>0.05.
Defined as less than 2.5 percentile derived from 106 10-year old children with normal vision. Event rate is 11.6% (304/2624).
FIGURE 3.
Frequency distribution of contrast sensitivity in Longitudinal Study of Ocular Complication of AIDS patients (n=1,330) (vertical bars) vs. expected distribution from 10-year-old children with normal vision (n=106) (solid line).
The relationship between CD4+ T-cell count and contrast sensitivity was further explored in Table 6. The mean contrast sensitivity decreases with decreasing CD4+ T-cell count both crudely (p=0.001) but less significantly so (p=0.07) when adjusted for visual acuity (although the adjusted effect may be misleading if decreased contrast sensitivity and decreased visual acuity are part of the same pathological process). The relationship between CD4+ T-cell count and mean contrast sensitivity is among eyes with visual acuity 20/20 Snellen or better (p=0.02) whereas it is not seen in eyes with visual acuity less than 20/20 Snellen (p=0.90). In addition, there is also a strong significant relationship of CD4+ T-cell count with the left tail of the distribution of contrast sensitivity using cutpoints for abnormal of <1.50, <1.45, and <1.40 log contrast sensitivity score; but less so for <1.35 and <1.30 log contrast sensitivity score.
Table 6.
Vision function in HIV individuals without retinitis: contrast sensitivity by categories of CD4+ T-cell count
| CD4+ T-cell count (cells/μL)
|
||||||
|---|---|---|---|---|---|---|
| <50 | 50–99 | 100–199 | 200–349 | 350+ | P* | |
| Overall | ||||||
| Mean (log cs) | 1.61 | 1.61 | 1.63 | 1.64 | 1.64 | 0.001 |
| Mean adjusted for visual acuity | 1.62 | 1.62 | 1.63 | 1.63 | 1.64 | 0.07 |
| No. eyes | 522 | 306 | 570 | 642 | 584 | |
| Visual acuity 20/20 Snellen or better | ||||||
| Mean (log cs) | 1.64 | 1.64 | 1.66 | 1.67 | 1.66 | 0.02 |
| No. eyes | 400 | 245 | 461 | 535 | 490 | |
| Visual acuity worse than 20/20 Snellen | ||||||
| Mean (log cs) | 1.52 | 1.45 | 1.49 | 1.51 | 1.51 | 0.90 |
| No. eyes | 122 | 60 | 109 | 107 | 94 | |
| Cutpoints (log cs) | ||||||
| % < 1.50 | 14.9 | 15.0 | 10.5 | 10.1 | 9.4 | 0.006 |
| % < 1.45 | 11.9 | 12.1 | 7.9 | 7.3 | 6.0 | 0.001 |
| % < 1.40 | 10.5 | 10.8 | 6.8 | 6.5 | 4.8 | 0.001 |
| % < 1.35 | 4.4 | 4.6 | 3.9 | 3.4 | 2.6 | 0.11 |
| % < 1.30 | 3.4 | 4.2 | 3.5 | 2.5 | 2.2 | 0.13 |
Based on Wald test from generalized linear model with robust variance estimation.
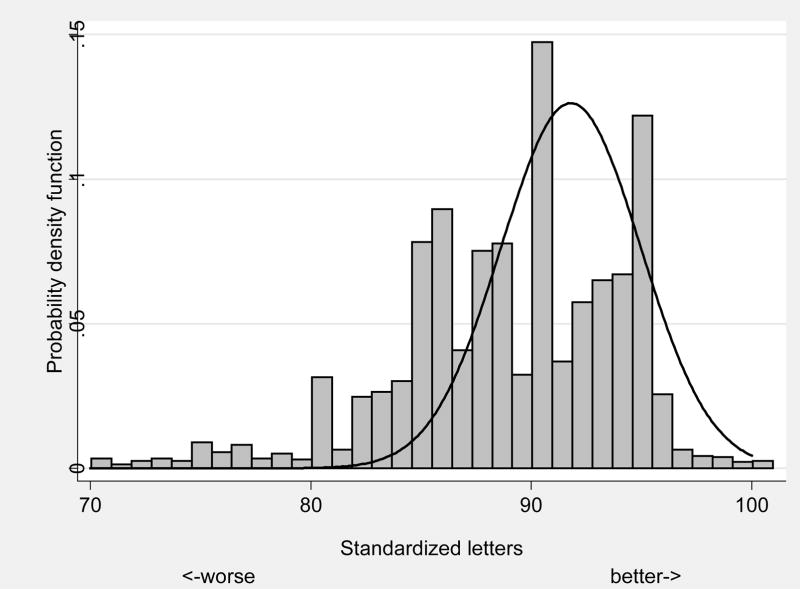
The distribution of visual acuity in the LSOCA population vs. the expected distribution from a group of similarly aged participants with normal vision is shown in Figure 4. The results of significant associations between demographic, health, virologic and immunologic measures with impaired visual acuity, defined as worse than 20/40 Snellen equivalents are shown in Table 5. Approximately 3% of eyes had impaired visual acuity. Risk factors for impaired visual acuity include no vs. any history of opportunistic infections (p<0.001) and decreasing Karnofsky score (p=0.001). There were no significant risk factors for legal blindness, defined as visual acuity worse than 20/100 Snellen equivalents.
FIGURE 4.
Frequency distribution of visual acuity in Longitudinal Study of Ocular Complication of AIDS patients (n=1,347) (vertical bars) vs. expected distribution from similarly aged subjects with normal vision (n=72) (solid line).
Table 5.
Vision function in HIV individuals without retinitis: significant* associations between demographic, health, virologic, and immunologic measures with impaired visual acuity† (worse than 20/40 Snellen)
| No. eyes | Odds ratio of Impaired VA (95% CI) | P | |
|---|---|---|---|
| Number of opportunistic infections | <0.001 | ||
| 0 | 582 | 2.9 (1.7 – 4.9) | |
| 1+ | 2084 | 1.0 (reference) | |
| Karnofsky score | 0.001 | ||
| < 80 | 431 | 5.0 (1.9 – 13.3) | |
| 80 | 805 | 2.2 (0.9 – 5.6) | |
| 90 | 1033 | 2.0 (0.8 – 4.8) | |
| 100 | 397 | 1.0 (reference) |
Based on backward stepwise logistic regression of 18 covariates (cohort, sex, race, age, education, insurance status, hemoglobin level, Karnofsky score, HIV risk factor, time since AIDS diagnosis, no. of OIs, HAART status, HIV viral load, CD4 count, nadir CD4 count, CD8 count, CMV viral load, and right vs. left eye) with probability of removal p>0.05.
Event rate is 2.9% (76/2666).
Dysfunction in one measure of visual function is highly associated with dysfunction in another. The relative odds of abnormal mean deviation is 9.6 (95% CI=7.4–12.3), 3.7 (95% CI=2.6–5.3), and 3.1 (95% CI=2.4–4.1) times higher in eyes with abnormal pattern standard deviation, abnormal contrast sensitivity, and visual acuity worse than 20/40, respectively. The relative odds of abnormal pattern standard deviation is 1.9 (95% CI=1.3–2.7) and 2.1 (95% CI=1.6–2.8) times higher in eyes with abnormal contrast sensitivity and visual acuity worse than 20/40, respectively. And finally, the relative odds of abnormal contrast sensitivity is 5.4 (95% CI=4.0–7.1) times higher in eyes with visual acuity worse than 20/40.
Discussion
The present large multicentered observational cohort study of HIV-positive patients who do not have cytomegalovirus retinitis has demonstrated that visual impairments are commonly found. The most prevalent visual dysfunction is loss of visual field. We measured visual field using achromatic Humphrey automated perimetry. Using this method, 39% of our HIV patients without CMV retinitis were below the 2.5 percentile of a normal population in terms of the Humphrey pattern standard deviation. In addition, 33% of the eyes from LSOCA patients were above the 97.5 percentile of a normal population with respect to Humphrey pattern standard deviation. The LSOCA population is probably not representative of patients living with AIDS in the United States with respect to eye problems since most patients were enrolled after referral to study ophthalmologists. Nonetheless, given that nearly 40% of eyes in LSOCA patients have some abnormal visual field suggests there is a substantial proportion of patients living with AIDS in the United States with visual field loss. The distribution curve of visual field performance in HIV patients is skewed to the left for the mean Humphrey deviation and pattern standard deviation leaving only a relatively small percentage of HIV patients in the upper half of the normal range.
A major limitation of these data is the lack of comparison to an internal group of similarly aged patients without HIV. Typically when control data are not collected, comparisons are made to normative data from a large national survey such as National Health and Nutrition Examination Surveys (NHANES) - but these data were not available. Because we thought it important to put the visual function of patients with AIDS in context to normals, we used external sources in which the procedures used to collect the visual function data were identical to LSOCA. Although the exact magnitude of the visual function abnormality may be incorrect because of the choice of the comparison group, clearly there is a large percentage of patients with AIDS with reduced visual function.
In the present study, we found certain associations, or risk factors, for visual field loss including minority status, intravenous drug use, anemia and lower Karnofsky scores (for mean deviation) and lack of private health insurance, which were risk factors in addition to the above for decreased pattern standard deviation. One could interpret these risk factors as a general index of disease severity or less access to care for HIV disease. Unfortunately, we do not have access to the entire HIV history of our patient population. Such data are difficult to procure. It would be interesting to know if laboratory indices of disease severity changed over time, and whether such indices, such as duration of low CD4 count or high viral load predict visual dysfunction. Unfortunately, our data set did not lend itself to such analyses.
Contrast sensitivity functions (CSF) were also abnormal in our patients. In 12% of the eyes from HIV-positive but retinitis-free patients, contrast was below the 2.5 percentile. However, CSFs were not seriously impaired. Similarly, Snellen acuity was below 20/40 in only 3% of these eyes. The prevalence of contrast sensitivity loss was comparable to the 7% found in a smaller population that included some patients from the current study.4 Taken as a whole, the modest losses of CSF and visual acuity are not so surprising. Diffuse damage in the retina or anterior visual pathways can, if not severe, be compensated for at the level of the visual cortex through various processing paradigms. The loss of contrast sensitivity and visual acuity are both likely to be pathophysiologically caused by the same process and statistically are thus closely related. On the other hand, visual field defects are less likely to be amenable to cortical compensation as retinopy precludes some cortical associations. Interestingly, for measures of central vision, systemic disease severity measures were also predictive. Intravenous drug use and lower education and low Karnofsky scores were predictive of decreased contrast sensitivity. More opportunistic infections and lower Karnofsky scores were predictive of decreased Snellen vision.
The anatomical site of damage to the anterior visual pathways cannot be determined from this study. The pattern of vision loss is consistent with inner retinal damage possibly from microinfarctions and retinal vascular loss. These have been well documented in this population, particularly those who have been most ill. However, other forms of optic nerve or retinal disease could also contribute to the visual impairments.27 28 29 30 Central nervous system dysfunction is also present in HIV patients and may be due to nonspecific HIV cytokine induced disease as well as opportunistic infection.
Our group has previously demonstrated that a primary optic neuropathy associated with cytokine expression is seen in HIV-positive patients in the absence of retinitis.31 Furthermore, cytokines, especially tumor necrosis factor (TNF) can replicate a similar optic neuropathy in an animal model.29 Finally, inhibition of TNF expression can prevent the optic neuropathy in this TNF model of optic neuropathy.30 Other studies from our group have described a similar optic neuropathy in HIV-positive patients without CMV retinitis.31
Clearly there is visual dysfunction in HIV patients without retinitis.32 The cause of this cannot be determined and immunosuppression and systemic diseases appear to only partially explain the vision loss. Further studies to analyze the association between vision dysfunction and central nervous system disease would be helpful in understanding our findings as would structural studies of the retina such as nerve fiber layer analysis and retinal imaging. Such studies have preliminarily suggested diffuse inner retinal damage is present. Electrophysiology, as well as imaging studies such as spectral OCT, could also help separate retinal from other sites of disease.33 Whatever the pathophysiology, it is possible that if the disorder is progressive, vision loss will become more prevalent and symptomatic with time as HIV patients live longer.
Acknowledgments
Funding/Support: LSOCA grant support include the following: Cooperative agreements from the National Eye Institute to The Johns Hopkins University School of Medicine (U10 EY 08052), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 08057), and the University of Wisconsin, Madison School of Medicine (U10 EY 08067).
Additional support provided by National Center for Research Resources through General Clinical Research Center grants:
5M01 RR 00350 (Baylor College of Medicine)
5M01 RR 05096 (LSU/Tulane/Charity Hospital)
5M01 RR00096 (New York University Medical Center, New York)
5M01 RR 00865 (University of California, Los Angeles)
5M01 RR00046 (University of North Carolina)
5M01 RR00043 (University of Southern California)
5M01 RR00047 (Weill Medical College of Cornell University)
Support also provided through cooperative agreements:
U01 AI 27674 (Louisiana State University/Tulane)
U01 AI 27660 (University of California, Los Angeles)
U01 AI 27670 (University of California, San Diego)
U01 AI 27663 (University of California, San Francisco)
U01 AI25868 (University of North Carolina)
U01 AI32783 (University of Pennsylvania)
Support also provided by RPB inc, New York; Dr. Freeman is the recipient of an RPB Physician Scientist award.
Other Acknowledgments: Participating clinical centers can be found in a prior printed publication.34
Biography
William R. Freeman, MD, Professor of Ophthalmology and Director of the UCSD Jacobs Retina Center, La Jolla, California. Dr. Freeman is a vitreoretinal surgeon with a strong interest in HIV disease, retinal imaging, and macular degeneration. He has published over 400 peer-reviewed papers on retinal diseases. He is on the editorial board of Retina and Ophthalmology and is Principal Investigator on two National Eye Institute clinical trials grants and on one laboratory research grant.

Footnotes
Financial Disclosures: None of the members of the study group have any financial disclosures related to the study.
Contributions to Authors in each of these areas: Design of the study (WRF, GNH); Conduct of the study (WRF, MLVN, DJ, PAS, AAS, JT, KHS, GNH); Collection of the data (WRF, MLVN, DJ, PAS, AAS, JT, KHS, GNH); Management of the data (MLVN); Analysis of the data (MLVN, DJ, PAS, AAS, JT, KHS); Interpretation of the data (MLVN, DJ, PAS, AAS, JT, KHS); Preparation of the manuscript (WRF, MLVN, DJ, PAS, AAS, JT, KHS, GNH); Review of the manuscript (WRF, MLVN, DJ, PAS, AAS, JT, KHS, GNH); Approval of the manuscript (MLVN, GNH, DJ, PAS, AAS, JT, KHS).
Statement about Conformity with Author Information: All authors, including William R. Freeman, Mark L. Van Natta, Douglas Jabs, Pamela A. Sample, Alfredo A. Sadun, Jennifer Thorne, Kayur H. Shah, and Gary N. Holland, confirm that the study and data accumulation were carried out with approval from the appropriate Institutional Review Board (IRB), informed consent was obtained from the patients, and the study is in accordance with HIPAA regulations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iragui VJ, Kalmijn J, Plummer D, Sample PA, Trick GL, Freeman WR. Pattern electroretinograms and visual evoked potentials in HIV infection: evidence of asymptomatic retinal and postretinal impairment in the absence of infectious retinopathy. Neurology. 1996;47:1452–1456. doi: 10.1212/wnl.47.6.1452. [DOI] [PubMed] [Google Scholar]
- 2.Mueller AJ, Plummer DJ, Dua R, et al. Analysis of visual dysfunctions in HIV-positive patients without retinitis. Am J Ophthalmol. 1997;124:158–167. doi: 10.1016/s0002-9394(14)70780-9. [DOI] [PubMed] [Google Scholar]
- 3.Quiceno JI, Capparelli E, Sadun AA, et al. Visual dysfunction without retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:8–13. doi: 10.1016/s0002-9394(14)75745-9. [DOI] [PubMed] [Google Scholar]
- 4.Shah KH, Holland GN, Yu F, Van Natta M, Nusinowitz S in collaboration with the Studies of the Ocular Complications of AIDS Research Group. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol. 2006;142:284–292. doi: 10.1016/j.ajo.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Sample PA, Plummer DJ, Mueller AJ, et al. Pattern of early visual field loss in HIV-infected patients. Arch Ophthalmol. 1999;117(6):755–760. doi: 10.1001/archopht.117.6.755. [DOI] [PubMed] [Google Scholar]
- 6.Plummer DJ, Marcotte TD, Sample PA, et al. Neuropsychological impairment-associated visual field deficits in HIV infection. HNRC Group. HIV Neurobehavioral Research Center. Invest Ophthalmol Vis Sci. 1999;40:435–442. [PubMed] [Google Scholar]
- 7.Perrin L, Telenti A. HIV treatment failure: testing for HIV resistance in clinical practice. Science. 1998;280:1871–1873. doi: 10.1126/science.280.5371.1871. [DOI] [PubMed] [Google Scholar]
- 8.Shapley R, Kaplan E, Soodak R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature. 1981;292:543–545. doi: 10.1038/292543a0. [DOI] [PubMed] [Google Scholar]
- 9.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 10.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–491. [PubMed] [Google Scholar]
- 11.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38:413–425. [PubMed] [Google Scholar]
- 13.Freeman WR, Chen A, Henderly DE, et al. Prevalence and significance of acquired immunodeficiency syndrome-related retinal microvasculopathy. Am J Ophthalmol. 1989;107:229–235. doi: 10.1016/0002-9394(89)90304-8. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Green WR, Fox R, et al. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology. 1989;96:1092–1099. doi: 10.1016/s0161-6420(89)32794-1. [DOI] [PubMed] [Google Scholar]
- 15.Tenhula WN, Xu SZ, Madigan MC, et al. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- 16.Kozak I, Bartsch DU, Cheng L, Kosobucki BR, Freeman WR. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. 2005;139:295–301. doi: 10.1016/j.ajo.2004.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire. Results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 18.Plummer DJ, Sample PA, Arévalo JF, et al. Visual field loss in HIV-positive patients without infectious retinopathy. Am J Ophthalmol. 1996;122:542–549. doi: 10.1016/s0002-9394(14)72115-4. [DOI] [PubMed] [Google Scholar]
- 19.Geier SA, Hammel G, Bogner JR, Kronawitter U, Berninger T, Goebel FD. HIV-related ocular microangiopathic syndrome and color contrast sensitivity. Invest Ophthalmol Vis Sci. 1994;35:3011–3021. [PubMed] [Google Scholar]
- 20.Geier SA, Nöhmeier C, Lachenmayr BJ, Klauss V, Goebel FD. Deficits in perimetric performance in patients with symptomatic human immunodeficiency virus infection or acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119:335–344. doi: 10.1016/s0002-9394(14)71177-8. [DOI] [PubMed] [Google Scholar]
- 21.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD for the Studies of the Ocular Complications of AIDS Research Group. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Lovie-Kitchin JE, Brown B. Repeatability and intercorrelations of standard vision tests as a function of age. Optom Vis Sci. 2000;77:412–420. doi: 10.1097/00006324-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Elliott DB, Bullimore MA, Baily IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vis Sci. 1991;6:471–475. [Google Scholar]
- 24.Myers VS, Gidlewski N, Quinn GE, Miller D, Dobson V. Distance and near visual acuity, contrast sensitivity, and visual fields of 10-year-old children. Arch Ophthalmol. 1999;117:94–99. doi: 10.1001/archopht.117.1.94. [DOI] [PubMed] [Google Scholar]
- 25.Choplin NT, Edwards RP. Visual field test with the Humphrey field analyzer. New Jersey: Slack, Inc.; 1995. pp. 1–256. [Google Scholar]
- 26.Brown B, Yap MK. Differences in visual acuity between eyes: determination of normal limits in a clinical population. Ophthalmic Physiol Opt. 1995;15:163–169. doi: 10.1046/j.1475-1313.1995.9590568m.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin XH, Kashima Y, Khan M, Heller KB, Gu XZ, Sadun AA. An immunohistochemical study of TNF-alpha in optic nerves from AIDS patients. Curr Eye Res. 1997;16:1064–1068. doi: 10.1076/ceyr.16.10.1064.9017. [DOI] [PubMed] [Google Scholar]
- 28.Saadati HG, Khan IA, Lin XH, Kadakia AB, Heller KB, Sadun AA. Immunolocalization of IL-1beta and IL-6 in optic nerves of patients with AIDS. Curr Eye Res. 1999;19:264–268. doi: 10.1076/ceyr.19.3.264.5319. [DOI] [PubMed] [Google Scholar]
- 29.Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol Res. 1996;18:176–184. doi: 10.1080/01616412.1996.11740399. [DOI] [PubMed] [Google Scholar]
- 30.Petrovich MS, Hsu HY, Gu X, Dugal P, Heller KB, Sadun AA. Pentoxifylline suppression of TNF-alpha mediated axonal degeneration in the rabbit optic nerve. Neurol Res. 1997;19:551–554. doi: 10.1080/01616412.1997.11740856. [DOI] [PubMed] [Google Scholar]
- 31.Tenhula WN, Xu SZ, Madigan MC, Heller K, Freeman WR, Sadun AA. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- 32.Quiceno JI, Capparelli E, Sadun AA, et al. Visual dysfunction without retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:8–13. doi: 10.1016/s0002-9394(14)75745-9. [DOI] [PubMed] [Google Scholar]
- 33.Sadun AA, Pepose JS, Madigan MC, Laycock KA, Tenhula WN, Freeman WR. AIDS-related optic neuropathy: a histological, virological and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233:387–398. doi: 10.1007/BF00180941. [DOI] [PubMed] [Google Scholar]
- 34.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD for the Studies of the Ocular Complications of AIDS Research Group. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114:780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]