Abstract
PURPOSE
To evaluate the effect of two-dimensional wavelet-based computed tomographic (CT) image compression according to the Joint Photographic Experts Group (JPEG) 2000 standard on computer-assisted assessment of nodule volume.
MATERIALS AND METHODS
This HIPAA-compliant study was approved by the research board at the authors’ institution; patients’ informed consent was not required. Fifty-one nodules in 23 patients (seven men, 16 women; mean age, 59 years; age range, 39–75 years) were selected on low-dose CT scans that were compressed to levels of 10:1, 20:1, 30:1, and 40:1 by using a two-dimensional JPEG 2000 wavelet-based image compression method. Nodules were classified according to size (≤5 mm or >5 mm in diameter), location (central, peripheral, or abutting pleura or fissures), and attenuation (solid, calcified, or subsolid). Regions of interest were placed on the original images and transposed onto compressed images. Nodule volumes on original (noncompressed) and compressed images were measured by using a computer-assisted method. A mixed-model analysis of variance was conducted for statistical evaluation.
RESULTS
Nodule volumes averaged 388.1 mm3 (range, 34–3474 mm3). There were three calcified, 33 solid noncalcified, and 15 subsolid nodules (13 with ground-glass attenuation). Average volume decreased with increasing compression level, to 383 mm3 (10:1), 370 mm3 (20:1), 360 mm3 (30:1), and 354 mm3 (40:1). No significant difference was identified between measurements obtained on original images and those compressed to a level of 10:1. Significant differences were noted, however, between original images and those compressed to a level of 20:1 or greater (P < .05). Compression level significantly interacted with nodule size, location, and attenuation (P < .001). The effect of compression was greater for nodules with ground-glass attenuation than for those with higher attenuation values. The difference in mean volumes between original images and those compressed to a level of 20:1 was 34.9 mm3 for nodules with ground-glass attenuation, compared with 8.3 mm3 for higher-attenuation nodules, a 4.2-fold difference.
CONCLUSION
Nodule volumes measured on images compressed to a level of 20:1 differed significantly from those measured on noncompressed images, especially for nodules with ground-glass attenuation. This difference could affect the assessment of nodule change in size as measured with computer-assisted methods.
Recent progress in radiologic imaging technology and image analysis has enabled assessment of the volume of small pulmonary nodules. Images of contiguous sections with a thickness of 1 mm through the entire thorax can be obtained routinely in 10 or 20 seconds by using a multi–detector row computed tomographic (CT) scanner with 16 or four detector rows, respectively. The use of volumetric thin-section CT was shown to improve nodule detection by human readers (1). More important, the use of high-spatial-resolution imaging has been shown to decrease error in computer-assisted methods for nodule volume measurement (2). Some investigators therefore have advocated the monitoring of nodule volumes instead of unidimensional measurements, which are used in the Response Evaluation Criteria in Solid Tumors guidelines, for the assessment of tumor response or progression in oncology patients (3).
While the ability to obtain contiguous 1-mm sections is potentially beneficial, the reconstructed thin-section images from a chest CT examination result in a CT data set of approximately 400 images or 200 MB, thus proving a challenge for data archival systems and teleradiology (4). For this reason, the use of high-performance lossy two-dimensional compression methods and, to a much lesser degree, lossy three-dimensional compression methods has been proposed to decrease the volume of data that must be archived and to reduce transmission times by more than an order of magnitude. Investigators in prior studies have primarily evaluated the effect of image compression on diagnostic image interpretation for chest radiography (5,6) and chest and abdominal CT (7–9). Few studies have been directed toward the effect of image compression on quantitative analysis. To our knowledge, the effect of CT image compression on nodule volume measurement has not been assessed previously. Given the increasing availability of a Joint Photographic Experts Group (JPEG) wavelet-based compression method that was proposed as a compression standard (ie, JPEG 2000), the objective of our study was to evaluate the effect of a two-dimensional JPEG 2000 wavelet-based image compression method on computer-assisted assessment of nodule volume.
MATERIALS AND METHODS
Because of the recent interest in screening and in decreasing the radiation dose associated with chest CT, we chose to restrict our study to CT image data from patients who underwent screening CT at our medical center after March 2000. Reports of the screening CT studies were reviewed, and 51 nodules were selected from 23 low-dose chest CT studies from 23 patients by a chest radiologist with 5 years of experience (J.P.K.). The patients were seven men and 16 women with a mean age of 59 years (age range, 39–75 years). Nodules were included in the study if their maximal diameter was larger than 2 mm. Nodules were specifically selected to be equally distributed throughout the thorax, including the upper part (above the inferior aspect of the aortic arch), middle part (from below the aortic arch to the right inferior pulmonary vein), and lower part of the thorax (below the right inferior pulmonary vein). The nodules were also selected so that subsolid nodules would constitute approximately one-third of the sample, as these commonly observed lesions may be particularly susceptible to artifacts related to image compression. CT images were obtained by using a multi–detector row CT scanner with four detector rows (Volume Zoom; Siemens Medical Solutions, Iselin, NJ) and a low-dose technique with 120 kVp, 20–40 mAs, 0.5-second rotation time, 512 × 512 reconstructed image matrix, and high-frequency reconstruction algorithm. The study was approved by the research board at our institution; patients’ informed consent was not required. The study complied with the Health Insurance Portability and Accountability Act.
Nodule Characteristics
The attenuation, location, and size of each nodule were assessed and recorded by the same radiologist who selected the cases. Nodules were classified according to their attenuation characteristics as solid, calcified, or subsolid. Subsolid nodules included nodules with only ground-glass attenuation, as well as those with mixed ground-glass and solid attenuation components. The nodule location was categorized as peripheral if it was within the peripheral one-third of a lobe and as central if it was not. Nodules that abutted the pleura, including those adjacent to fissures, were noted. Nodule size was determined on the basis of the largest cross-sectional dimension (diameter) measured by using the electronic calipers on an image workstation (Wizard; Siemens Medical Systems, Iselin, NJ). Nodules were categorized into two groups: those with a diameter less than or equal to 5 mm (smaller nodules) and those with a diameter greater than 5 mm (larger nodules).
Image Compression and Nodule Volume Measurement
JPEG 2000 is a recently developed standard based on wavelet technology. JJ 2000 4.1, a two-dimensional Java-based implementation of a version of JPEG 2000 (jj2000.epfl.ch), was adapted for use in CT data compression. The compression software was installed on a workstation (Ultra 10; Sun Microsystems, Santa Clara, Calif) with a Unix-based operating system (Solaris 8; Sun Microsystems). Reconstructed images were compressed to four levels (with compression ratios of 10:1, 20:1, 30:1, and 40:1) for comparison with the original noncompressed images (compression ratio of 1:1). Thus, there were five compression levels, for a total of 255 observations in 51 nodules.
Volume Measurement Algorithm
A C-language program was developed to measure the volume of each nodule by using an improved version of a previously described “partial-volume method” (10). The measurement algorithm was based on a three-dimensional approach and required the construction of an over-inclusive volume of interest around each nodule. The volume of interest encompassed the entire nodule not only in the transverse direction but also in the craniocaudal dimension and included some lung background and voxels affected by partial volume averaging (specifically, voxels that included both a part of the nodule and a small amount of lung background). The volume of interest was constructed from regions of interest (ROIs) that were placed by a single observer (J.C., 3 years of medical school training) around the nodule, in every CT section that contained the nodule, on the original noncompressed images. ROIs were placed on the nodule by using an ellipse that, if desired, could easily be re-sized and edited (without necessarily maintaining its original ellipsoid shape) to fit the nodule more closely. ROIs were positioned so that about 1–3 mm of lung was included between the edge of the nodule and the ROI for the entire nodule circumference, except in areas where there was an abutting soft-tissue structure, such as a vessel or the chest wall. ROIs were drawn with the goal of excluding adjacent normal structures such as vessels, fissures, and chest wall, as well as pathologic manifestations such as atelectasis and consolidation. The ROIs were electronically transferred to the corresponding compressed images by using the same matrix coordinates. Each ROI was reviewed on images at each compression level to ensure appropriate placement.
The C-language program estimated the “pure” lung attenuation (Li) for the volume of interest by averaging the attenuation of all voxels that were located at the periphery of the volume of interest and that had attenuation values of less than −700 HU. The “pure” nodule attenuation (Ni) was estimated next by averaging the attenuation of a fixed fraction F of the highest-attenuation voxels in the volume of interest. The optimal value of F was 5%, as had been determined experimentally by using phantoms. The algorithm then considered the set Wi of voxels in the volume of interest that were above the variable threshold attenuation Ti, which was midway between the attenuation of the surrounding lung (Li) and the attenuation of the central region of the nodule (Ni), as described by the equation Ti = (Li + Ni)/2. The algorithm used Wi to create the two sets (a superset of Wi) and (a subset of Wi). was obtained by morphologic growing of Wi by 1.5 mm, and was obtained by erosion of Wi by 1.5 mm. The set difference , a hollow shell 3 mm thick, was then constructed to maximize the likelihood that the set of voxels would contain the lung-nodule interface (partial-volume-averaged voxels) and to minimize the likelihood that it would contain both the nodule core and the surrounding lung. Finally, the partial-volume method (10) was applied to dW, and the resultant volume was added to the interior nodule volume of to obtain the volume of the entire nodule.
Statistical Analysis
A mixed-model analysis of variance was performed for each of two outcome measures. The two outcome measures used were (a) the measured volumes in cubic millimeters and (b) the absolute volume error (ie, the magnitude of the difference between volume measurements obtained on images at a given compression level and on original images). Thus, volumes determined on the basis of original images were assumed to have an absolute error of zero. The outcome measures were the dependent variable, and the model to predict outcome included the indicator variables that defined the level of compression (1:1, 10:1, 20:1, 30:1, 40:1) as well as the selected nodule characteristics, such as size (≤5 mm vs >5 mm in diameter), attenuation (ground glass vs not ground glass), and location (central vs peripheral). The covariance structure was modeled by assuming that observations were either correlated or independent when they were associated with nodules in the same patient or in different patients, respectively. The strength of the correlation depended on whether or not the observations were for the same nodule on images acquired at different compression levels. When a significant effect was detected for a multilevel nominal factor (eg, compression level or location), the Tukey honestly significant difference procedure was used to perform all pairwise comparisons among levels of that factor while maintaining the experimentwise type I error rate for the set of comparisons at or below the nominal 5% level. A P value of less than .05 was considered to indicate a statistically significant difference.
The mixed-model analysis of variance was also used to determine whether the standard deviation of the absolute error of the volume assessment changed as a function of compression level and whether there was a difference between solid nodules and those with ground-glass attenuation in terms of volume assessment with compression level. All statistical computations were performed by using statistical software (SAS, version 9.0; SAS Institute, Cary, NC).
RESULTS
The nodule volumes averaged 388.1 mm3 (range, 34–3474 mm3). There were 18, 19, and 14 nodules in the upper, middle, and lower zones of the lung, respectively. There were three calcified, 33 solid noncalcified, and 15 subsolid nodules, 13 of which had ground-glass attenuation alone and two of which included some solid components as well as areas of ground-glass attenuation. The two nodules that had both areas of ground-glass attenuation and solid components were considered to have ground-glass attenuation, and the three calcified nodules were grouped with nodules that had solid components, because of the small numbers of such nodules. Thirty-one nodules were located in the right lung, and 20 in the left lung. Thirty-two nodules were adjacent to the pleura, 24 abutted the costal pleura, seven were adjacent to a fissure, and one contacted both the costal pleura and the fissure. Seven nodules were located centrally, and 44, peripherally. When measured with calipers, 23 nodules had a diameter greater than 5 mm.
Overall, measured volume decreased with an increasing level of compression (Table 1). When measured volumes and absolute volume errors were analyzed, a significant interaction was found between the level of image compression (P < .001 for each characteristic) and nodule size, location, and attenuation. A follow-up analysis showed no significant difference between measurements obtained on original images and those obtained on images compressed to 10:1 for all nodule characteristics (smaller vs larger, ground-glass attenuation vs non–ground-glass attenuation, central vs peripheral) (Table 2); however, there was a significant difference between original images and those compressed to a level of 20:1 (P < .05) or more. Whether with or without ground-glass attenuation, nodules had significantly smaller volume measurements with increasing compression levels. The magnitude of the effect of increasing image compression was greater, however, for nodules with ground-glass attenuation than for those with non–ground-glass attenuation (Figure). For example, the mean volume measurement on images compressed to a level of 20:1 was 34.9 mm3 less than that on original (compression level, 1:1) images for nodules with ground-glass attenuation, compared with 8.3 mm3 less for solid nodules; in other words, there was a 4.2-fold difference in the volume measurement decrease between nodules with ground-glass attenuation and solid nodules (Table 2). The magnitude of absolute volume error increased with increases in compression level (Table 3). Therefore, there was a significant difference in the absolute volume difference between nodules measured at an image compression level of 10:1 and those measured at 20:1 (P < .05) (Table 3).
TABLE 1.
Nodule Volume Measurements on Compressed Images
| Compression Level | Nodule Volume (mm3)* |
|---|---|
| 1:1 | 388 (34–3474) |
| 10:1 | 383 (26–3445) |
| 20:1 | 370 (34–3314) |
| 30:1 | 360 (33–3225) |
| 40:1 | 354 (33–3187) |
Numbers are the mean of actual measured values in 51 nodules. Numbers in parentheses are the range.
TABLE 2.
Estimated Difference in Mean Nodule Volume for Each Compression Level Comparison according to Nodule Characteristics
| Nodule Attenuation
|
Nodule Location
|
Nodule Diameter
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ground Glass
|
Not Ground Glass
|
Central
|
Peripheral
|
>5 mm
|
≤5 mm
|
|||||||
| Compression Levels Compared | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value |
| 1:1 vs 10:1 | 7.7 | .968 | 2.4 | .825 | 6.2 | .998 | 3.9 | .734 | 5.4 | .978 | 3.3 | .885 |
| 1:1 vs 20:1 | 34.9 | .010 | 8.3 | .001 | 45.9 | .039 | 12.0 | .008 | 25.7 | .018 | 10.5 | .007 |
| 1:1 vs 30:1 | 57.5 | <.001 | 11.7 | <.001 | 72.3 | .038 | 18.5 | <.001 | 40.2 | <.001 | 16.0 | .002 |
| 1:1 vs 40:1 | 71.9 | <.001 | 14.1 | <.001 | 87.0 | .008 | 23.2 | <.001 | 46.9 | <.001 | 21.7 | <.001 |
| 10:1 vs 20:1 | 27.2 | .037 | 6.0 | .025 | 40.0 | .054 | 8.1 | .021 | 20.3 | .036 | 7.2 | .046 |
| 10:1 vs 30:1 | 49.8 | .003 | 9.3 | .016 | 66.1 | .006 | 14.6 | .001 | 24.7 | .007 | 12.6 | .044 |
| 10:1 vs 40:1 | 64.1 | <.001 | 11.8 | .001 | 80.7 | .001 | 19.2 | <.001 | 41.5 | 3.001 | 18.4 | <.001 |
| 20:1 vs 30:1 | 22.6 | .327 | 3.3 | .880 | 26.4 | .728 | 6.5 | .407 | 14.4 | .487 | 5.4 | .777 |
| 20:1 vs 40:1 | 37.0 | .029 | 5.8 | .036 | 41.0 | .031 | 11.1 | .027 | 21.2 | .137 | 11.1 | .128 |
| 30:1 vs 40:1 | 14.4 | .794 | 2.4 | .924 | 14.6 | .959 | 4.7 | .737 | 6.8 | .948 | 5.7 | .737 |
The difference in mean nodule volume between compression levels was calculated as the mean volume at the lower level of compression (eg, 10:1) minus the mean volume at the higher compression level (eg, 20:1).
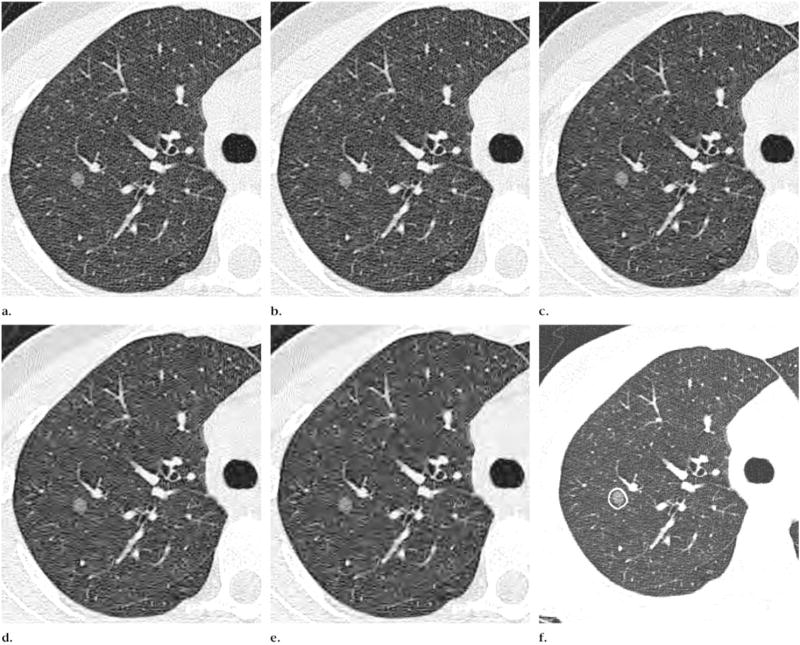
Figure.
Transverse CT images at the same anatomic level show a nodule with ground-glass attenuation at image compression levels of (a) 1:1 (no compression), (b) 10:1, (c) 20:1, (d) 30:1, and (e) 40:1. Note the increasing loss in definition of parenchymal architecture and nodule border with increase in compression level, particularly nodule border in e. (f) Noncompressed image shows delineation of ROI (contour line).
TABLE 3.
Difference in Mean Absolute Volume Error for Each Compression Level Comparison according to Nodule Characteristics
| Nodule Attenuation
|
Nodule Location
|
Nodule Diameter
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ground Glass
|
Not Ground Glass
|
Central
|
Peripheral
|
>5 mm
|
≤5 mm
|
|||||||
| Compression Levels Compared | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value | Estimated Difference (mm3)* | P Value |
| 10:1 vs 20:1 | −24.6 | .031 | −5.1 | .011 | −35.4 | .044 | −7.5 | .017 | −17.9 | .034 | −7.3 | .027 |
| 10:1 vs 30:1 | −47.0 | .002 | −9.4 | <.001 | −62.0 | .035 | −14.5 | <.001 | −31.6 | .005 | −14.1 | .003 |
| 10:1 vs 40:1 | −62.2 | <.001 | −11.6 | <.001 | −76.5 | .007 | −19.3 | <.001 | −40.0 | <.001 | −18.9 | <.001 |
| 20:1 vs 30:1 | −22.4 | .182 | −4.3 | .258 | −26.6 | .492 | −7.0 | .11 | −13.8 | .292 | −6.8 | .282 |
| 20:1 vs 40:1 | −37.6 | .010 | −6.5 | .016 | −41.2 | .161 | −11.8 | <.001 | −22.1 | .041 | −11.6 | .012 |
| 30:1 vs 40:1 | −15.2 | .619 | −2.2 | .648 | −14.5 | .776 | −4.7 | .44 | −8.4 | .790 | −4.7 | .539 |
The difference between compression levels in terms of the absolute volume error was calculated as the absolute volume error at the lower compression level (eg, 10:1) minus the absolute volume error at the higher compression level (eg, 20:1).
There was a systematic increase in the variance of the absolute error of the volume assessments as the level of compression increased, with significant increases between 10:1 and 20:1 (P < .01), 20:1 and 30:1 (P < .01), and 30:1 and 40:1 (P < .05) (Table 4). There was a significant difference between solid nodules and those with ground-glass attenuation in the variance for absolute volume error (P < .01), with ground-glass attenuation associated with a significantly higher absolute volume error.
TABLE 4.
Standard Deviation of Absolute Error in Nodule Volume Measurement at Each Compression Level
| Compression Level | Standard Deviation (mm3) |
|---|---|
| 1:1 | 0 |
| 10:1 | 8.5 |
| 20:1 | 30.3 |
| 30:1 | 48.2 |
| 40:1 | 57.6 |
DISCUSSION
The effect of image compression on quantitative measures obtained from imaging studies has not been evaluated extensively. In particular, to our knowledge, its effect on volumetric measurements on CT images has not been assessed previously. We demonstrated that the use of a two-dimensional JPEG 2000 wavelet-based image compression method introduced differences in error from that with the original noncompressed images on the order of 3–8 mm3 for 10:1 compressed images and on the order of 8–46 mm3 for 20:1 compressed images. Significantly, still larger differences were identified at an image compression level of 20:1 or greater. Additionally, the variances of the absolute volume errors increased significantly between all levels.
Our results are not unexpected, given that the identification of nodules by readers on both CT images and radiographs has been previously demonstrated to be detrimentally affected by image compression levels ranging between 10:1 and 20:1 (5,8,9,11). In an analysis of 100 coronary angiographic sequences, Kerensky et al (12) demonstrated that use of a JPEG cosine-transform method with an image compression ratio of 16:1 decreased sensitivity for the detection of diagnostic features and with a compression ratio of 10:1 reduced reader diagnostic confidence. Tuinenberg et al (13) studied the influence of image compression on measurement of coronary artery stenoses on arteriograms. Using a JPEG method, they demonstrated significant systematic and random differences in the calibration factor and vessel measurements at a compression level of 10:1.
There was a significant interaction between image compression and nodule size, location, and attenuation, with greater measurement errors associated with larger nodule size, central location, and ground-glass attenuation. It is not surprising that errors were greater for nodules with ground-glass attenuation; because such nodules have lower contrast with the surrounding lung, the quality of their depiction is less tolerant to image compression. In a study of reader diagnostic performance (9), the detection of nodules with ground-glass attenuation also was more affected by image compression than was that of solid nodules. Larger errors for central nodules in our study may be due to the greater likelihood of their being in contact with adjacent vessels and of this contact resulting in complex and extensive interfaces that hinder easy and accurate delineation of the border of a nodule. Such interfaces may be more susceptible to blurring-related distortion than are interfaces between solid peripheral nodules and the chest wall.
It is necessary to consider whether the volume errors that occur with compression at a level of 10:1 or even 20:1 are acceptable for clinical practice. Measurement accuracy on the order of 2–5 mm3 for simulated nodules in a realistic phantom environment was reported by Ko et al (10). Using their quantitative methods for measuring synthetic nodules imaged in air, Yankelevitz et al (2) demonstrated accuracy of approximately 3% and precision of approximately 2%. The compression-related error from our study was 8–46 mm3 for a compression level of 20:1. Therefore, image compression may significantly increase error in volume measurement of nodules. With regard to in vivo reproducibility, however, a larger variation that is at present poorly understood may occur because of respiratory motion, differences in respiratory phase, CT artifacts, and/or differences in nodule positioning between studies (14,15).
In this study, we tested only one image compression method, and therefore our results may not be reflective of those obtained from images compressed with different algorithms. The use of three-dimensional compression algorithms that exploit the similarity of data along the z-axis and that are not only based on data within a transverse section may better preserve image characteristics and minimize the effects of compression on quantitative measurements. We also assessed the influence of image compression on nodule volume assessment on low-dose chest CT images reconstructed with a high-frequency algorithm and a particular volume measurement method. Therefore, the effect of compression on different measurement techniques, diagnostic-quality CT images, and images reconstructed with the use of other algorithms has not been evaluated. The ROIs were placed by an observer who was not a radiologist but who completed 3 years of medical school and underwent training in lung anatomy on CT scans to supplement previous knowledge of lung anatomy. ROIs were transposed from the original images to the compressed images, a method that helped to minimize the variability related to the computer method itself. ROI placement with a similar technique was found in a prior study not to cause significant volume measurement changes (10).
In conclusion, nodule volume measurements obtained on images compressed to a level of 20:1 differed significantly from those obtained on original noncompressed images, especially for nodules with ground-glass attenuation. There was no statistically significant effect with a 10:1 compression level. The depiction of nodules with ground-glass attenuation was more affected by image compression than was that of solid nodules. These data have clear implications for the use of computer-assisted measurement of lung nodules, especially when follow-up CT studies are performed to assess interval change in nodule size. Therefore, the comparison of noncompressed images with compressed images would affect the assessment of nodule size and interval change as measured with computer-assisted methods.
Acknowledgments
J.P.K. supported by National Institutes of Health grant K23 CA096604
The authors acknowledge the expert CT technical assistance provided by Emilio Vega, RT, and Bernard Assadourian, RT, at New York University Department of Radiology.
Abbreviations
- JPEG
Joint Photographic Experts Group
- ROI
region of interest
Footnotes
Authors stated no financial relationship to disclose.
References
- 1.Awai K, Murao K, Ozawa A, et al. Pulmonary nodules at chest CT: effect of computer-aided diagnosis on radiologists’ detection performance. Radiology. 2004;230:347–352. doi: 10.1148/radiol.2302030049. [DOI] [PubMed] [Google Scholar]
- 2.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217:251–256. doi: 10.1148/radiology.217.1.r00oc33251. [DOI] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Rubin GD. Data explosion: the challenge of multidetectorrow CT. Eur J Radiol. 2000;36:74–80. doi: 10.1016/s0720-048x(00)00270-9. [DOI] [PubMed] [Google Scholar]
- 5.Slone RM, Foos DH, Whiting BR, et al. Assessment of visually lossless irreversible image compression: comparison of three methods by using an image-comparison workstation. Radiology. 2000;215:543–553. doi: 10.1148/radiology.215.2.r00ap47543. [DOI] [PubMed] [Google Scholar]
- 6.Erickson BJ, Manduca A, Persons KR, et al. Evaluation of irreversible compression of digitized posterior-anterior chest radiographs. J Digit Imaging. 1997;10:97–102. doi: 10.1007/BF03168595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg MA, Gazelle GS, Boland GW, et al. Focal hepatic lesions: effect of three-dimensional wavelet compression on detection at CT. Radiology. 1997;202:159–165. doi: 10.1148/radiology.202.1.8988206. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Sone S, Takashima S, et al. Effects of JPEG and wavelet compression of spiral low-dose CT images on detection of small lung cancers. Acta Radiol. 2001;42:156–160. doi: 10.1080/028418501127346657. [DOI] [PubMed] [Google Scholar]
- 9.Ko JP, Rusinek H, Naidich DP, et al. Wavelet compression of low-dose chest CT data: effect on lung nodule detection. Radiology. 2003;228:70–75. doi: 10.1148/radiol.2281020254. [DOI] [PubMed] [Google Scholar]
- 10.Ko JP, Rusinek H, Jacobs E, et al. Volume measurement of small pulmonary nodules on chest CT: a phantom study. Radiology. 2003;228:864–870. doi: 10.1148/radiol.2283020059. [DOI] [PubMed] [Google Scholar]
- 11.Cosman PC, Davidson HC, Bergin CJ, et al. Thoracic CT images: effect of lossy image compression on diagnostic accuracy. Radiology. 1994;190:517–524. doi: 10.1148/radiology.190.2.8284409. [DOI] [PubMed] [Google Scholar]
- 12.Kerensky RA, Cusma JT, Kubilis P, et al. American College of Cardiology/European Society of Cardiology International Study of Angiographic Data Compression phase I: the effects of lossy data compression on recognition of diagnostic features in digital coronary angiography. Eur Heart J. 2000;21:668–678. doi: 10.1053/euhj.1999.2100. [DOI] [PubMed] [Google Scholar]
- 13.Tuinenburg JC, Koning G, Hekking E, et al. American College of Cardiology/European Society of Cardiology International Study of Angiographic Data Compression phase II: the effects of varying JPEG data compression levels on the quantitative assessment of the degree of stenosis in digital coronary angiography. Joint Photographic Experts Group. J Am Coll Cardiol. 2000;35:1380–1387. doi: 10.1016/s0735-1097(99)00611-7. [DOI] [PubMed] [Google Scholar]
- 14.Kostis WJ, Yankelevitz DF, Reeves AP, Fluture SC, Henschke CI. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology. 2004;231:446–452. doi: 10.1148/radiol.2312030553. [DOI] [PubMed] [Google Scholar]
- 15.Wormanns D, Kohl G, Klotz E, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol. 2004;14:86–92. doi: 10.1007/s00330-003-2132-0. [DOI] [PubMed] [Google Scholar]